Abstract
Function of the mammalian translocator protein (TSPO; previously known as the peripheral benzodiazepine receptor) remains unclear because its presumed role in steroidogenesis and mitochondrial permeability transition established using pharmacological methods has been refuted in recent genetic studies. Protoporphyrin IX (PPIX) is considered a conserved endogenous ligand for TSPO. In bacteria, TSPO was identified to regulate tetrapyrrole metabolism and chemical catalysis of PPIX in the presence of light, and in vertebrates, TSPO function has been linked to porphyrin transport and heme biosynthesis. Positive correlation between high TSPO expression in cancer cells and susceptibility to photodynamic therapy based on their increased ability to convert the precursor 5-aminolevulinic acid (ALA) to PPIX appeared to reinforce this mechanism. In this study, we used TSPO knock-out (Tspo−/−) mice, primary cells, and different tumor cell lines to examine the role of TSPO in erythropoiesis, heme levels, PPIX biosynthesis, phototoxic cell death, and mitochondrial bioenergetic homeostasis. In contrast to expectations, our results demonstrate that TSPO deficiency does not adversely affect erythropoiesis, heme biosynthesis, bioconversion of ALA to PPIX, and porphyrin-mediated phototoxic cell death. TSPO expression levels in cancer cells do not correlate with their ability to convert ALA to PPIX. In fibroblasts, we observed that TSPO deficiency decreased the oxygen consumption rate and mitochondrial membrane potential (ΔΨm) indicative of a cellular metabolic shift, without a negative impact on porphyrin biosynthetic capability. Based on these findings, we conclude that mammalian TSPO does not have a critical physiological function related to PPIX and heme biosynthesis.
Keywords: PBR, protoporphyrin IX, mitochondria, bone marrow, photodynamic therapy, cancer
Introduction
Mammalian translocator protein (TSPO),2 previously known as the peripheral benzodiazepine receptor (1), is a highly conserved protein enriched in the outer mitochondrial membrane (2). Despite extensive efforts to characterize TSPO, its precise physiological function remains elusive (3, 4). High levels of TSPO expression in steroidogenic cells, its localization to the outer mitochondrial membrane, and increased steroid production upon pharmacological binding led to the primary prospective model that TSPO was a mitochondrial cholesterol transporter essential for steroidogenesis (5). In recent studies using precise genetic tools, we and others have systematically refuted the involvement of TSPO in this process (6–10). Similarly, copurification of TSPO with putative members of the mitochondrial permeability transition pore (MPTP) (11) and effects mediated by TSPO binding drugs on modulating apoptosis (12, 13) resulted in a secondary model that TSPO was associated with MPTP function and cell death (14). Again, recent discovery of the molecular identity of MPTP (15) and direct testing of MPTP function in the absence of TSPO (16) disputed its direct involvement in cell death processes (reviewed in Ref. 4). These new developments have shifted focus of TSPO function to yet another molecule considered to be an endogenous ligand, protoporphyrin IX (PPIX) (17).
Binding of porphyrins to TSPO has been a consistent property reported in bacteria (18), plants (19), and animals (17). In Rhodobacter sphaeroides, TSPO was found localized to the outer membrane (18) and played a role in negatively regulating photosynthesis genes in response to oxygen (20). Examining tetrapyrrole metabolism after providing the precursor ALA to different Tspo mutants of R. sphaeroides led to a conclusion that TSPO facilitated the export of excessive intermediates in the tetrapyrrole pathway (21). In Bacillus cereus, TSPO mediated a light-induced degradation of PPIX (22). In Arabidopsis thaliana, it was observed that TSPO attenuated ALA-induced porphyria through a potential scavenging mechanism (23). In Danio rerio embryos, pharmacological binding of TSPO protein using PK11195 (N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide) or morpholino antisense oligonucleotides resulted in a loss of circulating erythrocytes (24). In Gallus gallus, pharmacological binding of TSPO using PK11195 decreased globin levels indicating a function in the regulation of heme availability for the assembly of functional hemoglobin (25).
Early studies using TSPO-binding drugs have suggested a functional link between mammalian (Mus musculus and Homo sapiens) TSPO and the induction of hemoglobin synthesis (26, 27). Subsequent studies identified that TSPO could bind the heme precursor PPIX with nanomolar affinity (17) and that TSPO induction that was concomitant with heme biosynthetic enzymes, suggesting a possible involvement in porphyrin transport important for heme biosynthesis (28, 29). It was also demonstrated that knockdown of TSPO expression resulted in mitochondrial accumulation of exogenous PPIX, suggesting that TSPO could be involved in heme metabolism (30).
Pathological overexpression of TSPO has been reported in several cancers like colon, breast, and skin cancers (31, 32). A strong correlation between the aggressive cancer phenotype and expression levels of TSPO has been established in several studies (33–35). Cancer cells also show an increased ability to produce endogenous PPIX when supplemented with ALA (36, 37), a photosensitization mechanism that forms the basis of porphyrin-mediated PDT. In this context, experiments have suggested that TSPO plays a major role in PDT by its action on porphyrins (38, 39).
In search of a functional basis for this proposed relationship between mammalian TSPO and PPIX, we use genetic models, Tspo gene deleted (Tspo−/−) mice, Tspo−/− primary cells, and TspoΔ/Δ cancer cells to investigate TSPO-specific actions. Our results demonstrate that TSPO is not essential for porphyrin and heme biosynthesis or porphyrin-mediated phototoxic cell death, but that TSPO deficiency affects mitochondrial energy homeostasis that may impact multiple cellular events and responses.
Experimental Procedures
Tspo−/− Mice
Generation and validation of Tspo−/− mice has been previously described (7). Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of Cornell University approved all procedures.
Cells
All cell culture procedures were performed at 37 °C under 5% CO2. Primary murine fibroblasts were cultured from day 14 embryos in growth medium (DMEM containing 10% FBS and 1% nonessential amino acids). In brief, embryos were aseptically collected, and carcasses were minced and plated in cell culture dishes for attachment and growth. The cells were passaged once and frozen for use in experiments. Generation and culture of TSPO deleted MA-10 cells (MA-10:TspoΔ/Δ cells) has been previously described (8). Human colon cancer cell lines were obtained from ATCC and cultured using recommended methods. Cell lines HCT116 and HT29 were cultured in McCoy's 5A medium containing 10% FBS. Cell line LOVO was cultured in F12K medium containing 10% FBS. Cell line DLD-1 was cultured in RPMI1640 containing 10% FBS.
Hematology and Bone Marrow Histology
Blood was collected in heparinized tubes (BD Biosciences). Erythrocyte counts, platelet, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were obtained using an automated analyzer (Advia 120, Siemens). Erythrocyte counts were also confirmed manually using a hemocytometer. Femurs were processed for histology by fixation in 10% formaldehyde, followed by slow decalcification and paraffin embedding. Thin 4-μm sections were cut and stained using hematoxylin and eosin. Bone marrow cytology was assessed on brush preparations made from the marrow and stained using modified Wright's stain. Marrow hematopoietic cellularity and percentage of erythroid cells were assessed on bone marrow histology and cytology preparations by a board certified veterinary pathologist, and the percentage of erythroid precursors was calculated. Images were captured under a light microscope (DM1000; Leica) using a color camera (ICC 50HD; Leica).
Measurement of Heme in Tissues
Heme content was determined using a fluorometric method as described (40). Liver, spleen, and bone marrow samples were homogenized in 1% Triton X-100 in Tris-buffered saline. Homogenized samples were centrifuged at 5000 × g for 10 min. 1 μl of the homogenate was added to 500 μl of saturated (2 m) oxalic acid and boiled for 30 min to produce PPIX from heme. Samples were then centrifuged at 10,000 × g for 10 min at 4 °C, and PPIX fluorescence in the supernatant was measured using a fluorescence spectrophotometer (Infinite 200; Tecan), under excitation at 400 nm and emission at 660 nm, and concentrations were calculated using a standard curve. Pre-existing non-heme PPIX was subtracted from total heme by using a duplicate, unboiled sample. Heme values were normalized to protein content in each sample.
PPIX Uptake and Phototoxicity
For estimating PPIX uptake, fibroblasts treated with 0 (control), 0.5, 1, or 1.5 μm PPIX (Sigma) for 4 h in serum-free medium were collected by trypsinization. The trypsin was neutralized using 0.7 mg/ml type II-O trypsin inhibitor (Sigma) in DMEM to avoid exposure to serum. Cells were then resuspended, fixed using 1% formaldehyde, and assayed using a flow cytometer at an excitation of 488 nm and an emission range of 620–630 nm (Gallios; Beckman Coulter) to estimate the median PPIX uptake by individual cells. For evaluating phototoxicity, fibroblasts were treated with PPIX (0 (control), 0.5, 1, or 1.5 μm) for 4 h as described above were exposed to light at 450 (± 60)-nm wavelength at 160, 240, or 320 mJ using an 800 milliwatt mercury lamp light path fitted with a band-pass filter and a neutral density filter (OD 1.0). After exposure, fibroblasts were provided with growth medium and incubated for 6 h before labeling using propidium iodide (20 μg/ml) and Hoescht 33342 (1 μg/ml) to determine live and dead cells. Populations were counted after acquiring images with an inverted epifluorescence microscope (DM3000; Leica) using a monochromatic cooled camera (DFC365FX; Leica).
Bioconversion of ALA to PPIX
For in vivo experiments, ALA (25 mg/ml in PBS; Sigma) was administered to mice (250 mg/kg body weight intraperitoneal) and euthanized at 0 (baseline), 1, 4, or 8 h after ALA administration. Plasma, bone marrow (femurs), livers, and spleens were collected for estimating PPIX concentrations. For estimation of PPIX, samples were extracted using 1:1 methanol-1N perchloric acid (MeOH-PCA) on ice for 10 min, and the lysates were cleared by centrifugation at 10,000 × g for 10 min at 4 °C. Supernatants (100 μl) for each sample were analyzed for PPIX in black 96-well plates using a fluorescence spectrophotometer as described for heme, and concentrations were calculated using a standard curve. For plasma, data were represented as PPIX concentration per milliliter. For tissues, acidic pellets were neutralized with 1.5 m Tris acetate buffer and used to measure protein content using the bicinchoninic acid assay; PPIX concentrations were normalized to protein content. In a separate experiment, at 1 h after ALA administration, bone marrow (femurs) were collected for RNA extraction and quantitative PCR as described below.
