Abstract
Parathyroid hormone (PTH) is an important regulator of osteoblast function and is the only anabolic therapy currently approved for treatment of osteoporosis. The PTH receptor (PTH1R) is a G protein-coupled receptor that signals via multiple G proteins including Gsα. Mice expressing a constitutively active mutant PTH1R exhibited a dramatic increase in trabecular bone that was dependent upon expression of Gsα in the osteoblast lineage. Postnatal removal of Gsα in the osteoblast lineage (P-GsαOsxKO mice) yielded markedly reduced trabecular and cortical bone mass. Treatment with anabolic PTH(1–34) (80 μg/kg/day) for 4 weeks failed to increase trabecular bone volume or cortical thickness in male and female P-GsαOsxKO mice. Surprisingly, in both male and female mice, PTH administration significantly increased osteoblast numbers and bone formation rate in both control and P-GsαOsxKO mice. In mice that express a mutated PTH1R that activates adenylyl cyclase and protein kinase A (PKA) via Gsα but not phospholipase C via Gq/11 (D/D mice), PTH significantly enhanced bone formation, indicating that phospholipase C activation is not required for increased bone turnover in response to PTH. Therefore, although the anabolic effect of intermittent PTH treatment on trabecular bone volume is blunted by deletion of Gsα in osteoblasts, PTH can stimulate osteoblast differentiation and bone formation. Together these findings suggest that alternative signaling pathways beyond Gsα and Gq/11 act downstream of PTH on osteoblast differentiation.
Keywords: G protein, G protein-coupled receptor (GPCR), osteoblast, osteoporosis, parathyroid hormone, PTH/PTH-related peptide receptor
Introduction
Osteoporosis is one of the most common degenerative diseases of aging with an estimated 50% of postmenopausal women and 25% of older men at risk of sustaining a fragility fracture and economic costs exceeding $19 billion annually in the United States (1). The low bone mass underlying osteoporosis results from an imbalance between bone formation and bone resorption that leads to bone loss that becomes more pronounced with age (2). The most commonly prescribed therapies for osteoporosis target the inhibition of bone resorption but as such are not curative.
Anabolic therapy to increase osteoblast numbers and function and thereby stimulate bone formation is appealing as a potential route to curing osteoporosis. Administered once daily, recombinant parathyroid hormone (PTH(1–34);2 teriparatide) reduces fracture risk and significantly increases bone mineral density and bone mass (3). Teriparatide is currently the only Food and Drug Administration-approved anabolic therapy for osteoporosis. However, the mechanisms by which intermittent PTH stimulates bone anabolism have not been fully clarified.
Intermittent PTH treatment enhances bone formation and increases osteoblast numbers by a variety of mechanisms including stimulation of osteoblast proliferation and differentiation, inhibition of osteoblast apoptosis, and activation of quiescent lining cells (4–11). PTH also suppresses the expression of sclerostin, an inhibitor of canonical Wnt signaling produced by osteocytes and encoded by the Sost gene (12, 13). Canonical Wnt signaling plays a crucial role in regulating osteoblast differentiation and bone formation (14–16), and patients lacking sclerostin have high bone mass (17, 18). Highlighting the clinical relevance of this pathway, neutralizing antibodies targeted against sclerostin are now in clinical trials for the treatment of osteoporosis (17–19) and are being tested in preclinical models of other conditions of bone fragility such as osteogenesis imperfecta (20, 21).
The PTH receptor PTH1R is a G protein-coupled receptor. Stimulation of PTH1R by PTH activates a variety of G proteins including the stimulatory G protein Gs. Gs activates adenylyl cyclase, thereby increasing cyclic AMP (cAMP) levels and activating the protein kinase A (PKA) gene transcription pathway (22). Several lines of evidence suggest that Gs is a major mediator of the anabolic actions of PTH. PTH induces the expression of several osteoblast-specific target genes including osteocalcin (Bglap) (23), Mmp13 (24), and Tnfsf11 (25) in a PKA-dependent manner. Targeting of a constitutively active mutant form of PTH1R (caPTH1R), identified in patients with Jansen metaphyseal chondrodysplasia, to osteoblasts in mice results in profound increases in trabecular bone mass (26). In vitro, this mutant version of PTH1R predominantly activates Gs-dependent signaling pathways (27). Furthermore, constitutive activation of Gs-dependent signaling by an engineered Gs-coupled receptor also significantly increases trabecular bone mass (28). In addition to Gs, PTH1R couples to several other G proteins, activating phospholipase Cβ via Gq/G11 to stimulate protein kinase C and G12/G13 to stimulate phospholipase D (29, 30). We have previously reported that phospholipase C (PLC) signaling via PTH1R is essential for stromal cell response to PTH in growing mice (31). Activation of PTH1R also recruits β-arrestins 1 and 2, leading to receptor internalization and activation of ERK1/2 (32).
In the osteoblast lineage, the α subunit of the heterotrimeric Gs protein Gsα is required for normal skeletal development during embryogenesis (33–35). Deletion of Gsα early in the osteoblast lineage using Cre recombinase under control of the osterix (Osx) promoter (16) markedly impairs bone formation such that more immature woven bone is observed (35). There are at least two distinct roles for Gsα in the osteoblast lineage during skeletal development. Gsα is required for commitment of mesenchymal progenitors to the osteoblast lineage rather than the adipocyte lineage, likely mediated at least in part by alterations in Wnt signaling (33, 35). In contrast, in cells committed to the osteoblast lineage, Gsα restrains osteoblast differentiation (35).
Constitutive activation of Gs-dependent signaling in osteoblasts throughout embryogenesis results in a dramatic increase in trabecular bone volume (28). However, when activation of Gs is delayed until birth, there is a much milder increase in bone (36), and if delayed until 4 weeks of age, there is no discernible skeletal phenotype (28). Therefore Gsα-dependent signaling may have different functions during embryogenesis compared with postnatal skeletal homeostasis. Mice with Gsα deleted throughout embryonic development in Osx-expressing osteoprogenitors die before weaning (35). By administering doxycycline to suppress the expression of Cre recombinase, we delayed ablation of Gsα in the osteoblast lineage until birth (postnatal or P-GsαOsxKO mice), allowing us to examine the role of Gsα in osteoblasts in the postnatal skeleton. Here we report that Gsα is required for the high trabecular bone mass observed with constitutive activation of PTH1R. P-GsαOsxKO mice have severe osteoporosis, and when treated with anabolic PTH, there was no increase in trabecular bone volume or cortical thickness. However, osteoblast numbers and bone formation rate still increased along the trabecular surface of P-GsαOsxKO mice. We also examined the effects of anabolic PTH on PLC-defective mutant (D/D) mice that express only a mutated PTH1R that activates adenylyl cyclase normally but cannot activate PLC and found that PTH significantly increased almost all parameters of bone formation in tibiae of both wild type (WT) and D/D mice. Together these data demonstrate the involvement of other downstream mediators beyond activation of PKA and protein kinase C (PKC) in the anabolic action of PTH.
