Abstract
Background
The incidence of well-differentiated thyroid cancer (WDTC) has increased in recent years. Despite its excellent prognosis, increasing morbidity from recurrent diseases continues to affect long-term outcomes. Among at-risk populations, Filipinos have the highest incidence of thyroid cancer worldwide, characterized by a highly aggressive and recurrent form of disease. Here, we sought to identify risk factors associated with disease recurrence among Filipinos with WDTC.
Methods
This retrospective cohort study examined 723 patients diagnosed with WDTC seen at Philippine General Hospital. Affected individuals were classified based on the presence or absence of disease recurrence. Multivariate logistic regression analyses were used to determine significant predictors of recurrence.
Results
Multiple risk factors, including age >45 years (odds ratio [OR], 1.44), multifocality of cancer (OR, 1.43), nodal involvement (OR, 4.0), and distant metastases at presentation (OR, 2.78), were significantly associated with a recurrence of papillary thyroid cancer (PTC). In contrast, follicular variant histology (OR, 0.60) and postsurgical radioactive iodine therapy (OR, 0.31) were protective for PTC recurrence. Distant metastases at presentation (OR, 19.4) and postsurgical radioactive iodine therapy (OR, 0.41) were associated with follicular thyroid cancer (FTC) recurrence.
Conclusion
Lymph node metastases at presentation was the strongest predictor of recurrence in PTC, whereas distant metastases at presentation was the strongest for FTC recurrence. Among Filipinos, stratification of WDTC patients based on recurrence risk factors identified in this study will be helpful in guiding the intensity of treatment strategies and long-term thyroid cancer surveillance.
Keywords: Thyroid neoplasms; Thyroid cancer, follicular; Thyroid cancer, papillary
INTRODUCTION
The incidence of well-differentiated thyroid cancer (WDTC) has steadily increased over the past few decades, due largely to improvements in diagnostic modalities. This increase in both tumor incidence and improved detection has also led to significant increases in the incidence of thyroid cancer recurrence [1]. These improvements have led to earlier diagnosis and treatment, particularly in patients with recurrent disease, resulting in improved survival, even for those with distant metastases at presentation [2]. Although overall prognosis in WDTC remains excellent in comparison with that in other malignancies [3], recent improvements in WDTC diagnostics have had little effect on mortality, with the disease-specific death rate remaining stable at -1.3% [4].
Recurrence of thyroid cancer is often associated with increased morbidity, although it is also thought to have an impact on mortality. Patients who developed a recurrent disease after curative treatment have shorter survival compared with disease-free counterparts, with 50% to 60% eventually dying of the disease [4]. In response, current management guidelines recommend aggressive detection and control of recurrent disease while minimizing morbidity to the patient [5]. Recent studies estimate recurrence rates of WDTC at -30%, with the majority of recurrences occurring within the first 10 years after primary surgical treatment [6]. Age, male gender, increased tumor size, extra-thyroidal extension, nodal metastases at presentation, and aggressive histological type are just some of the factors known to increase the likelihood of cancer recurrence [7].
Recent studies conducted in Hawaii [8,9] and Los Angeles, California [10] have identified Filipinos as the ethnic group with the highest incidence of thyroid cancer. Among this population, thyroid cancers were also found to be more aggressive and recurrent in nature [11], with a significantly higher risk of malignancy in Filipinos with thyroid nodules compared with in other racial ethnicities [12]. However, despite this greater risk, no study has directly examined the recurrence rates, risk factors, and long-term outcomes of Filipinos with WDTC. Here, we examined recurrence rates and investigated risk factors associated with recurrence in Filipino patients diagnosed with WDTC.
METHODS
Study design
We performed a retrospective cohort study of patients diagnosed with WDTC (papillary or follicular) seen at the thyroid outpatient clinic of Philippine General Hospital (PGH), Manila, Philippines. This study was approved by the Research Ethics Board Panel of PGH. Informed consent was obtained from all participants. All patient information was anonymized and kept confidential.