For in vitro experiments, fibroblasts (density of 2,500 cells/cm2), MA-10 cells (5,000 cells/cm2), and colon cancer cell lines (5,000 cells/cm2) were treated with 1 mm of ALA and incubated for baseline and 24 h. After incubation, cells were collected by trypsinization followed by neutralization using trypsin inhibitor in DMEM. Cells were lysed in 1:1 MeOH-PCA, and PPIX fluorescence was estimated as described above for tissue samples.
Western Blots
Samples were processed in Laemmli sample buffer as previously described (41), and protein concentrations were determined using a bichionic acid assay. Equal amounts of protein (25–50 μg/sample) were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were then blocked using 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20. Incubations were carried out using a rabbit anti-TSPO monoclonal antibody (Abcam), a rabbit anti-IDH2 monoclonal antibody (Abcam), or a mouse anti-VDAC1 monoclonal antibody (Abcam) and a control mouse anti-actin monoclonal antibody (Li-Cor). Fluorescent IRDye800 goat anti-rabbit IgG and IRDye680 goat anti-mouse IgG were used for fluorescent labeling; membranes were imaged using a laser fluorescence scanner (Odyssey; Li-Cor).
PPIX and Mitochondrial Colocalization
Fibroblasts cultured in glass-bottomed 35-mm dishes (MatTek corporation) were treated with 1 μm PPIX in serum-free medium for 4 h. MitoTracker Green (Life Technologies) was added to label the mitochondria in live cells for the final 30 min of this incubation. Labeled cells were then provided with fresh medium, and high resolution images were acquired using a confocal microscope (Meta 510; Zeiss). Image analyses for colocalization of PPIX and MitoTracker were performed using the intensity correlation plugin in ImageJ (National Institutes of Health) (42) and calculating the Mander's overlap coefficient and Pearson's coefficient. At least 40 cells for each genotype were imaged and analyzed.
Quantitative PCR
Gene expression analyses using quantitative PCR were performed for both in vitro (fibroblasts and MA-10 cells) and in vivo (bone marrow) samples. Total RNA was extracted using TRIzol reagent (Life Technologies); reverse transcription of 1.5 μg of total RNA was performed using Multiscribe reverse transcriptase (Life Technologies). Gene expression assays for the different samples were performed using SYBR-green using intron-spanning primers for ALA synthase (Alas: 5′-TGTCGGTCTGGTGCAGTAATG-3′ and 5′-GGCATCATCTTAGCCAGGGT-3′), ALA dehydratase (Alad: 5′-GTTCTGCACAGCGGCTACTT-3′ and 5′-AGCTGGTTTACGCCATACCTG-3′), hydroxymethylbilane synthase (Hmbs: 5′-ATGAGGGTGATTCGAGTGGG-3′ and 5′-TTGTCTCCCGTGGTGGACATA-3′), uroporphyrinogen III synthase (Uros: 5′-CCAGCATCACGTTTTTCAGTCC-3′ and 5′-GTACTGGGGCCAATGGCTAT-3′), uroporphyrinogen decarboxylase (Urod: 5′-GGGTTCGGACTCCAGAATTTC-3′ and5′-CCTGTCTCATGCACCAAACG-3′), coproporphyrinogen oxidase (Cpox: 5′-GCCATTTACTGCTATGGGTGT and 5′-AGCTTCCTTTAGAGTACGGTGG-3′), protoporphyrinogen oxidase (Ppox: 5′-ATGGGCCGGACTGTGATAGTA-3′ and 5′-CTTGCTGCCCTCCACTAAGA-3′), ferrochelatase (Fech: 5′-CAGACAGATGAGGCTATCAAAGG-3′ and 5′-CACAGCTTGTTGGACTGGATG-3′), and Tspo (5′-TGGGTGCCTTCACTCTGG-3′ and 5′-AATCACCATGCCTGAATCCT-3′). Samples were analyzed after normalization to the expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh: 5′-GCCTTCCGTGTTCCTACC-3′ and 5′-GCCTGCTTCACCACCTTC-3′) or TATA box binding protein (Tbp: 5′-CCTTGTACCCTTCACCAATGAC-3′ and 5′-ACAGCCAAGATTCACGGTAGA-3′). Relative quantifications of fold change were performed comparing Ct values from individual samples by applying the 2−ΔΔCt method (43). Because of difficulty in designing intron-spanning primers, translocator protein 2 (Tspo2) expression was quantified using a TaqMan probe (Mm01281420_m1) with normalization to Gapdh (4352339E) (Life Technologies).
Adenoviral Expression of TSPO2
Adenoviruses that express TSPO2 were generated using the AdEasy system (44). In brief, Tspo2 cDNA was cloned from a bone marrow sample into a shuttle vector (pAd-CMV), linearized using PacI, and transformed into AdEasier bacteria containing the adenoviral backbone plasmid (pAdEasy-1). Adenoviruses were packaged by transfection into HEK-293A cells and amplified for two cycles in the same cell line. Titers were estimated by quantifying viral DNA, and infection rates were assessed by Tspo2 mRNA expression in the target cells by quantitative PCR. Control adenoviruses expressing tdTomato (Vector Biolabs) were amplified in HEK-293A cells and used as matched titers. Fibroblasts infected with adenoviral Tspo2 or tdTomato were used after 48 h for experiments evaluating the bioconversion of ALA to PPIX as described above.
Oxygen Consumption Rate
For measurements of mitochondrial respiration, oxygen consumption rates (OCR) were measured using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience). Fibroblasts (Tspofl/fl and Tspo−/−) were plated at a density of 30,000 cells in the XF24 cell culture microplates in regular culture medium (10% FBS, 25 mm glucose, 1 mm pyruvate) overnight. One hour before assay, cells were washed and incubated in XF assay medium (no bicarbonate) supplemented with 25 mm glucose and 1 mm pyruvate at 37 °C in a CO2-free atmosphere. OCR was measured real time every 3–5 min. Oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), antimycin A, and rotenone were sequentially injected into each well to assess basal respiration, coupling of respiratory chain, proton leak, and mitochondrial spare respiratory capacity.
Mitochondrial Volume, Mass, and Membrane Potential
Fibroblasts, Tspofl/fl and Tspo−/−, were seeded on 35-mm glass-bottomed dishes (MatTek) overnight in high glucose DMEM, 10% FBS. Cells were stained with 100 nm MitoTracker Green FM at 37 °C for 1 h in the dark, washed with PBS, and changed to phenol red-free high glucose DMEM, 10% FBS, 10 mm HEPES for live cell imaging using Zeiss LSM 510 confocal microscopy. Images were collected as z-stacks and analyzed for volume after adjustment of threshold using three-dimensional Manager plugin in ImageJ (45).
For mitochondrial membrane potential measurement, cells were costained with 100 nm MitoTracker Green FM and 20 nm tetramethylrhodamine methyl ester (TMRM) and imaged as above or intensities measured using a flow cytometer (Gallios; Beckman Coulter). After baseline values were taken, the cells were incubated with 5 μm oligomycin at 37 °C for 30 min before imaging. Depolarization was then induced by 10 μm FCCP, and images were taken after 2 min. Intensities of TMRM were normalized to those of MitoTracker Green for comparisons of Δψm between the two genotypes. Mitochondrial masses were assessed by comparing the intensities of MitoTracker green and were also confirmed by estimating the amounts of mitochondrial proteins IDH2 and VDAC1 using Western blots (as described above).
PK11195 Treatments
For ALA to PPIX bioconversion, fibroblasts were treated with PK11195 (1 μm) in 0.1% DMSO (control) in medium containing 1 mm of ALA and incubated for 24 h. After incubation, cells were collected and lysed, and PPIX fluorescence was estimated as described above. For measuring Δψm, fibroblasts treated with PK11195 (0 and 100 nm and 1 μm) were incubated for 60 min and stained with MitoTracker Green FM and TMRM, and intensities were measured using flow cytometry as described above.
Statistics
The responses measured for two experimental groups were compared using Student's t test; comparisons for more than two groups were performed using analysis of variance and post hoc Tukey's test (p < 0.05 was considered significant). All analyses were performed using Prism5 (GraphPad), and the data are represented as means ± S.E. of the mean.
Results
Erythropoiesis Is Not Affected in Tspo−/− Mice
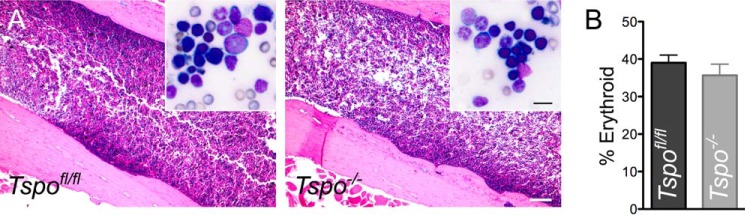
Blood collected from Tspo−/− mice showed that all their erythrocyte/hemoglobin parameters were within physiological intervals and were not different from Tspofl/fl mice (Table 1). Bone marrow from both Tspofl/fl and Tspo−/− mice exhibited identical morphological features and cellular composition after hematoxylin and eosin staining (Fig. 1A). Percentages of bone marrow erythroid cells were not different between Tspofl/fl and Tspo−/− mice (Fig. 1B). Hematology analyses showed no significant differences between Tspofl/fl and Tspo−/− mice (Table 1), and values were within physiological intervals observed for C57BL/6 mice. Measurements that indicated hemoglobin concentration also showed no differences between Tspofl/fl and Tspo−/− mice. Bone marrow gene expression of enzymes involved in PPIX biosynthesis showed some differences between Tspofl/fl and Tspo−/− mice (Fig. 2). No significant changes were observed for Hmbs, Uros, Urod, and Ppox expression. Baseline expression of Fech was significantly higher in Tspo−/− compared with Tspofl/fl bone marrow, but expression of Fech and Alas was significantly down-regulated to similar levels after ALA treatment in both Tspofl/fl and Tspo−/− bone marrow. In Tspo−/− bone marrow, expression of Alad significantly increased after ALA treatment; this trend was not observed in Tspofl/fl bone marrow. Mean baseline expression of Cpox was higher but not statistically different between Tspofl/fl and Tspo−/− bone marrow, but with ALA treatment Cpox significantly decreased in both Tspo−/− and Tspofl/fl bone marrow. Expression of Tspo2 showed a decreasing trend after ALA treatment in both Tspofl/fl and Tspo−/− bone marrow but was not significant. Expression of Tspo was significantly up-regulated compared with baseline after ALA treatment in Tspofl/fl bone marrow.
TABLE 1.