Experimental Procedures
Mice
Generation of GsαOsxKO mice has been described previously (34, 35). Postnatal deletion of Gsα was achieved by administering 10 μg/ml doxycycline in drinking water from conception until birth. Rosa26 (R26R, stock 3309) (37) and mTmG reporter mice (38) were obtained from The Jackson Laboratory (Bar Harbor, ME). Because GsαOsxKO mice are of mixed genetic background, littermate controls (Gsα(fl/fl) except where otherwise specified) were used for all experiments described. Generation of D/D mice has been described previously (39). Genotyping was performed on tail genomic DNA using protocols published previously (34, 35, 39). All animals were housed in the Center for Comparative Medicine at the Massachusetts General Hospital where all experiments were approved by the hospital's Subcommittee on Research Animal Care or at the Veterinary Services Center at Stanford University School of Medicine where all experiments were approved by the Stanford Administrative Panel on Laboratory Animal Care.
Histology
Mouse limbs were fixed in 10% buffered formalin, paraffin-embedded, and sectioned. Immunohistochemical analysis was performed on deparaffinized sections using biotinylated mouse anti-SOST antibody (R&D Systems). For X-Gal staining of β-galactosidase activity, limbs were fixed in 0.2% glutaraldehyde and 1.5% formaldehyde followed by overnight staining at 37 °C in X-Gal solution containing 1 mg/ml X-Gal (Takeda, Osaka, Japan), 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide, 2 mm MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40 (10).
Skeletal Preparations
Skeletons were fixed in 95% ethanol and then stained overnight in 0.015% Alcian blue in acetic acid/ethanol to highlight cartilage. Soft tissues were cleared in 1% KOH and then stained overnight in 0.01% alizarin red to detect mineralized bone (40).
Bone Mineral Density
Mice were anesthetized with tribromoethanol (600 mg/kg). Bone mineral density of the whole skeleton (excluding calvariae) or individual bones was measured by dual energy x-ray absorptiometry on a Lunar Piximus (GE Medical Systems, Milwaukee, WI).
Micro-computed Tomography (μCT) Analysis
Assessment of bone morphology and microarchitecture was performed using a desktop high resolution μCT (μCT40, Scanco Medical, Brüttisellen, Switzerland) as described previously (41). Briefly, the distal femoral metaphysis (P-GsαOsxKO mice) or L5 vertebrae (D/D mice) was scanned for trabecular bone assessment, and mid-diaphyses were scanned for cortical bone assessment using x-ray energy of 70 KeV, an integration time of 200 ms, and a 12-μm isotropic voxel size. Trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) were calculated using a threshold computed for control samples. For cortical bone analysis, cortical thickness (Co.Th) was assessed using a predefined fixed threshold.
Histomorphometry
Double calcein labeling was performed by injecting mice with 20 mg/kg calcein 3 and 10 days before sacrifice. Bones were fixed in 10% buffered formalin and embedded in methylmethacrylate as described previously (42). 5-μm sections were stained with toluidine blue or xylenol orange. Histomorphometric analysis of undecalcified trabecular bone was performed using the Osteomeasure system (OsteoMetrics, Decatur, GA) (42).
Quantitative Real Time PCR
Total RNA was prepared from flushed long bones and primary osteoblasts using the RNeasy kit (Qiagen), and cDNA was synthesized with the SuperScript III First Strand synthesis system for real time PCR (Invitrogen). Quantitative real time PCR was performed using primers for Gnas (43), Runx2 (44), Osx (44), collagen Iα1 (ColIα1) (40), osteopontin (Opn) (44), osteocalcin (Bglap) (45), and Sost (46) according to previously published protocols with mRNA levels normalized relative to β-actin expression. Total RNA samples subjected to cDNA synthesis reactions in the absence of reverse transcriptase were included as negative controls.
Serum Biochemistries
Blood was harvested by cardiac puncture at the time of euthanasia. Fasting serum levels of procollagen type I N-terminal propeptide (P1NP) and type I collagen C-terminal telopeptide (CTX; RatLaps) were measured by enzyme immunoassay (Immunodiagnostics Systems, Scottsdale, AZ) according to the manufacturer's protocol.
Cell Culture
Gsα(fl/fl) calvarial osteoblasts were harvested by serial collagenase digestion as described previously (47) and subjected to adenoviral infection with adeno-Cre or adeno-β-galactosidase (35). Cells were plated at 5 × 103 cells/cm2 and induced to undergo osteogenic differentiation with ascorbic acid (50 μg/ml) and β-glycerophosphate (10 mm). Rat PTH(1–34) (H-5460, Bachem, Dubendorf, Switzerland) was added to culture medium at 50 ng/ml for the first 6 h of every 48-h incubation cycle for 14 days.
Statistics
Statistical analyses were performed using a two-tailed Student's t test, and group differences were analyzed by two-way analysis of variance followed by post hoc Tukey's test. All values are expressed as means ± S.E.