Inclusion criteria
Adult patients (age >18 at the time of diagnosis) diagnosed with WDTC (papillary or follicular) were included in this study. Diagnoses were confirmed using final histopathological biopsy reports following thyroid surgery, regardless of the extent of surgical intervention.
Exclusion criteria
Patients diagnosed with follicular adenoma, poorly differentiated thyroid cancer, thyroid lymphoma, metastatic cancer, hurtle cell carcinoma, insular thyroid carcinoma, and anaplastic thyroid cancer were excluded from this study; patients lost to follow-up were also excluded.
Chart retrieval
Outpatient charts of all included patients were retrieved using their hospital case numbers via the PGH medical record database. Only charts that were active in circulation and those available and retrievable from the medical records database were included. Once retrieved, all charts were then carefully assessed and documented by a single investigator.
Data collection
Age at diagnosis (years); sex; duration of goiter (in months); family history; social history; associated comorbidities; metastasis, age at presentation, completeness of surgical resection, invasion (extrathyroidal), and size (MACIS) score; stage at diagnosis (local, regional, distant, tumor-node-metastasis [TNM] classification); therapy received (surgical intervention, radioisotope therapy with surgery, chemotherapy); and survival (months from diagnosis until death) were all documented and recorded using descriptive statistical analysis (mean, median, mode, standard deviation). These variables were then analyzed as possible predictors of tumor recurrence.
Tumor recurrence was assessed only after thyroidectomy and assessment of its time of appearance in months post-thyroidectomy. All recurrences were reported if one of the following was present: (1) elevated stimulated (>2 µg/L) or unstimulated (>1 µg/L) serum thyroglobulin after thyroidectomy and radioactive ablation; (2) recurrent or new-onset lymphadenopathies proven to be thyroid cancer by biopsy or radioiodine scan; and (3) recurrent or new-onset distant metastases proven to be thyroid cancer by biopsy or radioiodine scan.
Statistical analysis
Patients were separated into two groups (those with tumor recurrence and those without), and groups were compared using a chi-square t test. Multivariate logistic regression analyses were then used to determine significant predictors of tumor recurrence. Kaplan-Meier analysis was then used to compare each of the significant predictors. The predictive power of each variable was calculated and expressed using odds ratio, 95% confidence interval (CI), and P values.
RESULTS
A total of 649 patients with papillary thyroid cancer (PTC) and 79 patients with follicular thyroid cancer (FTC) were included in this study, with a mean follow-up duration of 53±12 and 83±23 months, respectively. Only five patients were lost to follow-up and therefore excluded from analysis. Of this group, 214 patients (32.9%) with PTC and 23 patients (29.1%) with FTC developed a recurrence, with mean intervals of 13±6 and 26±15 months from thyroidectomy, respectively. Patients with FTC were older at presentation and had less lymph node involvement at presentation. Tumor sizes for recurrent PTC and FTC were nearly similar (3.30±2.0 cm vs. 3.27±2.0 cm, P=0.36). More than 50% of patients with a recurrence underwent repeated radioactive iodine ablation therapy, averaging two sessions of -200 to 300 mCi total received dose. Nearly 25% of patients underwent repeated surgical resection to remove tumor remnants or lymph node metastases. Compared with patients with recurrent PTC, more patients with recurrent FTC underwent additional external beam radiotherapy. Chemotherapy was not a common treatment modality used in this population. Recurrent FTC had a higher mortality rate, relative to PTC (8.7% vs. 0.9%, respectively) (Table 1).
Table 1. Baseline Characteristics of Filipino Patients with Recurrent Well-Differentiated Thyroid Cancer.
| Characteristic | Papillary thyroid cancer (n=214) | Follicular thyroid cancer (n=23) |
|---|---|---|
| Age, yr | 44.3±13.4 | 48.9±11.4 |
| Sex male, % | 20.1 | 13.0 |
| Lymph node involvement at presentation | 85 (39.7) | 6 (26.1) |
| Tumor size, cm | 3.3±2.0 | 3.27±2.0 |
| Interval between recurrence and thyroidectomy, mo | 13±6 | 26±15 |
| Follow-up duration, mo | 53±12 | 83±23 |
| Repeated surgery | 47 (22.0) | 6 (26.1) |
| Repeated radioactive iodine therapy | 110 (51.4) | 12 (52.2) |
| External beam radiotherapy | 7 (3.3) | 5 (21.7) |
| Chemotherapy | 1 (0.4) | 0 |
| Mortality | 2 (0.9) | 2 (8.7) |
Values are expressed as mean±SD or number (%).