Erythrocyte characteristics in Tspo−/− mice
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; CHCM, cell hemoglobin concentration mean; RDW, red blood cell distribution width.
| Parameters | TSPOfl/fl | TSPO−/− | p value |
|---|---|---|---|
| Erythrocytes (106 cells/μl) | 9.8 ± 0.05 | 9.8 ± 0.15 | 1.0000 |
| Platelets (103/μl) | 1284.4 ± 182.24 | 1508.6 ± 196.58 | 0.4272 |
| Hemoglobin (g/dL) | 15.7 ± 0.15 | 15.8 ± 0.16 | 0.5028 |
| Hematocrit (%) | 52.8 ± 0.66 | 52.0 ± 0.44 | 0.3466 |
| MCV (fL) | 54.20 ± 0.8 | 53.00 ± 1.09 | 0.4021 |
| MCH (pg) | 15.6 ± 0.24 | 16.0 ± 0.31 | 0.3466 |
| MCHC (g/dL) | 29.0 ± 0.00 | 30.0 ± 0.31 | 0.3411 |
| CHCM (g/dL) | 27.0 ± 0.00 | 27.4 ± 0.24 | 0.1411 |
| RDW (%) | 12.6 ± 0.24 | 12.4 ± 0.25 | 0.5796 |
FIGURE 1.
Bone marrow histology and evaluation of erythroid cells. A, sections of Tspofl/fl and Tspo−/− mice femur bone showing the marrow. Histological evaluation of the marrow showed no difference in overall hematopoietic cellularity. Scale bar, 200 μm. Inset, bone marrow cytology; there was no difference in relative density of erythroid precursor cells. Scale bar, 15 μm. B, enumeration of erythroid cells showed no differences in the percentage of erythroid precursors between the two groups (n = 3/group).
FIGURE 2.
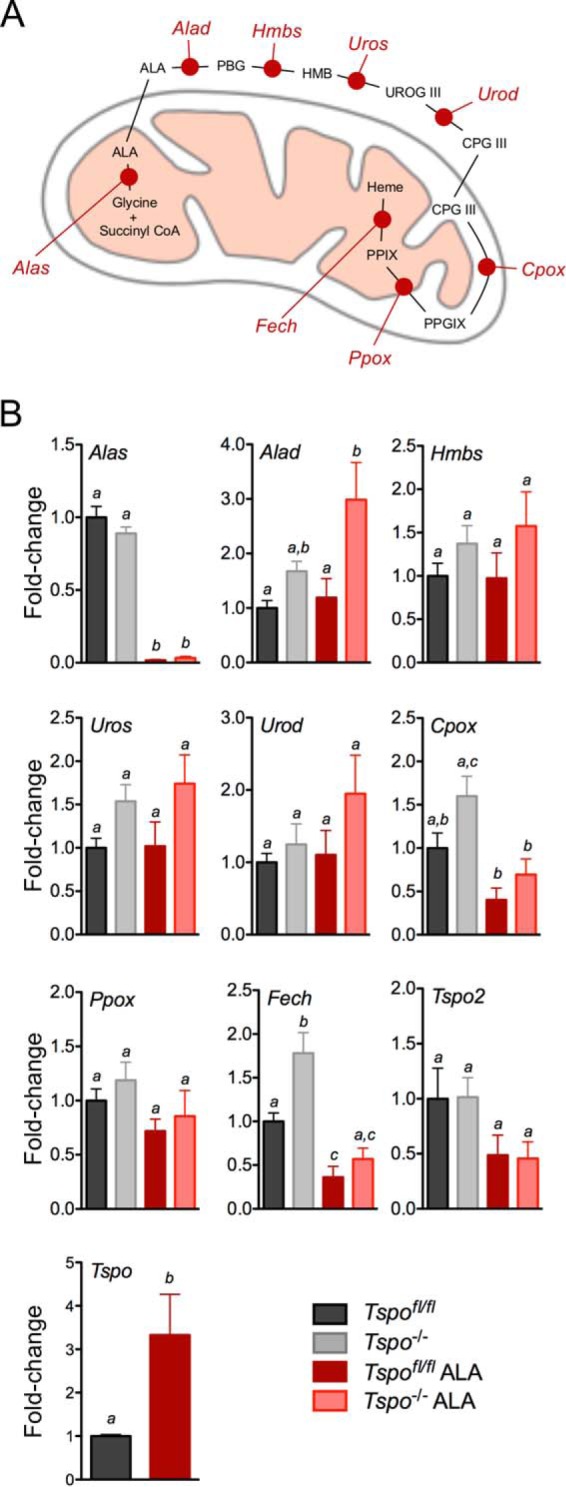
Expression of genes involved in PPIX synthesis in Tspo−/− bone marrow. A, schematic showing the different enzymatic steps involved in the conversion of ALA to PPIX and heme. The enzymes were ALA synthase (Alas), ALA dehydratase (Alad), hydroxymethyl bilane synthase (Hmbs), uroporphyrinogen III synthase (Uros), uroporphyrinogen decarboxylase (Urod), coproporphyrinogen oxidase (Cpox), protoporphyrinogen oxidase (Ppox), and ferrochelatase (Fech). The intermediates were porphobilinogen (PBG), hydroxymethyl bilane (HMB), uroporphyrinogen III (UROG III), coproporphyrinogen III (CPG III), and protoporphyrinogen IX (PPGIX). B, expression levels of transcripts coding enzymes involved in the conversion of ALA to PPIX in Tspofl/fl and Tspo−/− bone marrow at both baseline and after treatment with ALA. a, b, and c indicate p < 0.05 (n = 6/group).
Tissue Heme Levels in Tspo−/− Mice
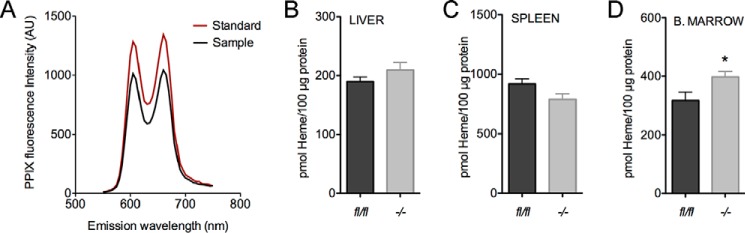
In analytical preparations from tissues, emission spectra for PPIX measurements were highly specific when compared with standards allowing for the precise quantification of heme (Fig. 3A). Baseline heme levels were not different between Tspofl/fl and Tspo−/− livers and spleens (Fig. 3, B and C). However, heme levels were modestly higher but significant in bone marrow from Tspo−/− mice compared with Tspofl/fl cohorts (Fig. 3D).
FIGURE 3.
Heme levels in tissues from Tspo−/− mice. A, PPIX standard and a representative analytical spleen sample showing the emission spectrum of PPIX with excitation at 400 nm; two emission maximums at 605 and 660 nm specific for PPIX are observed in both standard and prepared sample. B–D, normalized baseline heme levels in tissues (liver, spleen, and bone marrow) from Tspofl/fl and Tspo−/− mice. *, p < 0.05 (n = 6/group).
Systemic Conversion of ALA to PPIX in Tspo−/− Mice
Baseline PPIX concentrations in plasma, and tissue lysates (bone marrow, livers and spleens) were not different between Tspofl/fl and Tspo−/− mice (Fig. 4). After ALA administration, increases in PPIX levels at 1, 4, and 8 h were similar in both Tspofl/fl and Tspo−/− mice in plasma and all tissues.
FIGURE 4.
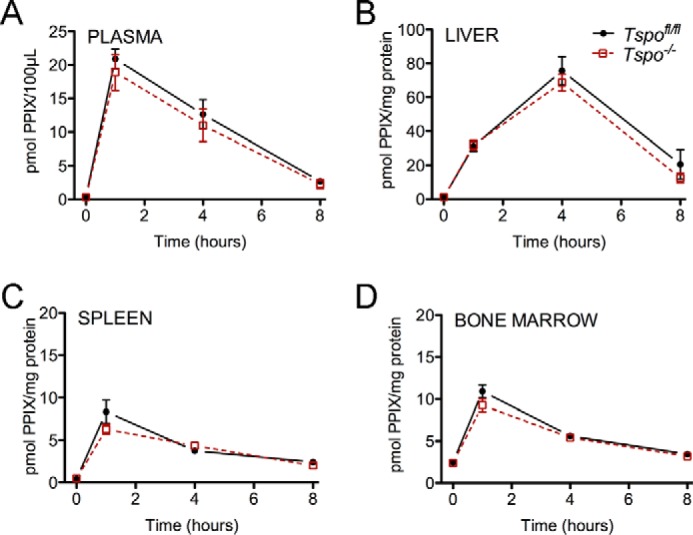
PPIX levels in plasma and tissues from Tspo−/− mice. Panels show normalized PPIX fluorescence in blood plasma (A), liver (B), spleen (C), and bone marrow (D), at baseline and at 1, 4, and 8 h after administration of ALA (n = 6–8/group).
TSPO Deficiency Does Not Affect PPIX Uptake and Localization
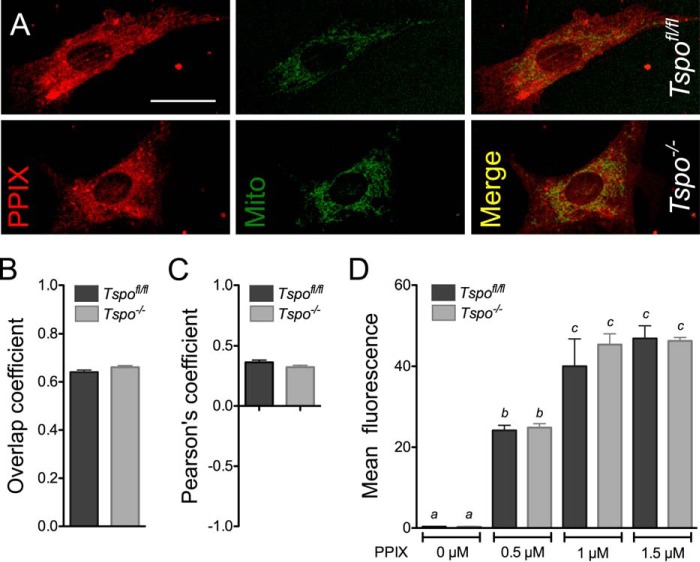
When Tspofl/fl and Tspo−/− fibroblasts were treated with PPIX, the extent of PPIX localization to the mitochondria computed as overlap with MitoTracker® Green fluorescence was identical between the two genotypes (Fig. 5, A–C). Treatments of Tspofl/fl and Tspo−/− fibroblasts with increasing concentrations of PPIX showed dose-dependent increases in intracellular fluorescence that were not different between the two genotypes (Fig. 5D). PPIX uptake by fibroblasts approached saturation at 1 μm PPIX treatment, with no difference observed when treatment was increased to 1.5 μm PPIX (Fig. 5D).