Results
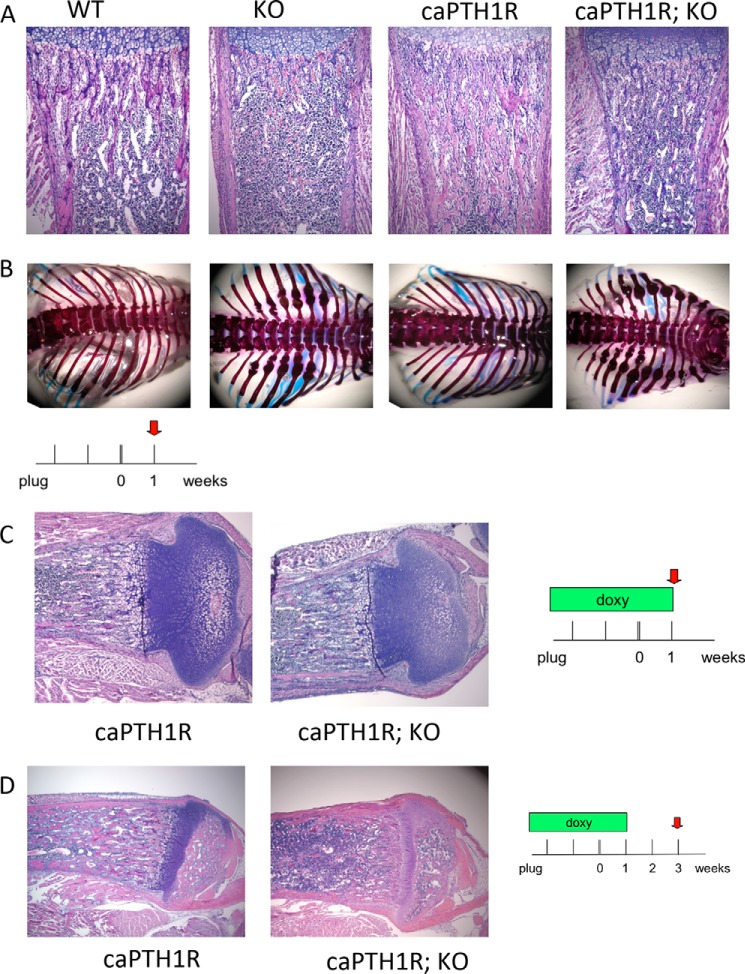
To determine whether Gsα is required for the expansion of trabecular bone mediated by caPTH1R in vivo, we crossed GsαOsxKO mice with caPTH1R transgenic mice. At 1 week of age, histological analysis of proximal tibiae revealed that trabecular bone was nearly absent in GsαOsxKO mice but was greater than controls in caPTH1R mice (Fig. 1A). Skeletal preparations at 1 week of age demonstrated that healing calluses marked sites of rib fractures in GsαOsxKO but not caPTH1R mice (Fig. 1B). Double mutant mice (caPTH1R;GsαOsxKO mice) still exhibited rib fractures, and histological analysis revealed a paucity of trabecular bone that more closely resembled GsαOsxKO mice, although there were a few remaining fragments of trabecular bone (Fig. 1, A and B).
FIGURE 1.
Gsα is required for increase of trabecular bone by constitutively active PTH1R. Hematoxylin- and eosin-stained sections of proximal tibiae (A) and skeletal preparations of rib cages of 7-day-old control (WT), GsαOsxKO (KO), caPTH1R, or double mutant (caPTH1R; KO) mice are shown. C, H&E-stained sections of proximal tibiae of caPTH1R or double mutant (caPTH1R; KO) mice treated with doxycycline (doxy) from plug until 1 week of age. D, H&E-stained sections of proximal tibiae of caPTH1R or double mutant (caPTH1R; KO) mice treated with doxycycline from plug until 1 week of age and analyzed at 3 weeks of age. Images are representative of at least three mice per genotype.
Constitutive expression of Osx-driven Cre recombinase throughout embryonic development resulted in severe osteoporosis at birth with the majority of GsαOsxKO mice dying by 3 weeks of age (34, 35), precluding analysis of Gsα conditional knock-out mice in adulthood. However, the osterix promoter-driven GFP::Cre fusion protein is regulated by a Tet-Off tetracycline transactivator (16). Doxycycline interferes with binding of tetracycline transactivator to its target and abolishes transcription of Osx1-GFP::Cre recombinase (48, 49). When doxycycline was administered in drinking water at 2 mg/ml to pregnant females from mating until 7 days after birth, caPTH1R;GsαOsxKO mice resembled caPTH1R mice with significantly greater trabecular bone mass, providing evidence that doxycycline prevented the deletion of Gsα (Fig. 1C). However, if doxycycline was withdrawn at day 7, then by day 21, caPTH1R;GsαOsxKO mice resembled GsαOsxKO mice by their marked reduction in trabecular bone mass (Fig. 1D). These results demonstrate that the caPTH1R mutant receptor requires expression of Gsα in osteoblasts to increase trabecular bone mass and suggest that Gsα expression in GsαOsxKO mice can be regulated by doxycycline administration.
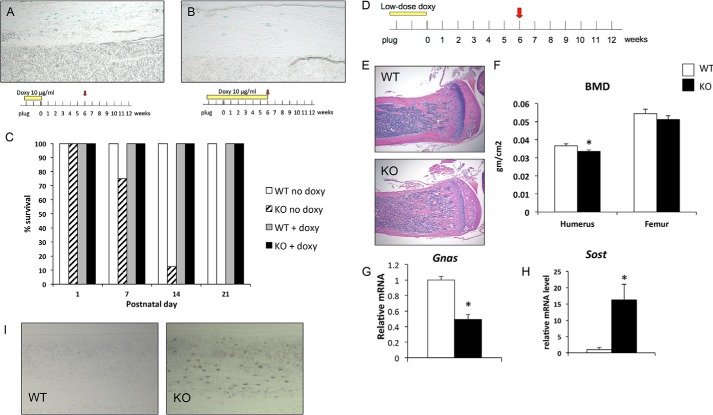
Hong et al. (50) reported that high doses of doxycycline can lead to prolonged suppression of Cre expression even after withdrawal of doxycycline, whereas lower doses of doxycycline allow improved titration of Cre expression. We therefore tested doxycycline at a 200-fold lower dose (10 μg/ml) in drinking water starting from conception. To determine whether Cre is expressed after withdrawal of doxycycline, we crossed GsαOsxKO mice to R26 reporter mice (37) in which a floxed stop cassette precedes the gene encoding β-galactosidase. Expression of Cre recombinase results in expression of β-galactosidase, which is detected by staining with X-Gal. Chen et al. (51) reported that the extraskeletal expression of Osx1-GFP::Cre is limited to the olfactory bulb, gastric, and intestinal epithelial cells; therefore we focused on X-Gal staining in bone. Following withdrawal of doxycycline at birth, we found abundant X-Gal staining of osteocytes and osteoblasts in Osx1-GFP::Cre+ mice, indicating Cre expression (Fig. 2A). In controls maintained on doxycycline until 6 weeks of age, X-Gal staining in the bone was significantly but not completely reduced (Fig. 2B). Despite incomplete suppression of Cre recombinase by doxycycline, the survival of P-GsαOsxKO mice, which in the absence of doxycycline do not survive to weaning, improved to 100% at 3 weeks of age (Fig. 2C).
FIGURE 2.