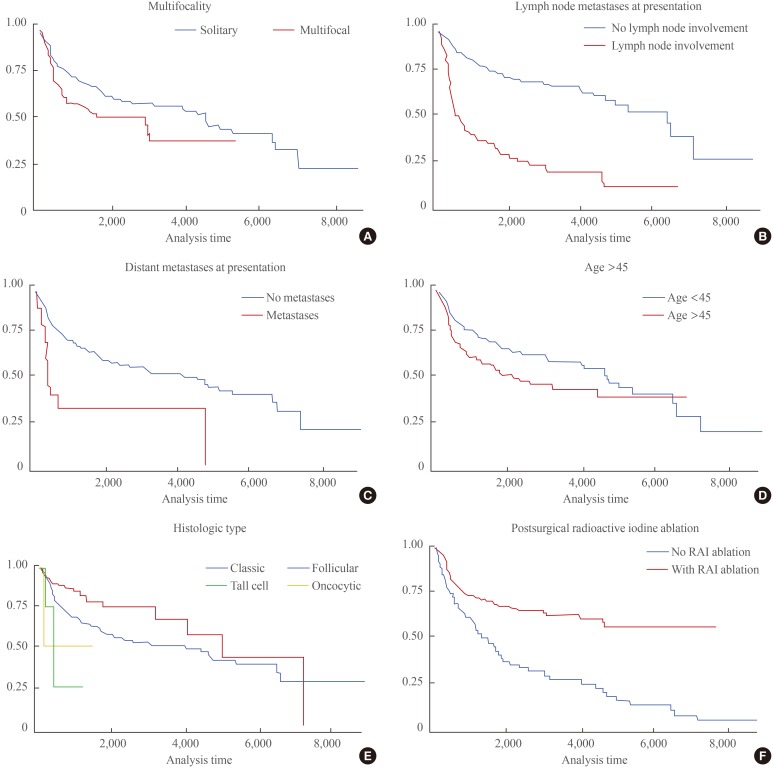
Male sex, age >45 years, history of smoking, alcohol intake, tumor size >4 cm, multifocality of cancer, bilateral lobe involvement, tall cell variant histology, lymph node metastases at presentation, distant metastases at presentation, and high MACIS scores (>7) were identified as significant risk factors for the recurrence of PTC in Filipinos. In contrast, follicular variant histology and postsurgical radioactive iodine ablation therapy were identified as significant protective factors against PTC recurrence (Table 2). A multivariate logistic regression analysis of all significant factors revealed that age >45 years, lymph node metastases at presentation, distant metastases at presentation, and multifocality of cancer were independent risk factors, whereas follicular variant histology and postsurgical radioactive iodine ablation therapy were protective against PTC recurrence regardless of stage at presentation (Table 3). The relationship of the significant variables involved in PTC recurrence, regardless of effect, are clearly visible using a Kaplan-Meier curve (Fig. 1).
Table 2. Predictive Variables for Recurrence in Filipinos with Well-Differentiated Thyroid Cancer.