FIGURE 5.
PPIX uptake and localization in Tspofl/fl and Tspo−/− fibroblasts. A, PPIX fluorescence and MitoTracker® Green (Mito) colocalization in Tspofl/fl and Tspo−/− fibroblasts. Scale bar, 50 μm. B, Mander's overlap coefficient for PPIX and MitoTracker® Green localization was not different between Tspofl/fl and Tspo−/− fibroblasts. C, Pearson's coefficient for PPIX and MitoTracker® Green localization was not different between Tspofl/fl and Tspo−/− fibroblasts. D, PPIX uptake by fibroblasts after treatment with increasing concentrations of PPIX (0, 0.5, 1.0, or 1.5 μm) for 4 h was not different between Tspofl/fl and Tspo−/− cells. Mean fluorescence intensity for PPIX was measured by flow cytometry (n = 6 independent primary cultures/group).
TSPO Deficiency Does Not Affect Porphyrin-mediated Phototoxicity
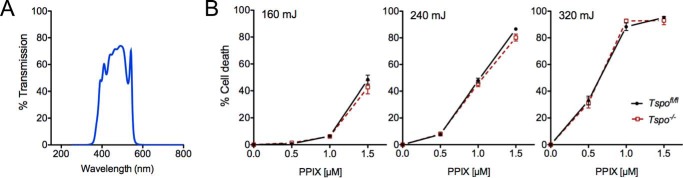
Without PPIX treatment, exposure of both Tspofl/fl and Tspo−/− fibroblasts to light at 450 ± 60-nm wavelengths with increasing energies (160, 240, and 320 mJ) did not induce phototoxicity and cell death (Fig. 6). When these Tspofl/fl and Tspo−/− fibroblasts were treated with PPIX, they displayed different levels of cell death that increased with both increasing concentration of PPIX and increasing energy of light (up to 95% cell death was observed with 1.5 μm of PPIX treatment and 320 mJ light energy). However, no significant differences in PPIX-mediated phototoxic cell death were observed between Tspofl/fl and Tspo−/− fibroblasts (Fig. 6).
FIGURE 6.
Porphyrin-mediated phototoxicity in Tspofl/fl and Tspo−/− fibroblasts. A, spectrum of band pass filter used for photoexcitation allowed transmission of light 450 ± 60 nm. B, photoexcitation-induced cell death was not different between Tspofl/fl and Tspo−/− after treatment with increasing concentrations of PPIX (0, 0.5, 1.0, or 1.5 μm) and subsequent exposure to increasing energies of light (160, 240, or 320 mJ; n = 6 independent primary cultures/group).
Bioconversion of ALA to PPIX Is Not Changed in Tspo−/− Fibroblasts
Baseline PPIX concentration in Tspofl/fl and Tspo−/− fibroblast lysates were not different (Fig. 7A). After ALA treatment, PPIX concentrations in fibroblast lysates showed a significant increase with no statistical difference between the two genotypes (Fig. 7A). Expression of genes involved in PPIX biosynthesis indicated some differences between Tspofl/fl and Tspo−/− fibroblasts (Fig. 7B). Baseline expression of Alas, Hmbs, Urod, Cpox, Ppox, and Fech were significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts; baseline expression of Alad and Uros was not different between the two genotypes. After ALA treatment, expression of Alas, Alad, Hmbs, and Cpox was not different between the two genotypes; expression of Uros, Urod, Ppox, and Fech was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. Expression of Tspo did not show any changes with ALA treatment in Tspofl/fl fibroblasts. Tspo2 expression was not detected in Tspofl/fl and Tspo−/− fibroblasts at both baseline and after treatment with ALA (not shown).
FIGURE 7.
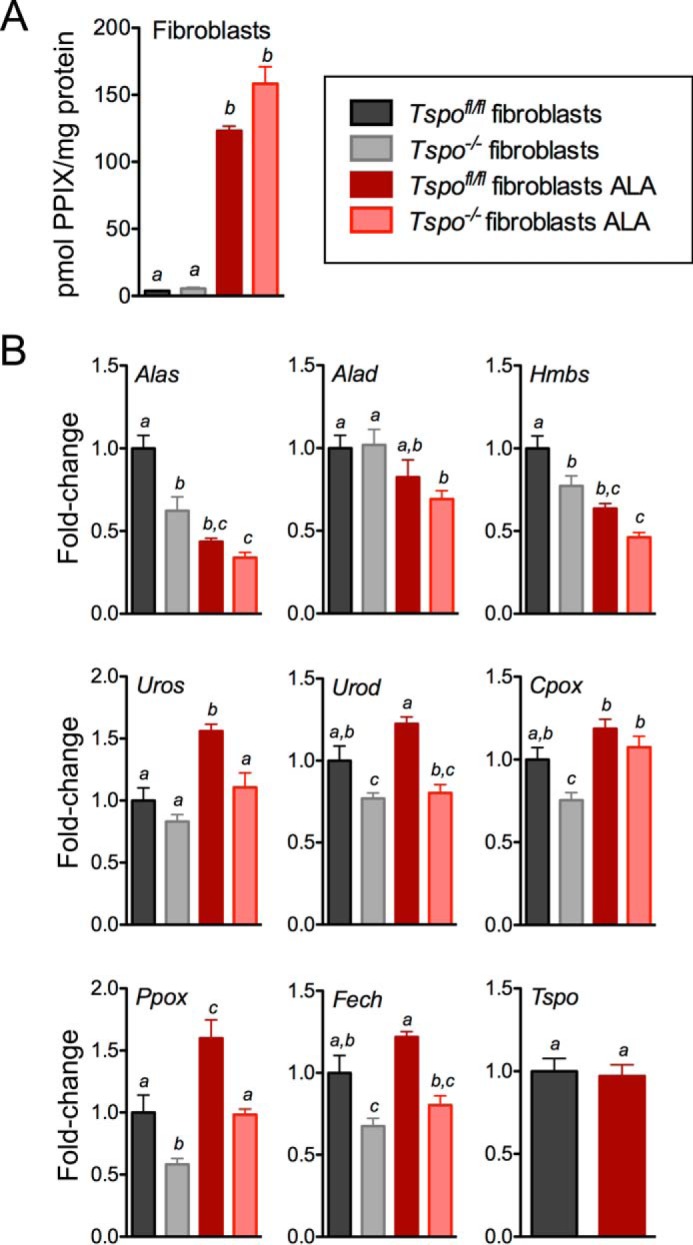
PPIX synthesis from ALA in Tspofl/fl and Tspo−/− fibroblasts. A, PPIX concentration in Tspofl/fl and Tspo−/− fibroblast cell lysates before and after treatment with ALA. a and b indicate p < 0.05 (n = 3/group). B, expression levels of transcripts coding enzymes involved in the conversion of ALA to PPIX in Tspofl/fl and Tspo−/− fibroblasts at both baseline and after treatment with ALA. a, b, and c indicate p < 0.05 (n = 6 independent primary cultures/group).
Bioconversion of ALA to PPIX Is Not Changed in MA-10:TspoΔ/Δ Cells
Baseline PPIX concentration in MA-10 cell lysates showed a modest but significantly lower level in MA-10:TspoΔ/Δ cells compared with MA-10 cells (Fig. 8A). After ALA treatment, PPIX concentrations in cell lysates showed a significant increase with no difference between MA-10:TspoΔ/Δ cells and MA-10 cells (Fig. 8A). Expression of genes involved in PPIX biosynthesis indicated some differences between MA-10:TspoΔ/Δ cells and MA-10 cells (Fig. 8B). Baseline expression of Alad, Uros, and Urod were significantly higher in MA-10:TspoΔ/Δ cells compared with MA-10 cells, and these differences did not change after ALA treatment. Baseline expression of Alas was not different between MA-10:TspoΔ/Δ cells and MA-10 cells, but expression significantly decreased upon ALA treatment in both genotypes. Baseline expression of Hmbs, Cpox, and Ppox was not different between MA-10:TspoΔ/Δ cells and MA-10 cells, but a significant increase in expression was observed after ALA treatment only in MA-10:TspoΔ/Δ cells compared with MA-10 cells. Baseline Fech expression was significantly decreased in MA-10:TspoΔ/Δ cells compared with MA-10 cells; this difference was also seen after ALA treatment. Expression of Tspo showed a modest but significant decrease with ALA treatment in MA-10 cells. Tspo2 expression was not observed in MA-10 cells and MA-10:TspoΔ/Δ cells at both baseline and after treatment with ALA (not shown).
FIGURE 8.