Postnatal deletion of Gsα leads to osteopenia and increased sclerostin expression. A, X-Gal staining for β-galactosidase activity in 6-week-old GsαOsxKO mice treated with doxycycline (Doxy) from plug until delivery (100× magnification). B, X-Gal staining of 6-week-old P-GsαOsxKO mice treated with doxycycline until 6 weeks of age (100×). C, survival frequency in control (WT no doxy; white bar; n = 18) and GsαOsxKO (KO no doxy; hatched bar; n = 8) mice without doxycycline treatment or in control (WT + doxy; gray bar; n = 7) or P-GsαOsxKO mice (KO + doxy; black bar; n = 10) on 10 μg/ml doxycycline from plug until birth. D, pregnant dams were administered 10 μg/ml doxycycline in drinking water from plug until delivery. The resulting mice were analyzed at 6 weeks of age. E, H&E-stained sections of proximal tibia from female 6-week-old mice treated with 10 μg/ml doxycycline until birth. F, bone mineral density (BMD) is significantly reduced in the humerus of P-GsαOsxKO mice. gm, gram. G, Gnas mRNA levels in femurs of WT and P-GsαOsxKO mice at 6 weeks. H, Sost mRNA levels in calvariae of 6-week-old mice. I, sclerostin immunostaining in cortical bone of WT and P-GsαOsxKO tibiae at 6 weeks of age. n = 5, WT; n = 6, P-GsαOsxKO. *, p < 0.05. Error bars represent S.E.
To assess the effects of postnatal Gsα deletion, we administered 10 μg/ml doxycycline throughout embryogenesis until birth (P0) and examined the skeleton at 6 weeks of age (Fig. 2D). Control mice did not express the Cre transgene; however, expression of the Cre transgene in wild-type mice did not significantly affect skeletal formation or bone mineral density, although Cre-expressing mice were slightly smaller (data not shown), consistent with previous reports of a transient decrease in body weight of Osx-Cre transgenic mice that resolves by 12 weeks of age (52). Histological analysis of P-GsαOsxKO mice at 6 weeks of age demonstrated low trabecular bone mass (Fig. 2E), and dual energy x-ray absorptiometry revealed reduced bone mineral density in the humerus (Fig. 2F). Serum calcium and phosphate levels did not differ between P-GsαOsxKO and control littermates (data not shown). We have previously reported that Gnas mRNA levels are reduced by almost 90% in sorted Osx1-GFP::Cre+ cells of GsαOsxKO mice in which Gsα is deleted throughout embryogenesis (34). However, the yield of Osx1-GFP::Cre+ cells from mineralized adult bone is extremely low. We therefore prepared RNA from bone samples flushed of marrow and found that Gnas mRNA levels were reduced by 50% (Fig. 2G). Because Gsα is ubiquitously expressed, the remaining Gnas mRNA detected was likely largely expressed in non-osteoblast lineage cells.
As another approach to determine the effectiveness of Gsα deletion, we examined expression of sclerostin. Sclerostin is an inhibitor of canonical Wnt signaling that is suppressed by PTH. Constitutive ablation of Gsα in the osteoblast lineage up-regulates Sost, the gene encoding sclerostin (35). We therefore examined Sost mRNA and sclerostin protein levels in P-GsαOsxKO mice. Sost mRNA increased >15-fold in bones of female P-GsαOsxKO mice (Fig. 2H). Immunohistochemical analysis for sclerostin protein also revealed a marked increase in sclerostin expression in P-GsαOsxKO osteocytes (Fig. 2I), suggesting deletion of Gsα with high efficiency from the osteoblast lineage.
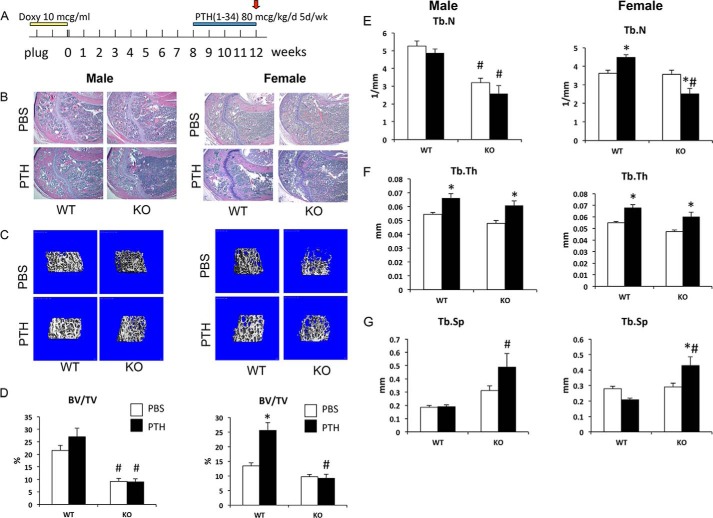
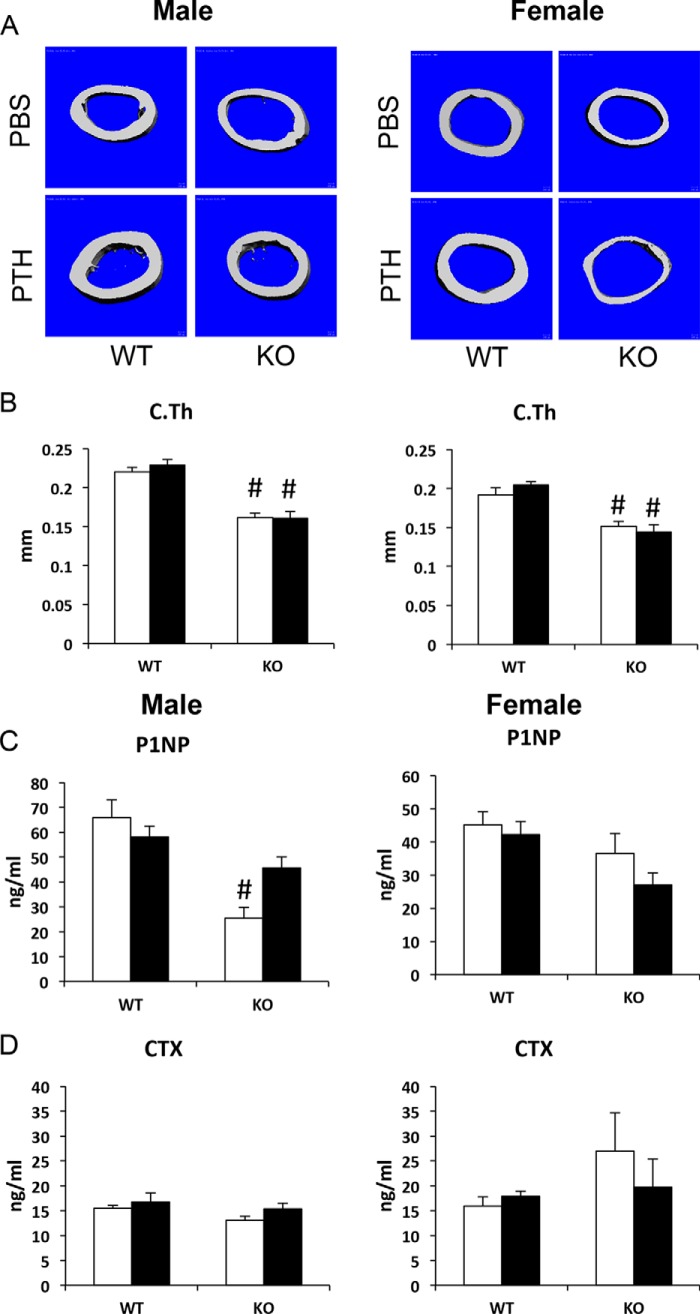