| Variable | Presence of recurrence | Absence of recurrence | Odds ratio | P value <0.05 |
|---|---|---|---|---|
| Papillary thyroid cancer | ||||
| Number | 214 | 435 | ||
| Male sex | 43 (20.1) | 53 (12.2) | 1.96 | <0.001 |
| Age >45 yr | 110 (51.4) | 184 (42.3) | 1.60 | 0.001 |
| Family history | 4 (1.9) | 4 (0.9) | 1.50 | 0.422 |
| Smoking history | 39 (18.2) | 32 (7.4) | 2.66 | <0.001 |
| Alcohol intake | 48 (22.4) | 41 (9.4) | 2.89 | <0.001 |
| Diabetes | 20 (9.4) | 35 (8.1) | 1.17 | 0.521 |
| Hypertension | 59 (27.6) | 108 (24.8) | 1.11 | 0.490 |
| Hashimoto's thyroiditis | 8 (3.7) | 32 (7.4) | 0.66 | 0.257 |
| Graves' disease/hyperthyroidism | 12 (5.6) | 29 (6.7) | 1.11 | 0.710 |
| Second malignancy | 6 (2.8) | 14 (3.2) | 0.70 | 0.384 |
| Tumor size >4 cm (T3) | 65 (30.4) | 86 (19.8) | 1.80 | <0.001 |
| Multifocality of cancer | 60 (28.0) | 92 (21.2) | 1.62 | 0.002 |
| Bilateral lobe involvement | 45 (21.0) | 69 (15.9) | 1.70 | 0.002 |
| Follicular variant histology | 19 (8.9) | 82 (18.9) | 0.55 | 0.013 |
| Tall cell variant histology | 3 (1.4) | 2 (0.5) | 3.70 | 0.025 |
| Oncocytic variant histology | 1 (0.5) | 1 (0.2) | 2.15 | 0.444 |
| Lymph node metastases at presentation (N1) | 119 (55.6) | 75 (17.2) | 4.45 | <0.001 |
| Distant metastases at presentation (M1) | 14 (6.5) | 10 (2.3) | 3.35 | <0.001 |
| High MACIS score (>7) | 24 (11.2) | 19 (4.4) | 2.86 | <0.001 |
| Incomplete thyroidectomy | 14 (6.5) | 24 (5.5) | 0.94 | 0.804 |
| Postsurgical radioactive ablation | 130 (60.6) | 335 (77.0) | 0.43 | <0.001 |
| TSH suppression <0.1 mIU/L | 87 (40.9) | 53 (12.6) | 1.36 | 0.761 |
| Follicular thyroid cancer | ||||
| Number | 23 | 56 | ||
| Male sex | 3 (13.0) | 4 (7.1) | 3.15 | 0.077 |
| Age >45 yr | 14 (60.9) | 25 (44.6) | 2.50 | 0.043 |
| Family history | 1 (4.3) | 2 (3.6) | <0.01 | 1.000 |
| Smoking history | 2 (8.7) | 5 (8.9) | 2.93 | 0.163 |
| Alcohol intake | 2 (8.7) | 8 (14.3) | 1.16 | 0.844 |
| Diabetes | 2 (8.7) | 2 (3.6) | 3.67 | 0.089 |
| Hypertension | 4 (17.4) | 16 (28.6) | 0.67 | 0.470 |
| Hashimoto's thyroiditis | 1 (4.3) | 1 (1.8) | <0.01 | 1.000 |
| Second malignancy | 1 (4.3) | 1 (1.8) | <0.01 | 1.000 |
| Tumor size >4 cm (T3) | 5 (21.7) | 20 (35.7) | 0.86 | 0.775 |
| Multifocality of cancer | 3 (13.0) | 3 (5.4) | 4.25 | 0.027 |
| Bilateral lobe involvement | 3 (13.0) | 2 (3.6) | 5.54 | 0.009 |
| Lymph node metastases at presentation (N1) | 3 (13.0) | 3 (5.4) | 1.63 | 0.435 |
| Distant metastases at presentation (M1) | 9 (39.1) | 1 (1.8) | 21.56 | <0.001 |
| Incomplete thyroidectomy | 7 (30.4) | 1 (1.8) | 5.32 | <0.001 |
| Postsurgical radioactive ablation | 11 (47.8) | 47 (83.9) | 0.29 | 0.004 |
| TSH suppression <0.1 mIU/L | 15 (65.2) | 45 (80.4) | 0.686 | 0.410 |
Values are expressed as number (%).
MACIS, metastasis, age at presentation, completeness of surgical resection, invasion (extrathyroidal), and size; TSH, thyroid stimulating hormone.