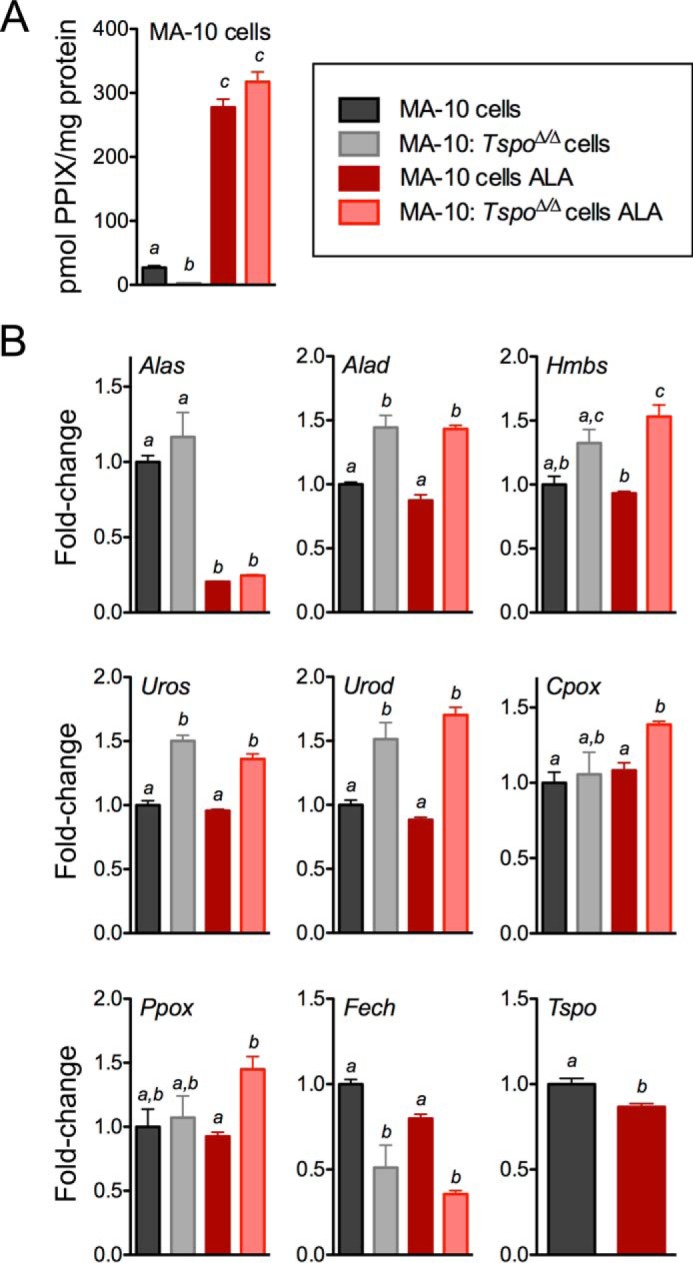
PPIX synthesis from ALA in MA-10 cells and MA-10:TspoΔ/Δ cells. A, PPIX concentration in MA-10 cells and MA-10:TspoΔ/Δ cell lysates before and after treatment with ALA. a, b, and c indicate p < 0.05 (n = 3/group). B, expression levels of transcripts coding enzymes involved in the conversion of ALA to PPIX in MA-10 cells and MA-10:TspoΔ/Δ cells at both baseline and after treatment with ALA. a, b, and c indicate p < 0.05 (n = 3/group).
TSPO Expression Does Not Correlate to PPIX Production by Colon Cancer Cells
In comparing fibroblasts and MA-10 cells with different human colon cancer cell lines, TSPO expression was highest in MA-10 cells and weakest in fibroblasts; among the colon cancer cell lines, DLD1 > HT29 > HCT116≥LOVO (Fig. 9A). Expression of IDH2, used as an indirect measure of mitochondrial mass in the different colon cancer lines, showed a range of differences between the cell lines, suggesting varied mitochondrial masses; relative expression of ACTB was also not perfectly consistent for baseline normalization between the cell lines (Fig. 9A). PPIX synthesis after ALA treatment in the colon cancer cell lines showed levels decreasing in order from HT29 > HCT116 > DLD1 > LOVO cells (Fig. 9B). In LOVO cells, we did not observe enhanced PPIX production after ALA treatment. These observations support the results seen in MA-10 cells, suggesting that the efficacy of PPIX synthesis after ALA treatment is not related to TSPO expression in colon cancer cells.
FIGURE 9.
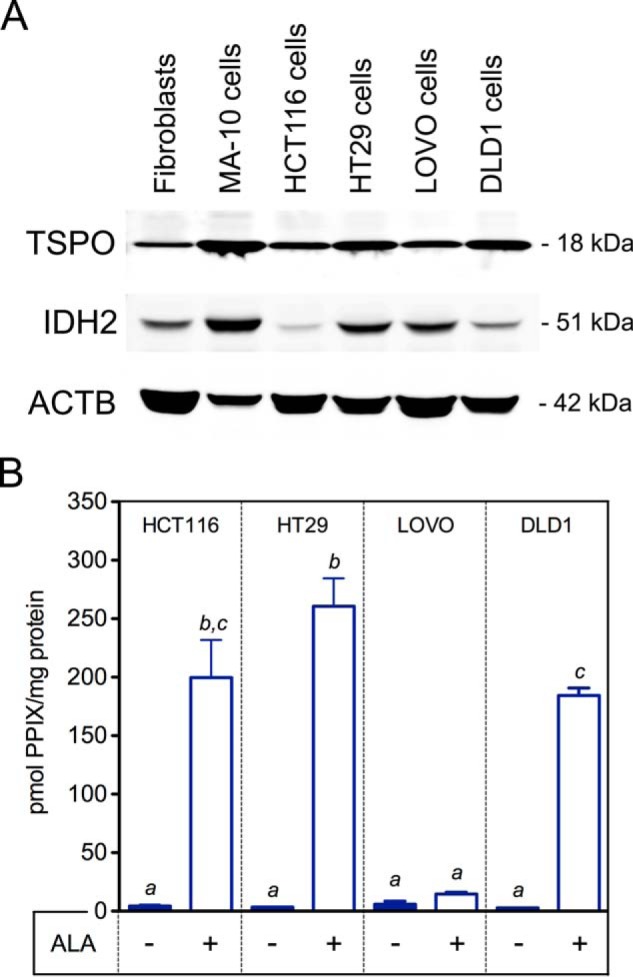
TSPO expression levels and PPIX synthesis from ALA in colon cancer cells. A, expression levels of TSPO and mitochondrial protein IDH2 in relation to ACTB in primary Tspofl/fl fibroblasts, MA-10 Leydig cells, and four different colon cancer cell lines (HCT116, HT29, LOVO, and DLD1 cells). Equal total protein (50 μg) was loaded to generate this representative Western blot. B, PPIX concentration in cell lysates before and after treatment with ALA in HCT116, HT29, LOVO, and DLD1 colon cancer cell lines. a, b, and c indicate p < 0.05 (n = 3/group).
Exogenous TSPO2 Expression Does Not Change ALA to PPIX Bioconversion in Fibroblasts
There was no expression of Tspo2 observed in fibroblasts and MA-10 cells (Fig. 10A). Exogenous adenoviral Tspo2 expression in Tspofl/fl and Tspo−/− fibroblasts resulted in ∼8-fold higher expression levels compared with endogenous expression observed in the bone marrow. PPIX synthesis before and after ALA treatment was not different between Tspofl/fl and Tspo−/− fibroblasts after exogenous expression of Tspo2 (Fig. 10B). In control tdTomato expression, ALA treatment showed a modest but significant increase in PPIX levels in Tspo−/− fibroblasts. However, this increase was not significantly different compared with Tspo2 expressing Tspo−/− fibroblasts. These results suggest that TSPO2 expression does not have a positive or negative effect on PPIX production from ALA.
FIGURE 10.
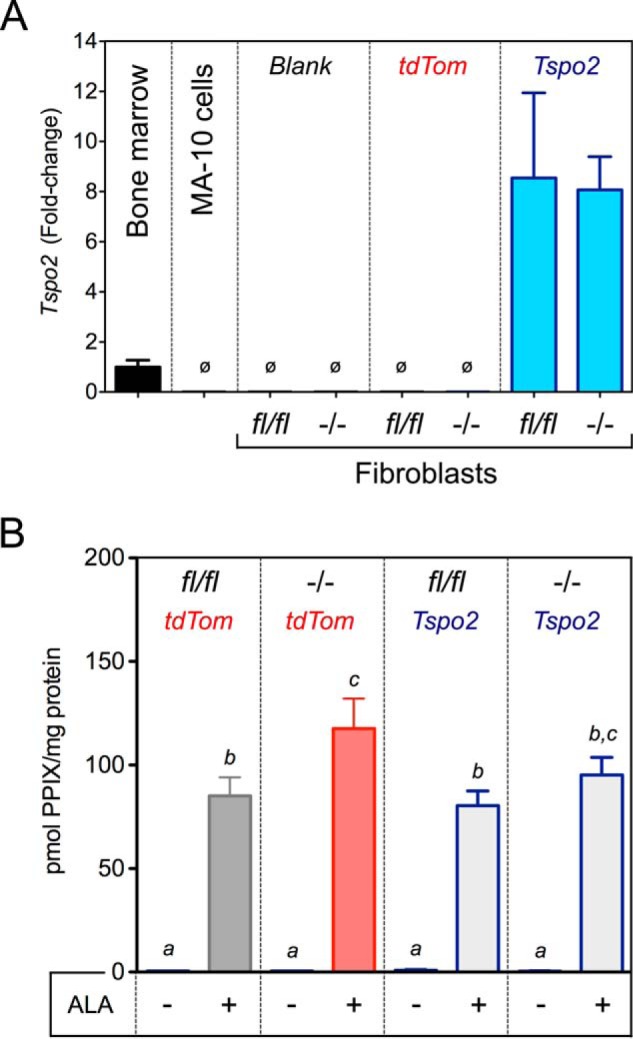
Effect of TSPO2 expression on PPIX synthesis from ALA. A, Tspo2 gene expression normalized to endogenous expression in the bone marrow. Expression of Tspo2 was not observed (ø) in MA-10 cells and fibroblasts (blank). Specific ∼8-fold expression of Tspo2 was induced by adenoviral expression in Tspofl/fl and Tspo−/− fibroblasts. Adenovirus expressing tdTomato (tdTom) was examined as a negative control. B, PPIX concentration in Tspofl/fl and Tspo−/− fibroblast lysates after induced expression of tdTom or Tspo2, before and after treatment with ALA. a, b, and c indicate p < 0.05 (n = 3/group).
Mitochondrial Homeostasis Is Altered in Tspo−/− Fibroblasts
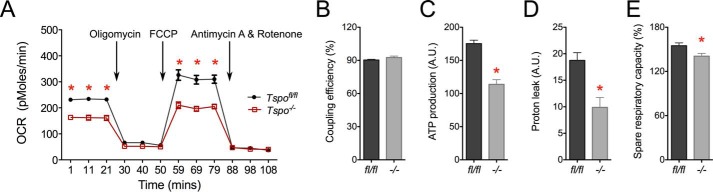
Comparing primary fibroblasts from Tspofl/fl and Tspo−/− mice showed that baseline and maximal OCR were significantly lower with the loss of TSPO (Fig. 11A). However, the percentages of oxidative phosphorylation coupling efficiencies were unchanged in Tspo−/− compared with Tspofl/fl fibroblasts (Fig. 11B), but absolute values were significantly lower in Tspo−/− fibroblasts (not shown). There was a decrease in calculated ATP production in Tspo−/− compared with Tspofl/fl fibroblasts (Fig. 11C). Proton leak was also decreased in Tspo−/− compared with Tspofl/fl fibroblasts (Fig. 11D). The spare respiratory capacity was significantly reduced in Tspo−/− compared with Tspofl/fl fibroblasts (Fig. 11E). These findings suggest a significant shift in mitochondrial homeostasis in Tspo−/− fibroblasts that could affect multiple mitochondrial and cellular functions.