To examine the role of Gsα in the anabolic response to PTH, control and P-GsαOsxKO mice were injected with once daily PTH(1–34) at 80 μg/kg/day beginning at 8 weeks of age for 4 weeks (Fig. 3A). In vehicle (PBS)-treated mice, histological analysis of the proximal humerus (Fig. 3B) and μCT analysis of distal femur trabecular bone (Fig. 3C) revealed low trabecular bone mass in P-GsαOsxKO male and female mice compared with controls. In females, PTH treatment increased BV/TV and Tb.N in WT compared with vehicle-treated mice, but this was not observed in P-GsαOsxKO mice where PTH treatment instead decreased Tb.N (Fig. 3, D and E). In males, PTH did not increase BV/TV or Tb.N in either WT or P-GsαOsxKO mice (Fig. 3, D and E). Trabecular thickness was significantly greater in PTH-treated WT and P-GsαOsxKO mice of both genders (Fig. 3F), and trabecular separation was increased in PTH-treated male and female P-GsαOsxKO mice as compared with WT controls (Fig. 3G). Cortical thickness was reduced in both male and female P-GsαOsxKO mice compared with WT controls; PTH treatment had no effect on this parameter (Fig. 4, A and B). Serum levels of P1NP, a marker of bone formation, did not differ in female mice, whereas in males P1NP was lower in PBS-treated P-GsαOsxKO mice than in WT, but PTH did not have any significant effect (Fig. 4C). In summary, in both male and female P-GsαOsxKO mice, PTH failed to increase trabecular bone volume or cortical thickness but did enhance trabecular thickness. In addition, PTH increased trabecular spacing in P-GsαOsxKO but not control mice.
FIGURE 3.
Intermittent PTH does not increase trabecular bone mass in P-GsαOsxKO male and female mice. A, mice were treated with 10 μg (mcg)/ml doxycycline from plug until delivery. At 8 weeks of age, control (WT) and P-GsαOsxKO (KO) mice were injected with 80 μg/kg/day (d) PTH(1–34) or PBS 5 days/week (wk). H&E-stained sections of proximal humerus (B), μCT analysis of distal femur trabecular bone (C), BV/TV (D), Tb.N (E), Tb.Th (F), and Tb.Sp (G) of male (left) and female (right) mice are shown. n = 6–9 for each group of male mice, and n = 5–8 for each group of female mice. *, p < 0.05 versus respective PBS control; #, p < 0.05 versus respective WT control. Error bars represent S.E.
FIGURE 4.
Cortical thickness is decreased in P-GsαOsxKO male and female mice. μCT analysis of femoral midshaft cortical bone (A) and cortical thickness (C.Th) in WT and P-GsαOsxKO (KO) male (left) and female (right) mice treated with PBS and PTH is shown. n = 6–9 for each group of male mice, and n = 5–8 for each group of female mice. Serum levels of P1NP (C) and CTX (D) from male and female WT and P-GsαOsxKO mice treated with PBS and PTH are shown. n = 8–11 for each group of male mice, and n = 6–9 for each group of female mice. *, p < 0.05 versus respective PBS control; #, p < 0.05 versus respective WT control. Error bars represent S.E.
Histomorphometric analysis of distal femora from male mice confirmed that BV/TV and trabecular number were lower in PBS-treated P-GsαOsxKO compared with WT mice and that PTH treatment had no effect on either of these parameters (Table 1). Again, PTH increased trabecular thickness in WT and P-GsαOsxKO mice; no significant change in trabecular separation was observed. PTH treatment increased both osteoblast surface and osteoblast numbers in control mice and, surprisingly, in P-GsαOsxKO male mice. Bone formation rate as assessed by double calcein labeling was also greater in both control and P-GsαOsxKO male mice treated with PTH. The failure of intermittent PTH to increase bone volume in P-GsαOsxKO mice despite increased osteoblast numbers and bone formation rate could potentially be explained by high osteoclast activity. However, PTH treatment did not significantly alter osteoclast surface in either WT or KO mice (Table 1). There were no differences in serum levels of CTX, a marker of bone resorption, in either female or male mice (Fig. 4D).
TABLE 1.
Histomorphometry of 12-week-old control (WT) or P-GsαOsxKO (KO) tibiae with intermittent PTH or vehicle for 4 weeks
Values are mean ± S.E. with n = 6–9 mice for each group. BFR, bone formation rate; BS, bone surface; MS, mineralizing surface; MAR, mineral apposition rate; Ob.S, osteoblast surface; N.Ob/B.Pm, number of osteoblasts per bone perimeter; O.Th, osteoid thickness; OS, osteoid surface; Os.S, osteoclast surface; N.Oc/B.Pm, number of osteoclasts per bone perimeter.