Table 3. Multivariate Logistic Regression Analysis of Significant Risk Factors for Recurrence in Filipinos with Well-Differentiated Thyroid Cancer.
| Risk factor | Odds ratio (95% CI) | P value |
|---|---|---|
| Papillary thyroid cancer | ||
| Age >45 yr | 1.44 (1.09-1.89) | 0.010 |
| Lymph node metastases at presentation (N1) | 4.00 (2.99-5.34) | <0.001 |
| Distant metastases at presentation (M1) | 2.78 (1.59-4.84) | <0.001 |
| Multifocality | 1.43 (1.05-1.95) | 0.023 |
| Follicular variant histology | 0.60 (0.37-0.97) | 0.037 |
| Postsurgical radioactive ablation | 0.31 (0.24-0.42) | <0.001 |
| Follicular thyroid cancer | ||
| Distant metastases at presentation (M1) | 19.4 (6.28-59.96) | <0.001 |
| Postsurgical radioactive ablation | 0.41 (0.17-0.98) | 0.044 |
CI, confidence interval.
Fig. 1. Kaplan-Meier disease-free estimate curves of significant factors for papillary thyroid cancer recurrence. (A) Multifocality. (B) Lymph node metastases at presentation. (C) Distant metastases at presentation. (D) Age >45. (E) Histologic type. (F) Postsurgical radioactive iodine (RAI) ablation.
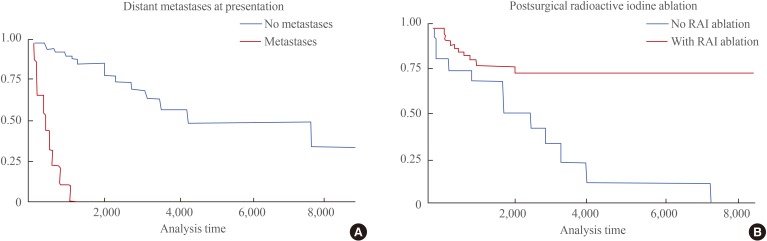
Factors including age >45 years, tumor size >4 cm, multifocality of cancer, bilateral lobe involvement, distant metastases at presentation, and incomplete thyroidectomy were all identified as significant risk factors for the recurrence of FTC in Filipinos. In contrast, postsurgical radioactive iodine ablation therapy was the only statistically significant protective factor against FTC recurrence (Table 2). A multivariate logistic regression analysis of all significant factors revealed distant metastases at presentation as the only independent risk factor, whereas postsurgical radioactive iodine ablation was further validated as a protective factor for FTC recurrence (Table 3). The relationships between variables significantly related to the recurrence of FTC, whether positively or negatively, are shown using a Kaplan-Meier curve (Fig. 2).
Fig. 2. Kaplan-Meier disease-free estimate curves of significant factors for follicular thyroid cancer recurrence. (A) Distant metastases at presentation. (B) Postsurgical radioactive iodine (RAI) ablation.
DISCUSSION
The incidence of WDTC continues to rise, due in part to steady progress in imaging and more sensitive monitoring methods [1]. Despite the excellent prognosis of WDTC, increasing morbidity from recurrences of this disease continues to affect long-term outcomes, leading to higher medical costs and poorer quality of life [3]. The impact of locoregional recurrence on survival continues to be controversial, as there is no established link between nodal recurrence and mortality [13,14], and only distant metastases have been clearly shown to affect disease-specific survival [13]. Indeed, one study directly addressing this issue found that all the disease-related deaths in their study were due to distant recurrence, with no patients dying from uncontrolled locoregional recurrent disease [2].
Although the high recurrence rates observed in our study closely resembled those observed in the study by Kus et al. [11] among Filipinos in Canada, this association can likely be attributed to the larger baseline tumor size and delayed medical access of most of the patients in this study. Whether Filipino ethnicity represents an independent factor contributing to the aggressiveness of thyroid cancer remains difficult to discern at this time. Further genetic studies are necessary to establish the claim for more aggressive thyroid cancer behavior among Filipinos.