FIGURE 11.
Analysis of mitochondrial bioenergetics in Tspofl/fl and Tspo−/− fibroblasts. A, baseline OCR and maximal OCR after addition of the protonophore FCCP was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. OCR declined upon blocking ATP synthase activity using oligomycin or inhibiting electron transport chain using antimycin A and rotenone, but they were not significantly different between the two genotypes. B, mitochondrial coupling efficiency was not different between Tspofl/fl and Tspo−/− fibroblasts. C, mitochondrial ATP production was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. D, proton leak was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. E, spare respiratory capacity was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. *, p < 0.05 (n = 5–7 independent primary cultures).
Mitochondrial Membrane Potential Is Decreased in Tspo−/− Fibroblasts
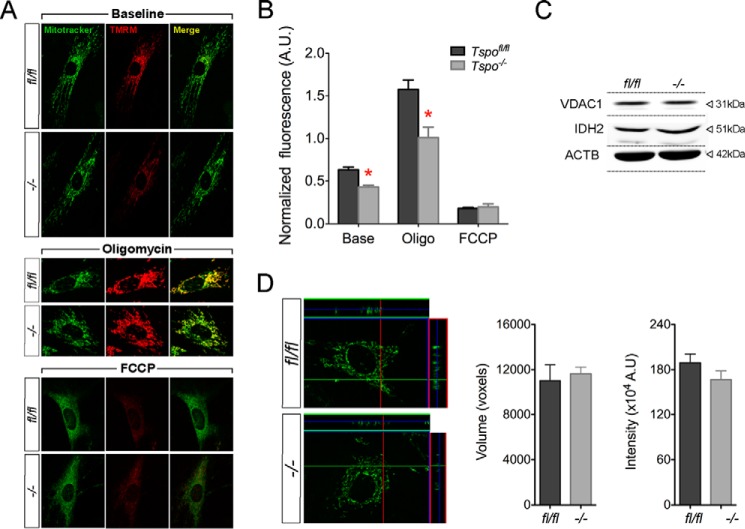
Assessment of ΔΨm showed that Tspo−/− fibroblasts had significantly lower baseline values compared with Tspofl/fl fibroblasts, and this difference was increased after inhibition of ATP synthase by oligomycin and completely dissipated as expected after FCCP in both genotypes (Fig. 12, A and B). Given the scale of these changes as a result of TSPO deletion, we verified that mitochondrial volume and mass were not different between Tspo−/− and Tspofl/fl fibroblasts (Fig. 12, C–E). Differences observed in Tspo−/− fibroblasts corroborate that loss of TSPO impacts mitochondrial homeostasis in primary cells.
FIGURE 12.
Analysis of mitochondrial membrane potential (Δψm) in Tspofl/fl and Tspo−/− fibroblasts. A, representative images of Tspofl/fl and Tspo−/− fibroblasts stained with TMRM and MitoTracker® Green from experiments measuring Δψm, showing baseline fluorescence, after treatment with oligomycin, and FCCP. B, normalized TMRM/MitoTracker® Green fluorescence intensity comparison between Tspofl/fl and Tspo−/− fibroblasts showed significantly decreased Δψm at baseline and after oligomycin treatment. *, p < 0.05 (n = 150 cells/group). C, expression of mitochondrial proteins VDAC1 and IDH2 were not different between Tspofl/fl and Tspo−/− fibroblasts. D, representative z-stacks of MitoTracker® Green stained Tspofl/fl and Tspo−/− fibroblasts used for calculating mitochondrial volume. Mitochondrial volume was not different between Tspofl/fl and Tspo−/− fibroblasts. Mitochondrial mass as measured by MitoTracker® Green labeling intensity was also not different between Tspofl/fl and Tspo−/− fibroblasts (n = 150 cells/group).
PK11195 Does Not Affect ALA to PPIX Bioconversion but Has Off Target Effects Affecting ΔΨm
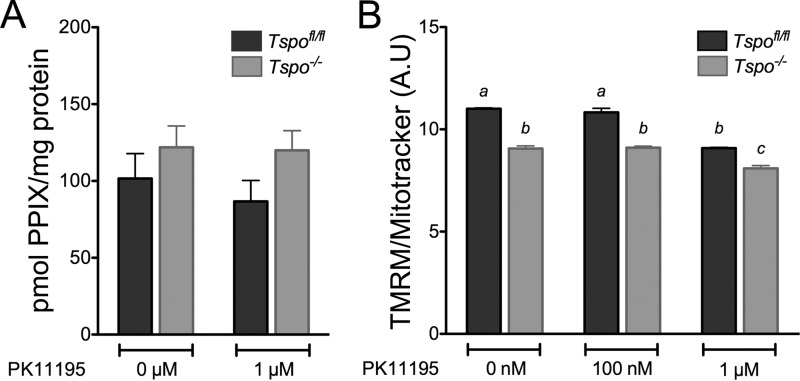
Treatment with PK11195 [1 μm, the concentration used in the zebrafish study (24)], did not affect PPIX production after ALA treatment in both Tspofl/fl and Tspo−/− fibroblasts (Fig. 13A). However, this same concentration of PK11195 significantly decreased ΔΨm in both Tspofl/fl and Tspo−/− fibroblasts (Fig. 13B), indicating that PK11195 treatment can result in off target effects at this concentration. Baseline values for ΔΨm measured using flow cytometry also showed a significantly lower ΔΨm in Tspo−/− fibroblasts compared with Tspofl/fl fibroblasts (Fig. 13B), consistent with the microscopic quantitation (Fig. 12A and B).
FIGURE 13.
Effect of PK11195 treatment on ALA to PPIX bioconversion and Δψm. A, PPIX concentration in Tspofl/fl and Tspo−/− fibroblast cell lysates after treatment with ALA in the presence of 0 (control) or 1 μm PK11195. Treatment with PK11195 did not have an effect PPIX production. B, normalized TMRM/MitoTracker® Green fluorescence intensity comparison between Tspofl/fl and Tspo−/− fibroblasts after treatment with 0 (control) and 100 nm and 1 μm PK11195. Baseline Δψm was significantly lower in Tspo−/− compared with Tspofl/fl fibroblasts. Treatment with PK11195 significantly decreased Δψm at 1 μm concentration in both Tspofl/fl and Tspo−/− fibroblasts. a and b indicate p < 0.05 (n = 4 independent primary cultures/group).
Discussion
Based on its conserved PPIX binding property (17), it was proposed that the ancestral role for TSPO could be enzyme catalysis of porphyrins, albeit only in the presence of light (46). Purified TSPO protein from Chlorobium tepidum (CtTSPO) and B. cereus (BcTSPO) indicated a property of photo-oxidative PPIX degradation (22, 46). In contrast, chordate TSPO (fish, birds, and mammals) has been suggested to be important for PPIX transport and heme biosynthesis (24, 25, 28, 47). The PPIX binding property of TSPO was also considered the basis of ALA-based PDT for cancers (38, 39, 48). Diverging from these conclusions, results in this manuscript suggest that TSPO does not have a physiological effect on PPIX metabolism and may not be involved in PDT.
Deficiency of TSPO did not affect heme synthesis and erythropoiesis in mice, suggesting an evolutionary shift distinct from its hematopoietic function reported in zebrafish (49). Bone marrow, the primary site of erythropoiesis, also expresses the TSPO paralog TSPO2 that can be found only in birds and mammals (49). TSPO2 has 35% homology to TSPO and is almost exclusively localized to the bone marrow (49). However, TSPO2 localization to the endoplasmic reticulum (49) suggests that it may not be involved in a mitochondrial function that is redundant to TSPO. Fibroblasts and MA-10 cells do not express TSPO2. Moreover, we confirmed that TSPO2 does not affect PPIX synthesis using adenovirus-induced Tspo2 expression in fibroblasts, ruling out the possibility of functional redundancy. In support, expression of the heme biosynthetic gene Fech showed a compensatory baseline increase in expression in Tspo−/− bone marrow, suggesting that TSPO deletion did induce a shift in mitochondrial homeostasis. However, this shift in homeostasis toward increased heme synthesis did not alter physiological hemoglobin concentration measured in circulation. Moreover, when Tspo−/− mice were treated with ALA, there were no differences in maximal PPIX production in plasma, liver, spleen, and bone marrow. Although Tspo up-regulation was observed after ALA treatment in Tspofl/fl bone marrow (functionally akin to previous reports of Tspo up-regulation during erythroid differentiation (28)), we infer that this is unrelated to PPIX biosynthesis or scavenging, because Tspo−/− mice had no differences in PPIX production. It is possible that administration of ALA promotes erythroid differentiation in the bone marrow, as observed in the widely used in vitro K562 erythroid differentiation model (50–52). A Tspo up-regulation response as reported in erythroid cells was not observed in fibroblasts or MA-10 cells, also suggesting that this is specific for the bone marrow.
To examine PPIX uptake and porphyrin-mediated phototoxicity in a system that expresses TSPO (but not TSPO2), we used primary fibroblasts as a model (53) to examine mechanisms. We found that TSPO deficiency did not affect the extent of PPIX association with the mitochondria and the level of uptake by fibroblasts. Moreover, PPIX-mediated phototoxic cell death was not different between Tspofl/fl and Tspo−/− fibroblasts. Although these observations were rather unexpected given that previous studies reported the contrary in other model systems (12, 30, 54), they corroborated a recent report demonstrating that PPIX accumulation was not different in between Tspofl/fl and Tspo−/− mitochondria isolated from the liver (16).
Early studies have established that CPOX is present in the mitochondrial intermembrane space and that coproporphyrinogen III needs to cross the outer mitochondrial membrane to gain access to this enzyme (55). Although weaker than its affinity for PPIX, TSPO was demonstrated to bind coproporphyrinogen III (28). Because TSPO-binding chemicals appeared to block the conversion of coproporphyrinogen III to PPIX via intermediate protoporphyrinogen IX, a possible function for TSPO as a porphyrin transporter was proposed (29). In support, it was demonstrated that TSPO was directly involved in PPIX transport in an experiment using bacterial protoplasts expressing murine TSPO (47). In the present study, when we examined the ability of fibroblasts to synthesize PPIX from precursor ALA, we found no difference between Tspo−/− and Tspofl/fl cells. The exact same experiment in MA-10 cells (that also do not express TSPO2 but express ∼14-fold higher TSPO compared with fibroblasts) showed no difference between MA-10 and MA-10:TspoΔ/Δ cells. These results agreed with our in vivo observations in Tspo−/− mice and confirmed that TSPO is not involved in PPIX biosynthesis.