| Parameters | WT |
KO |
||
|---|---|---|---|---|
| PBS (n = 8) | PTH (n = 9) | PBS (n = 6) | PTH (n = 6) | |
| BV/TV (%) | 15.50 ± 1.93 | 18.96 ± 2.27 | 5.22 ± 0.83a | 5.48 ± 1.47a |
| Tb.Th (μm) | 37.84 ± 1.79 | 48.98 ± 2.38b | 28.62 ± 1.29 | 37.29 ± 3.24a,b |
| Tb.N (/mm) | 4.00 ± 0.35 | 3.78 ± 0.31 | 1.79 ± 0.23a | 1.56 ± 0.41a |
| Tb.Sp (μm) | 229.57 ± 30.75 | 232.41 ± 27.59 | 576.35 ± 75.42 | 1324.96 ± 622.91 |
| MS/BS (%) | 22.50 ± 4.22 | 30.61 ± 2.22 | 27.68 ± 1.60 | 37.30 ± 5.59a |
| MAR (μm/day) | 1.57 ± 0.16 | 3.00 ± 0.26b | 1.21 ± 0.34 | 1.86 ± 0.07a |
| BFR/BS (μm3/μm2/day) | 0.35 ± 0.08 | 0.93 ± 0.12b | 0.33 ± 0.09 | 0.70 ± 0.11b |
| Ob.S/BS (%) | 6.53 ± 1.30 | 12.37 ± 1.67b | 1.21 ± 0.77 | 8.22 ± 2.27b |
| N.Ob/B.Pm (/mm) | 4.08 ± 0.89 | 8.19 ± 1.35b | 0.76 ± 0.49 | 5.35 ± 1.56b |
| OS/BS (%) | 2.05 ± 0.64 | 6.49 ± 1.91 | 0.16 ± 0.16 | 2.91 ± 1.05b |
| O.Th (μm) | 0.60 ± 0.11 | 0.87 ± 0.22 | 0.05 ± 0.05 | 0.48 ± 0.16 |
| Oc.S/BS (%) | 4.97 ± 0.71 | 5.31 ± 0.32 | 5.03 ± 0.73 | 6.67 ± 0.60 |
| N.Oc/B.Pm (/mm) | 2.27 ± 0.33 | 2.17 ± 0.13 | 2.86 ± 0.47 | 3.34 ± 0.35 |
a p < 0.05 versus respective WT control.
b p < 0.05 versus respective PBS control.
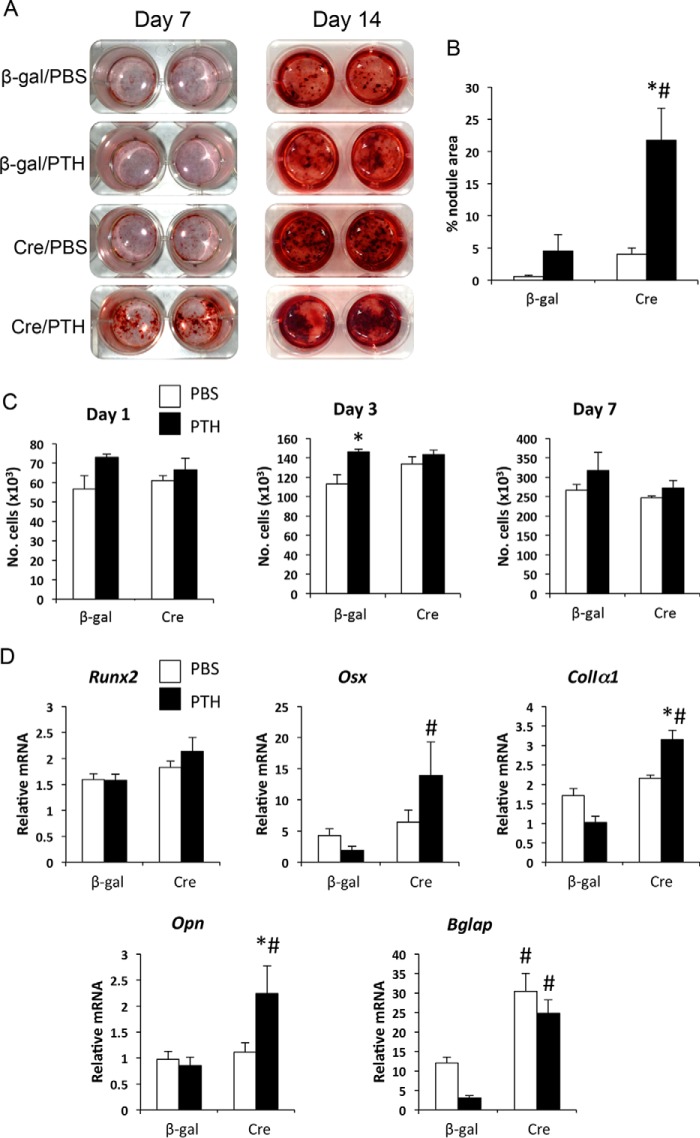
To determine whether the effects of PTH on osteoblast numbers and bone formation rate in P-GsαOsxKO mice were mediated by direct action of PTH on osteoblasts, we isolated calvarial osteoblasts from mice with two floxed alleles of Gsα. Infection of Gsα(fl/fl) calvarial osteoblasts in vitro with adenovirus encoding Cre recombinase leads to efficient (>90%) deletion of Gsα (35). We treated Gsα(fl/fl) calvarial osteoblasts infected with Cre recombinase or β-gal control and then cultured cells under osteogenic conditions with PTH or PBS for 6 h of every 48 h to stimulate osteoblast differentiation (53). In control osteoblasts, PTH increased mineralized nodule formation after 14 days, and as reported previously, deletion of Gsα led to markedly enhanced mineralization (35). Treatment with PTH further increased mineralized nodule formation by Gsα-deficient osteoblasts (Fig. 5, A and B). Mineralization can be increased by increased cell density. However, cell numbers were increased only transiently at day 3 in PTH-treated control osteoblasts (Fig. 5C). Examination of expression of markers of osteogenic differentiation confirmed that PTH treatment of Gsα-deficient osteoblasts significantly increased expression of a range of osteoblast marker genes including Osx, ColIα1, Opn, and Bglap as compared with PTH-treated control osteoblasts (Fig. 5D). This provides additional evidence that PTH can stimulate osteogenic differentiation in Gsα-deficient osteoblasts.
FIGURE 5.
PTH enhances osteogenic differentiation of Gsα-deficient osteoblasts. A, Alizarin red staining of Gsα(fl/fl) calvarial osteoblasts infected with adeno-β-gal (control) or adeno-Cre, then treated with PBS or PTH for 6 h of every 48 h, and subjected to osteogenic differentiation for 7 and 14 days. B, quantitation of mineralized nodule area at day 14. C, numbers of cells per well of Gsα(fl/fl) calvarial osteoblasts treated with adeno-β-gal or adeno-Cre and then with PBS or PTH. n = 3 experiments. D, mRNA expression levels of Runx2, Osx, ColIα1, Opn, and Bglap at day 7 in cells described above. Mean results from three replicate experiments are shown. *, p < 0.05 versus respective PBS control; #, p < 0.05 versus respective WT control. Error bars represent S.E.