Disease-associated factors identified in our study were broadly comparable with those identified in other studies. Lymph node metastases at presentation appears to be the only factor consistently predictive of thyroid cancer recurrence across studies. Distant metastases at presentation has been consistently identified as an important risk factor for tumor recurrence (Table 4) [7,15,16,17]. The majority of nodal metastases start at the central nodal compartment, followed by the ipsilateral lateral nodal compartment, which is consistent with the typical lymphatic drainage pathway of the thyroid gland. Distant recurrence occurs commonly in the lung and bone but can also be seen in other sites. It has been shown that the location of recurrence has a prognostic significance for patients with recurrent WDTC, with distant metastases at presentation predictive of poor prognosis [18].
Table 4. Comparison of Multivariate Analyses for Recurrence of Well-Differentiated Thyroid Cancer across Different International Studies.
| Risk factor | Our data | Palme et al. (2004) [15] | Baek et al. (2010) [7] | Kim et al. (2014) [16] | Ito et al. (2012) [17] |
|---|---|---|---|---|---|
| Age | √ | X | X | X | √ |
| Male sex | X | √ | X | √ | √ |
| Tumor size | X | √ | X | X | √ |
| Nodal metastases | √ | √ | √ | √ | √ |
| Distant metastases | √ | √ | NA | √ | NA |
| Histologic type | √ | X | NA | √ | NA |
| Multifocality | √ | X | X | X | NA |
| Incomplete thyroidectomy | X | √ | X | X | NA |
| Postsurgical radioactive iodine ablation | √ | X | NA | NA | NA |
NA, not assessed.
The 45-year age cutoff used by the International Union Against Cancer (UICC) TNM classification was also shown to be a significant risk factor for WDTC recurrence among Filipinos. Multifocality and bilaterality of thyroid malignancy were unique to this study as significant risk factors for WDTC recurrence. This observation may be indicative of greater aggressiveness and higher malignancy potential in thyroid nodules among Filipinos [12]. High MACIS scores in PTC (>7) were also shown to be predictive of PTC recurrence, which is consistent with its use as a potential prognostic scoring system [19].
Some variants of PTC have been associated with higher risk for disease recurrence and aggressiveness, including the tall cell variant of PTC, which is often seen as a more aggressive variant of PTC, increasing the risk for PTC recurrence [20]. On the other hand, the follicular variant of PTC was identified as a significant protective factor for PTC recurrence. This is consistent with the behavior observed in several studies on follicular variant PTC that showed lower nodal metastases at presentation with similar long-term prognoses as those of classic PTC [21,22].
Among the different therapeutic modalities for WDTC, only post-surgical radioactive iodine ablative therapy was shown to significantly reduce recurrence. In our study, complete thyroidectomy was a significant protective factor according to univariate analysis, but it turned out to be insignificant when multivariate analysis was applied. This clearly proves that surgery alone is insufficient as a means of risk reduction or complete prevention of future recurrences; it must therefore be combined with post-surgical radioactive therapy [23]. Surprisingly, adequate TSH suppression therapy did not significantly lower recurrence in our study, suggesting only a minor role in our population as opposed to previous reports [24].