Provided the prognostic significance proposed for TSPO expression in colon cancer (56) and evidence that TSPO density in cancers parallels their susceptibility to porphyrin-mediated phototoxicity (54), we examined TSPO and PPIX synthesis in cancer cells. Comparison of different human colon cancer cell lines showed that TSPO expression had no correlation with PPIX synthesis capability, similar to observations in fibroblasts and MA-10 cells. Therefore, earlier reports indicating that TSPO directs mitochondrial porphyrin transport as part of the biosynthetic machinery (29, 47) are not substantiated. Recent identification of an essential porphyrin transporter ABCB6 (ATP-binding cassette, subfamily A (ABC1), member 6) at the outer mitochondrial membrane could explain the mechanism of mitochondrial porphyrin uptake and heme biosynthesis (57), without a role for TSPO in this process.
Although the PPIX binding property of TSPO is highly conserved and photo-oxidative degradation of PPIX could be considered an enzymatic function for TSPO, the physiological relevance of this slow and light-dependent activity in multicellular organisms appears unclear (58). Recent high resolution structural studies on MmTSPO (M. musculus) (59), RsTSPO (R. sphaeroides) (60), and BcTSPO (22) highlight limited similarities between vertebrate (MmTSPO) and the bacterial forms (RsTSPO and BcTSPO) with respect to PPIX binding residues, side chain interactions, and dimerization (58). In addition to the <30% identity, conserved residues that are organized on the outside of MmTSPO are reversed in BcTSPO and RsTSPO. Moreover, MmTSPO was found to be a monomer, RsTSPO appears to form an obligate dimer, and BcTSPO could also dimerize but does so with a distinct interface, suggesting evolutionary separations if this organization is indeed functionally important. Although the PPIX photo-oxidative degradation mediated by CtTSPO and BcTSPO are often discussed in broad terms, there is no functional evidence for this property in MmTSPO. Experiments that used the vertebrate XtTSPO (Xenopus tropicalis), and HsTSPO (H. sapiens) A147T variant showed that the catalytic photo-oxidative degradation of PPIX is conserved in XtTSPO but not in HsTSPO (22). The photo-oxidative degradation property of the wild type HsTSPO and MmTSPO remains to be tested. Moreover, light-mediated reactions would be quite unusual for most tissues that express MmTSPO or HsTSPO. Therefore, results obtained for bacterial TSPO need to be extrapolated with caution, because they may not be conserved or relevant in higher vertebrates.
We find that TSPO deficiency significantly decreases OCR and ΔΨm, indicating a distinct effect on core mitochondrial bioenergetics in fibroblasts that does not affect PPIX synthesis. This also indicates a potential shift in cellular energy homeostasis in Tspo−/− cells, which could affect multiple cellular events and responses. This shift in metabolic homeostasis could also explain gene expression differences in the heme biosynthetic pathway that were not only inconsistent between Tspo−/− bone marrow, fibroblasts, and MA-10 cells but also did not result in changes to PPIX production. In Tspo−/− primary microglia, a similar decrease in mitochondrial OCR was reported (10). Deletion of TSPO in Drosophila melanogaster (dTspo−/−) also resulted in a decrease in mitochondrial OCR and oxidative phosphorylation complex activities (61). However, OCR changes were not observed in Tspo−/− mitochondria isolated from murine hepatocytes (16), suggesting that cell type-specific bioenergetics and other properties affected by the presence/absence or level of TSPO expression could influence observations.
Treatment of fibroblasts with PK11195 did not affect ALA to PPIX bioconversion. However, use of PK11195 decreased ΔΨm in both Tspofl/fl and Tspo−/− cells, suggesting that at least part of the effects observed using this agent is not mediated by TSPO. This observation indicates that some of the actions of PK11195 and other TSPO-binding drugs that have been linked to several seemingly disparate functions, without a physiological explanation (reviewed in Ref. 3 and 4), may be due to off target effects. Early studies that utilized TSPO-binding drugs, PK11195 and Ro5-4864 (4′-chlorodiazepam) demonstrated that they could induce a dose-dependent decrease in OCR (62) and inhibit mitochondrial respiratory control (63), similar to that observed in Tspo−/− cells. However, given our results using PK11195 in Tspo−/− cells, it is clear that specificity of TSPO binding and off target effects may preclude the ability to interpret results from pharmacological studies.
In conclusion, our results suggest that despite its reported PPIX binding property, MmTSPO does not have an effect on erythropoiesis, heme biosynthesis, bioconversion of ALA to PPIX, or in porphyrin-mediated phototoxic cell death. Because TSPO is highly expressed in a variety of cancers, the lack of functional definition for TSPO does not preclude it from being a target for localizing photosensitizing agents as in PDT. However, meaningful interpretation of pharmacological effects observed when using TSPO-binding drugs requires careful consideration of the limitations.
Author Contributions
V. S. and A. H. Z. conceived and designed the project. A. H. Z. performed most experiments, analyzed data, and interpreted results. L. N. T. performed experiments on mitochondrial bioenergetics, C. M. optimized the method and supervised analysis of lysates for PPIX levels, M. P. S. processed and interpreted bone marrow histology, V. V. P. built the Tspo2 adenoviral vector, K. M. supervised in vivo ALA to PPIX bioconversion experiments, and R. C. helped optimize and supervised mitochondrial membrane potential experiments. A. H. Z. and V. S. organized the figures and prepared the manuscript.
Acknowledgments
We thank the Diagnostic Center for Population and Animal Health at Michigan State University for hematology analysis, Dr. Rebecca M. Williams and Dr. Warren R. Zipfel for providing technical help with implementing the phototoxic cell death experiments, the Cornell Biotechnology Resource Center Imaging facility for providing the mercury light source, and Dr. Xiling Shen at Cornell University for sharing human colon cancer resources.
Note Added in Proof
In the original version of the manuscript that was published as a JBC Paper in Press, panels in Fig. 3 and Fig. 4 reporting heme and PPIX values in the y axis were in error due to a miscalculation of coordinates and scale. The correct y axis labels and values for heme and PPIX are now presented in the revised Fig. 3 and Fig. 4. This correction does not change interpretation of results or the conclusions.
This work was supported by funds from the Cornell College of Agriculture and Life Sciences (startup funds to V. S.); the Jane E Brody undergraduate research award and the Dextra undergraduate research endowment fund (to A. H. Z.); and fellowships from the Vietnam Education Foundation and the Cornell Center for Vertebrate Genomics (to L. N. T.). The authors declare that they have no conflicts of interest with the contents of this article.
- TSPO
- translocator protein
- PPIX
- protoporphyrin IX
- PDT
- photodynamic therapy
- ALA
- 5-aminolevulinic acid
- MPTP
- mitochondrial permeability transition pore
- OCR
- oxygen consumption rate
- PK11195
- N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide
- Alas
- ALA synthase
- Alad
- ALA dehydratase
- Hmbs
- hydroxymethylbilane synthase
- Uros
- uroporphyrinogen III synthase
- Urod
- uroporphyrinogen decarboxylase
- Cpox
- coproporphyrinogen oxidase
- Ppox
- protoporphyrinogen oxidase
- Fech
- ferrochelatase
- FCCP
- carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- TMRM
- tetramethylrhodamine methyl ester.
References
- 1. Braestrup C., and Squires R. F. (1977) Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc. Natl. Acad. Sci. U.S.A. 74, 3805–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anholt R. R., Pedersen P. L., De Souza E. B., and Snyder S. H. (1986) The peripheral-type benzodiazepine receptor: localization to the mitochondrial outer membrane. J. Biol. Chem. 261, 576–583 [PubMed] [Google Scholar]
- 3. Gavish M., Bachman I., Shoukrun R., Katz Y., Veenman L., Weisinger G., and Weizman A. (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 51, 629–650 [PubMed] [Google Scholar]
- 4. Selvaraj V., and Stocco D. M. (2015) The changing landscape in translocator protein (TSPO) function. Trends Endocrinol. Metab. 26, 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papadopoulos V., and Miller W. L. (2012) Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 26, 771–790 [DOI] [PubMed] [Google Scholar]
- 6. Morohaku K., Pelton S. H., Daugherty D. J., Butler W. R., Deng W., and Selvaraj V. (2014) Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tu L. N., Morohaku K., Manna P. R., Pelton S. H., Butler W. R., Stocco D. M., and Selvaraj V. (2014) Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 289, 27444–27454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tu L. N., Zhao A. H., Stocco D. M., and Selvaraj V. (2015) PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinology 156, 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selvaraj V., Stocco D. M., and Tu L. N. (2015) Translocator protein (TSPO) and steroidogenesis: a reappraisal. Mol. Endocrinol. 29, 490–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banati R. B., Middleton R. J., Chan R., Hatty C. R., Kam W. W., Quin C., Graeber M. B., Parmar A., Zahra D., Callaghan P., Fok S., Howell N. R., Gregoire M., Szabo A., Pham T., Davis E., and Liu G. J. (2014) Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 5, 5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McEnery M. W., Snowman A. M., Trifiletti R. R., and Snyder S. H. (1992) Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. U.S.A. 89, 3170–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pastorino J. G., Simbula G., Gilfor E., Hoek J. B., and Farber J. L. (1994) Protoporphyrin IX, an endogenous ligand of the peripheral benzodiazepine receptor, potentiates induction of the mitochondrial permeability transition and the killing of cultured hepatocytes by rotenone. J. Biol. Chem. 269, 31041–31046 [PubMed] [Google Scholar]
- 13. Chelli B., Falleni A., Salvetti F., Gremigni V., Lucacchini A., and Martini C. (2001) Peripheral-type benzodiazepine receptor ligands: mitochondrial permeability transition induction in rat cardiac tissue. Biochem Pharmacol 61, 695–705 [DOI] [PubMed] [Google Scholar]