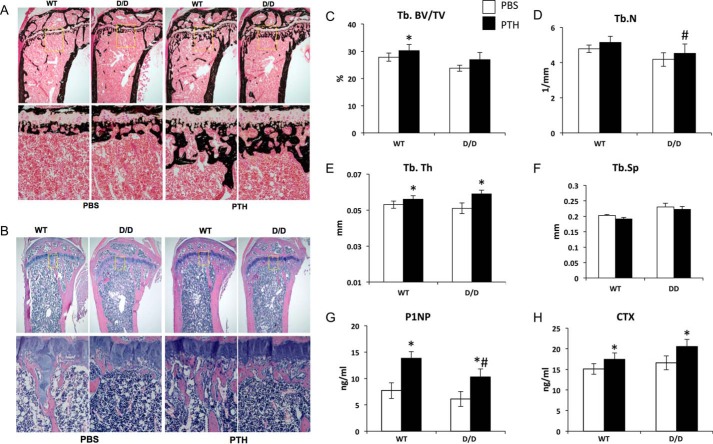
In addition to the Gsα-linked adenylyl cyclase-PKA signaling pathway, PTH is known to activate the Gq/G11-linked PLC-PKC signaling pathway. PLC-defective mutant (D/D) mice express only a mutated PTH1R that activates adenylyl cyclase normally but cannot activate PLC (39). To determine whether PLC signaling pathway via the PTH1R plays a role in bone anabolic action of PTH, we administered intermittent PTH to both PTH1R WT and D/D mice. Although a lowered bone mass was previously noted in growing tibiae of D/D mice at 6 and 10 weeks of age, at 16 weeks neither the tibia (determined by histomorphometry) nor the femur (examined by μCT) of D/D mice exhibited a significantly lower bone mass at baseline (Fig. 6 and Table 2). Intermittent PTH increased trabecular bone volume in the primary spongiosa in both WT and D/D mice; this area was excluded from standard measurement of histomorphometry (Fig. 6, A and B). Histomorphometry revealed no changes in bone architecture parameters (BV/TV, Tb.Th, and Tb.N) in the areas measured (Table 2). However, parameters of bone formation including mineral apposition rate, bone formation rate, bone volume and tissue volume, osteoid surface, osteoblast surface, and osteoblast number were increased by intermittent PTH in both WT and D/D mice (Table 2). In L5 vertebrae, as measured by μCT, PTH increased trabecular bone volume only in WT, but not D/D, mice (Fig. 6C). Trabecular number was not significantly altered by PTH treatment in either WT or D/D mice (Fig. 6D). PTH increased trabecular thickness in both WT and D/D mice (Fig. 6E) but had no effect on trabecular separation (Fig. 6F).
FIGURE 6.
Intermittent PTH increases bone formation in DSEL mice. von Kossa-stained plastic sections (A) and H&E stained paraffin sections (B) from proximal tibiae of 16-week old WT and DSEL (D/D) mice treated with vehicle (VEH) or intermittent PTH (80 μg/kg/day) from 12 weeks of age. n = 7–9 for each group. C–F, μCT analysis of 16-week-old L5 vertebrae from WT and DSEL (D/D) mice treated with vehicle (PBS) or intermittent PTH (80 μg/kg/day) for the last 4 weeks. Tb.BV/TV (%) (C), Tb.N (1/mm) (D), Tb.Th (mm) (E), and Tb.Sp (1/mm3) (F) were measured in eight to nine animals of each group. Serum P1NP (G) and CTX (H) were measured in 16-week-old WT and DSEL (D/D) mice treated with vehicle (VEH) or intermittent PTH (80 μg/kg/day) for the last 4 weeks. n = 8 per group. *, p < 0.05 versus respective PBS control; #, p < 0.05 versus respective WT control. Error bars represent S.E.
TABLE 2.
Histomorphometry of 16-week-old control (WT) or D/D tibiae with intermittent PTH or vehicle for 4 weeks
Values are mean ± S.E. with n = 7–9 mice for each group. BFR, bone formation rate; BS, bone surface; MS, mineralizing surface; MAR, mineral apposition rate; Ob.S, osteoblast surface; N.Ob/B.Pm, number of osteoblasts per bone perimeter; O.Th, osteoid thickness; OS, osteoid surface; Os.S, osteoclast surface; N.Oc/B.Pm, number of osteoclasts per bone perimeter.
| Parameters | WT |
D/D |
||
|---|---|---|---|---|
| PBS (n = 8) | PTH (n = 8) | PBS (n = 7) | PTH (n = 9) | |
| BV/TV (%) | 4.84 ± 0.80 | 5.97 ± 1.04 | 3.72 ± 0.54 | 5.10 ± 0.91 |
| Tb.Th (μm) | 33.62 ± 3.28 | 35.57 ± 2.34 | 28.73 ± 1.63 | 32.42 ± 1.80 |
| Tb.N (/mm) | 1.44 ± 0.19 | 1.61 ± 0.24 | 1.27 ± 0.15 | 1.54 ± 0.23 |
| Tb.Sp (μm) | 759 ± 109 | 708 ± 133 | 823 ± 94 | 839 ± 228 |
| MS/BS (%) | 21.27 ± 1.43 | 31.54 ± 1.66a | 24.97 ± 1.28 | 25.70 ± 2.24 |
| MAR (μm/day) | 1.90 ± 0.14 | 2.34 ± 0.12a | 1.56 ± 0.21 | 2.52 ± 0.13a |
| BFR/BS (μm3/μm2/year) | 147 ± 14 | 270 ± 23a | 141 ± 20 | 238 ± 26a |
| Ob.S/BS (%) | 14.25 ± 2.77 | 29.45 ± 2.15a | 17.97 ± 3.59 | 31.47 ± 2.77a |
| N.Ob/B.Pm (/mm) | 11.47 ± 1.95 | 23.64 ± 1.75a | 14.09 ± 3.05 | 22.87 ± 1.67a |
| OS/BS (%) | 4.36 ± 1.12 | 19.51 ± 1.86a | 7.45 ± 2.35 | 14.41 ± 1.51a |
| O.Th (μm) | 2.31 ± 0.30 | 2.83 ± 0.18 | 2.48 ± 0.12 | 2.73 ± 0.15 |
| Oc.S/BS (%) | 2.36 ± 0.69 | 5.24 ± 0.97a | 3.39 ± 0.64 | 5.82 ± 1.04 |
| N.Oc/B.Pm (/mm) | 0.94 ± 0.28 | 1.97 ± 0.36a | 1.37 ± 0.29 | 2.55 ± 0.61 |
a p < 0.05 versus respective PBS control.
We measured serum P1NP as a bone formation marker and serum CTX as a bone resorption marker (Fig. 6, G and H). Both P1NP and CTX were significantly increased by PTH in WT and D/D mice; the level of P1NP in PTH-treated D/D mice was lower than that of PTH-treated WT mice (Fig. 6G).