In conclusion, the recurrence rates for PTC and FTC in this study were 32.9% and 29.1% respectively. The most significant predictors of recurrence of PTC and FTC were nodal metastases at presentation and distant metastases at presentation, respectively. The factors contributing to the recurrence of WDTC among Filipinos that were identified in this study will be helpful in guiding treatment strategies and long-term thyroid cancer surveillance aimed to reduce future morbidity and mortality in this population.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Magarey MJ, Freeman JL. Recurrent well-differentiated thyroid carcinoma. Oral Oncol. 2013;49:689–694. doi: 10.1016/j.oraloncology.2013.03.434. [DOI] [PubMed] [Google Scholar]
- 3.Nixon IJ, Ganly I, Palmer FL, Whitcher MM, Patel SG, Tuttle RM, et al. Disease-related death in patients who were considered free of macroscopic disease after initial treatment of well-differentiated thyroid carcinoma. Thyroid. 2011;21:501–504. doi: 10.1089/thy.2010.0451. [DOI] [PubMed] [Google Scholar]
- 4.Coburn M, Teates D, Wanebo HJ. Recurrent thyroid cancer. Role of surgery versus radioactive iodine (I131) Ann Surg. 1994;219:587–593. doi: 10.1097/00000658-199406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 7.Baek SK, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20:147–152. doi: 10.1089/thy.2008.0243. [DOI] [PubMed] [Google Scholar]
- 8.Kolonel LN. Cancer incidence among Filipinos in Hawaii and the Philippines. Natl Cancer Inst Monogr. 1985;69:93–98. [PubMed] [Google Scholar]
- 9.Goodman MT, Yoshizawa CN, Kolonel LN. Descriptive epidemiology of thyroid cancer in Hawaii. Cancer. 1988;61:1272–1281. doi: 10.1002/1097-0142(19880315)61:6<1272::aid-cncr2820610636>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Haselkorn T, Bernstein L, Preston-Martin S, Cozen W, Mack WJ. Descriptive epidemiology of thyroid cancer in Los Angeles County, 1972-1995. Cancer Causes Control. 2000;11:163–170. doi: 10.1023/a:1008932123830. [DOI] [PubMed] [Google Scholar]
- 11.Kus LH, Shah M, Eski S, Walfish PG, Freeman JL. Thyroid cancer outcomes in Filipino patients. Arch Otolaryngol Head Neck Surg. 2010;136:138–142. doi: 10.1001/archoto.2009.206. [DOI] [PubMed] [Google Scholar]
- 12.Clark JR, Eski SJ, Freeman JL. Risk of malignancy in Filipinos with thyroid nodules: a matched pair analysis. Head Neck. 2006;28:427–431. doi: 10.1002/hed.20333. [DOI] [PubMed] [Google Scholar]
- 13.Patron V, Hitier M, Bedfert C, Le Clech G, Jegoux F. Occult lymph node metastases increase locoregional recurrence in differentiated thyroid carcinoma. Ann Otol Rhinol Laryngol. 2012;121:283–290. doi: 10.1177/000348941212100501. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Clinical outcomes of patients with papillary thyroid carcinoma after the detection of distant recurrence. World J Surg. 2010;34:2333–2337. doi: 10.1007/s00268-010-0712-0. [DOI] [PubMed] [Google Scholar]
- 15.Palme CE, Waseem Z, Raza SN, Eski S, Walfish P, Freeman JL. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:819–824. doi: 10.1001/archotol.130.7.819. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Park SY, Lee YJ, Lee EK, Kim SK, Kim TH, et al. Risk factors for recurrence after therapeutic lateral neck dissection for primary papillary thyroid cancer. Ann Surg Oncol. 2014;21:1884–1890. doi: 10.1245/s10434-014-3507-y. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–1278. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 18.Waseem Z, Palme CE, Walfish P, Freeman JL. Prognostic implications of site of recurrence in patients with recurrent well-differentiated thyroid cancer. J Otolaryngol. 2004;33:339–344. doi: 10.2310/7070.2004.04013. [DOI] [PubMed] [Google Scholar]
- 19.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LiVolsi VA. Papillary carcinoma tall cell variant (TCV): a review. Endocr Pathol. 2010;21:12–15. doi: 10.1007/s12022-010-9106-y. [DOI] [PubMed] [Google Scholar]
- 21.Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid. 2013;23:1263–1268. doi: 10.1089/thy.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HW, Bhattacharyya N. Clinical behavior of follicular variant of papillary thyroid carcinoma: presentation and survival. Laryngoscope. 2010;120:712–716. doi: 10.1002/lary.20828. [DOI] [PubMed] [Google Scholar]
- 23.Tsang RW, Brierley JD, Simpson WJ, Panzarella T, Gospodarowicz MK, Sutcliffe SB. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–388. [PubMed] [Google Scholar]
- 24.McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002;34:554–564. doi: 10.1080/078538902321117760. [DOI] [PubMed] [Google Scholar]