- 14. Sileikyte J., Petronilli V., Zulian A., Dabbeni-Sala F., Tognon G., Nikolov P., Bernardi P., and Ricchelli F. (2011) Regulation of the inner membrane mitochondrial permeability transition by the outer membrane translocator protein (peripheral benzodiazepine receptor). J. Biol. Chem. 286, 1046–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G. D., Petronilli V., Zoratti M., Szabó I., Lippe G., and Bernardi P. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 110, 5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sileikyte J., Blachly-Dyson E., Sewell R., Carpi A., Menabò R., Di Lisa F., Ricchelli F., Bernardi P., and Forte M. (2014) Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (translocator protein of 18 kDa (TSPO)). J. Biol. Chem. 289, 13769–13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verma A., Nye J. S., and Snyder S. H. (1987) Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc. Natl. Acad. Sci. U.S.A. 84, 2256–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeliseev A. A., and Kaplan S. (1995) A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 270, 21167–21175 [DOI] [PubMed] [Google Scholar]
- 19. Frank W., Baar K. M., Qudeimat E., Woriedh M., Alawady A., Ratnadewi D., Gremillon L., Grimm B., and Reski R. (2007) A mitochondrial protein homologous to the mammalian peripheral-type benzodiazepine receptor is essential for stress adaptation in plants. Plant J. 51, 1004–1018 [DOI] [PubMed] [Google Scholar]
- 20. Yeliseev A. A., Krueger K. E., and Kaplan S. (1997) A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. U.S.A. 94, 5101–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeliseev A. A., and Kaplan S. (1999) A novel mechanism for the regulation of photosynthesis gene expression by the TspO outer membrane protein of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 274, 21234–21243 [DOI] [PubMed] [Google Scholar]
- 22. Guo Y., Kalathur R. C., Liu Q., Kloss B., Bruni R., Ginter C., Kloppmann E., Rost B., and Hendrickson W. A. (2015) Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 347, 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vanhee C., Zapotoczny G., Masquelier D., Ghislain M., and Batoko H. (2011) The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 23, 785–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rampon C., Bouzaffour M., Ostuni M. A., Dufourcq P., Girard C., Freyssinet J. M., Lacapere J. J., Schweizer-Groyer G., and Vriz S. (2009) Translocator protein (18 kDa) is involved in primitive erythropoiesis in zebrafish. FASEB J. 23, 4181–4192 [DOI] [PubMed] [Google Scholar]
- 25. Nakazawa F., Alev C., Shin M., Nakaya Y., Jakt L. M., and Sheng G. (2009) PBRL, a putative peripheral benzodiazepine receptor, in primitive erythropoiesis. Gene Expr. Patterns 9, 114–121 [DOI] [PubMed] [Google Scholar]
- 26. Clarke G. D., and Ryan P. J. (1980) Tranquillizers can block mitogenesis in 3T3 cells and induce differentiation in Friend cells. Nature 287, 160–161 [DOI] [PubMed] [Google Scholar]
- 27. Wang J. K., Morgan J. I., and Spector S. (1984) Differentiation of Friend erythroleukemia cells induced by benzodiazepines. Proc. Natl. Acad. Sci. U.S.A. 81, 3770–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taketani S., Kohno H., Okuda M., Furukawa T., and Tokunaga R. (1994) Induction of peripheral-type benzodiazepine receptors during differentiation of mouse erythroleukemia cells. A possible involvement of these receptors in heme biosynthesis. J. Biol. Chem. 269, 7527–7531 [PubMed] [Google Scholar]
- 29. Taketani S., Kohno H., Furukawa T., and Tokunaga R. (1995) Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J. Biochem. 117, 875–880 [DOI] [PubMed] [Google Scholar]
- 30. Zeno S., Veenman L., Katz Y., Bode J., Gavish M., and Zaaroor M. (2012) The 18 kDa mitochondrial translocator protein (TSPO) prevents accumulation of protoporphyrin IX. Involvement of reactive oxygen species (ROS). Curr. Mol. Med. 12, 494–501 [DOI] [PubMed] [Google Scholar]
- 31. Batarseh A., and Papadopoulos V. (2010) Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 327, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Austin C. J., Kahlert J., Kassiou M., and Rendina L. M. (2013) The translocator protein (TSPO): a novel target for cancer chemotherapy. Int. J. Biochem. Cell Biol. 45, 1212–1216 [DOI] [PubMed] [Google Scholar]
- 33. Hardwick M., Fertikh D., Culty M., Li H., Vidic B., and Papadopoulos V. (1999) Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 59, 831–842 [PubMed] [Google Scholar]
- 34. Galiègue S., Casellas P., Kramar A., Tinel N., and Simony-Lafontaine J. (2004) Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin. Cancer Res. 10, 2058–2064 [DOI] [PubMed] [Google Scholar]
- 35. Maaser K., Grabowski P., Oezdem Y., Krahn A., Heine B., Stein H., Buhr H., Zeitz M., and Scherübl H. (2005) Up-regulation of the peripheral benzodiazepine receptor during human colorectal carcinogenesis and tumor spread. Clin. Cancer Res. 11, 1751–1756 [DOI] [PubMed] [Google Scholar]
- 36. Peng Q., Warloe T., Berg K., Moan J., Kongshaug M., Giercksky K. E., and Nesland J. M. (1997) 5-Aminolevulinic acid-based photodynamic therapy: clinical research and future challenges. Cancer 79, 2282–2308 [DOI] [PubMed] [Google Scholar]
- 37. Krieg R. C., Messmann H., Rauch J., Seeger S., and Knuechel R. (2002) Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem. Photobiol. 76, 518–525 [DOI] [PubMed] [Google Scholar]
- 38. Bisland S. K., Goebel E. A., Hassanali N. S., Johnson C., and Wilson B. C. (2007) Increased expression of mitochondrial benzodiazepine receptors following low-level light treatment facilitates enhanced protoporphyrin IX production in glioma-derived cells in vitro. Lasers Surg. Med. 39, 678–684 [DOI] [PubMed] [Google Scholar]
- 39. Furre I. E., Shahzidi S., Luksiene Z., Møller M. T., Borgen E., Morgan J., Tkacz-Stachowska K., Nesland J. M., and Peng Q. (2005) Targeting PBR by hexaminolevulinate-mediated photodynamic therapy induces apoptosis through translocation of apoptosis-inducing factor in human leukemia cells. Cancer Res. 65, 11051–11060 [DOI] [PubMed] [Google Scholar]
- 40. Morrison G. R. (1965) Fluorometric microdetermination of heme protein. Anal. Chem. 37, 1124–1126 [DOI] [PubMed] [Google Scholar]
- 41. Morohaku K., Phuong N. S., and Selvaraj V. (2013) Developmental expression of translocator protein/peripheral benzodiazepine receptor in reproductive tissues. PLoS One 8, e74509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 44. Luo J., Deng Z. L., Luo X., Tang N., Song W. X., Chen J., Sharff K. A., Luu H. H., Haydon R. C., Kinzler K. W., Vogelstein B., and He T. C. (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 [DOI] [PubMed] [Google Scholar]
- 45. Ollion J., Cochennec J., Loll F., Escudé C., and Boudier T. (2013) TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ginter C., Kiburu I., and Boudker O. (2013) Chemical catalysis by the translocator protein (18 kDa). Biochemistry 52, 3609–3611 [DOI] [PubMed] [Google Scholar]
- 47. Wendler G., Lindemann P., Lacapère J. J., and Papadopoulos V. (2003) Protoporphyrin IX binding and transport by recombinant mouse PBR. Biochem. Biophys. Res. Commun. 311, 847–852 [DOI] [PubMed] [Google Scholar]
- 48. Ratcliffe S. L., and Matthews E. K. (1995) Modification of the photodynamic action of delta-aminolaevulinic acid (ALA) on rat pancreatoma cells by mitochondrial benzodiazepine receptor ligands. Br. J. Cancer 71, 300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fan J., Rone M. B., and Papadopoulos V. (2009) Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J. Biol. Chem. 284, 30484–30497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawasaki N., Morimoto K., Tanimoto T., and Hayakawa T. (1996) Control of hemoglobin synthesis in erythroid differentiating K562 cells: I. role of iron in erythroid cell heme synthesis. Arch. Biochem. Biophys. 328, 289–294 [DOI] [PubMed] [Google Scholar]
- 51. Taketani S., Furukawa T., and Furuyama K. (2001) Expression of coproporphyrinogen oxidase and synthesis of hemoglobin in human erythroleukemia K562 cells. Eur. J. Biochem. 268, 1705–1711 [PubMed] [Google Scholar]
- 52. Chiabrando D., Marro S., Mercurio S., Giorgi C., Petrillo S., Vinchi F., Fiorito V., Fagoonee S., Camporeale A., Turco E., Merlo G. R., Silengo L., Altruda F., Pinton P., and Tolosano E. (2012) The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Invest. 122, 4569–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li G., Szewczuk M. R., Raptis L., Johnson J. G., Weagle G. E., Pottier R. H., and Kennedy J. C. (1999) Rodent fibroblast model for studies of response of malignant cells to exogenous 5-aminolevulinic acid. Br. J. Cancer 80, 676–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verma A., Facchina S. L., Hirsch D. J., Song S. Y., Dillahey L. F., Williams J. R., and Snyder S. H. (1998) Photodynamic tumor therapy: mitochondrial benzodiazepine receptors as a therapeutic target. Mol. Med. 4, 40–45 [PMC free article] [PubMed] [Google Scholar]
- 55. Elder G. H., and Evans J. O. (1978) Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem. J. 172, 345–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maaser K., Grabowski P., Sutter A. P., Höpfner M., Foss H. D., Stein H., Berger G., Gavish M., Zeitz M., and Scherübl H. (2002) Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin. Cancer Res. 8, 3205–3209 [PubMed] [Google Scholar]
- 57. Krishnamurthy P. C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K. E., Wang J., Sosa-Pineda B., Murti K. G., and Schuetz J. D. (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443, 586–589 [DOI] [PubMed] [Google Scholar]
- 58. Li F., Liu J., Garavito R. M., and Ferguson-Miller S. (2015) Evolving understanding of translocator protein 18kDa (TSPO). Pharmacol. Res. 99, 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jaremko L., Jaremko M., Giller K., Becker S., and Zweckstetter M. (2014) Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343, 1363–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li F., Liu J., Zheng Y., Garavito R. M., and Ferguson-Miller S. (2015) Protein structure: crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science 347, 555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin R., Angelin A., Da Settimo F., Martini C., Taliani S., Zhu S., and Wallace D. C. (2014) Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Aβ42-induced neurodegeneration. Aging Cell 13, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Larcher J. C., Vayssiere J. L., Le Marquer F. J., Cordeau L. R., Keane P. E., Bachy A., Gros F., and Croizat B. P. (1989) Effects of peripheral benzodiazepines upon the O2 consumption of neuroblastoma cells. Eur. J. Pharmacol. 161, 197–202 [DOI] [PubMed] [Google Scholar]
- 63. Hirsch J. D., Beyer C. F., Malkowitz L., Beer B., and Blume A. J. (1989) Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol. Pharmacol. 35, 157–163 [PubMed] [Google Scholar]