Discussion
We have found that Gsα signaling in the osteoblast lineage is important not only for embryonic skeletal development but also for adult skeletal homeostasis. Furthermore, we have demonstrated that Gsα is a downstream mediator of anabolic PTH signaling in vivo by two approaches. First, we crossed mice lacking Gsα in osteoblasts to mice expressing caPTH1R in osteoblasts and found that the postnatal absence of Gsα markedly attenuates the ability of caPTH1R to increase trabecular bone. Second, we administered intermittent PTH to adult mice lacking Gsα in osteoblasts and found that in the absence of Gsα the anabolic actions of intermittent PTH on trabecular bone are blunted in vivo.
Mice with postnatal ablation of Gsα in osteoblast progenitors (P-GsαOsxKO mice) have severe osteoporosis affecting both trabecular and cortical bone. Osteoclast surface and CTX levels are not elevated, indicating that their low bone mass is not due to enhanced bone resorption. These findings are consistent with our previous studies demonstrating failure of bone formation in mice with deletion of Gsα in osteoprogenitors throughout embryonic development (35).
PTH increased osteoblast numbers and bone formation rate in P-GsαOsxKO mice, a paradoxical finding because PTH failed to increase overall bone mass in those mice. A proportionally greater increase in resorption in response to PTH by P-GsαOsxKO mice would be one explanation; however, there was no significant increase in osteoclast surface, CTX levels, or trabecular separation in P-GsαOsxKO mice treated with intermittent PTH, and trabecular thickness was increased. An alternative explanation is that by 8 weeks of age when anabolic PTH was started P-GsαOsxKO mice have insufficient trabecular bone surface on which new bone can be formed; studies are now underway to begin PTH treatment earlier before bone loss has progressed to such an advanced degree in P-GsαOsxKO mice. Finally, in cultured osteoblasts, PTH can maximally activate PKA at concentrations significantly lower than required to increase total cAMP levels, suggesting that only a fraction of the total cAMP that can be generated by PTH stimulation is required for PKA activation (54). It is also important to note that Gsα-mediated PTH1R signaling in other tissues such as the kidney is presumably preserved. Careful analysis of postnatal extraskeletal Osx-Cre expression by Chen et al. (51) revealed that although Osx-Cre targets cells in the olfactory bulb, gastric, and intestinal epithelia there is no expression in the kidney, liver, or other organs. Therefore indirect effects of PTH, for example on systemic mineral metabolism, may also influence skeletal homeostasis. However, we found that PTH does have direct effects in osteoblasts that are altered by the removal of Gsα.
The mechanisms by which intermittent PTH stimulates osteoblast differentiation and function are incompletely understood. Because PTH treatment has a positive effect on osteoblast surface in Gsα-deficient mice, it is likely that PTH also stimulates signaling pathways downstream of PTH1R independent of Gsα. These may include pathways activated by other PTH1R-coupled G proteins including Gq/G11 and G12/G13 (29, 30). We found that although intermittent PTH in older mice did not significantly increase trabecular bone volume in long bones of either WT or D/D mice within the time frame of this experiment it did significantly increase almost all parameters of bone formation in both WT and D/D mice. Our data suggest that PLC signaling through the PTH1R is therefore not required for PTH-stimulated bone turnover in mature mice. However, our findings do not rule out the possibility that in the absence of Gsα activation of Gq/11 may mediate some of the anabolic effects of PTH.
In addition to PTH1R-coupled G proteins, β-arrestins have also been implicated in the anabolic response to PTH, and the anabolic effect of PTH is blunted in the absence of β-arrestin2 (55). Initially identified based on their roles in G protein-coupled receptor desensitization, growing evidence suggests that β-arrestins can themselves mediate signaling downstream of G protein-coupled receptors. Activation of PTH1R by PTH stimulates both Gsα- and β-arrestin-mediated activation of mitogen-activated protein kinases ERK1/2; although Gsα activation leads to an early increase in ERK activity, β-arrestins contribute to a later, sustained activation of ERK1/2 (32). A modified PTH peptide that attenuates Gsα-dependent signaling while stimulating β-arrestin-dependent signaling (PTH-βarr) (32) can still increase bone mass as well as bone formation rate and osteoblast surface in a β-arrestin2-dependent manner (56), although effects mediated by stimulation of endogenous PTH secretion were not ruled out. Studies are now underway to address the role of non-Gsα-mediated signaling pathways downstream of PTH1R.
Author Contributions
J. Y. W. conceived and coordinated the study and wrote the paper. J. Y. W., G. N., and E. S. designed, performed, and analyzed the experiments shown in Fig. 1. J. Y. W. and P. A. designed, performed, and analyzed the experiments shown in Fig. 2. P. S. and R. C. designed, performed, and analyzed the experiments shown in Figs. 3 and 4. I. J. P. and N. A. S. designed, performed, and analyzed the experiments shown in Table 1. H. S. designed, performed, and analyzed the experiments shown in Fig. 5. J. G. and H. M. K. designed, performed, and analyzed the experiments shown in Table 2. M. C. and L. S. W. generated the Gsα-floxed mice and contributed to experimental design and data interpretation. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Andrew McMahon for providing Osx1-GFP::Cre mice and the Massachusetts General Hospital Center for Comparative Medicine and Stanford Veterinary Services Center staff for care of the animals.
This work was supported by National Institutes of Health Grants AR054741 and AR059942 (to J. Y. W.), AR061228 (to P. S.), and DK011794 (to H. M. K.) and the NIDDK Intramural Research Program (to M. C. and L. S. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PTH
- parathyroid hormone
- PTH1R
- PTH receptor
- PLC
- phospholipase C
- caPTH1R
- constitutively active mutant form of PTH1R
- Osx
- osterix
- μCT
- micro-computed tomography
- BV
- bone volume
- TV
- tissue volume
- BV/TV
- trabecular bone volume fraction
- Tb.Th
- trabecular thickness
- Tb.Sp
- trabecular separation
- Tb.N
- trabecular number
- Co.Th
- cortical thickness
- P1NP
- procollagen type I N-terminal propeptide
- CTX
- type I collagen C-terminal telopeptide
- SOST
- sclerostin
- ColIα1
- collagen Iα1
- Opn
- osteopontin
- Bglap
- osteocalcin.
References
- 1. Burge R., Dawson-Hughes B., Solomon D. H., Wong J. B., King A., and Tosteson A. (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 22, 465–475 [DOI] [PubMed] [Google Scholar]
- 2. Raisz L. G. (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Investig. 115, 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neer R. M., Arnaud C. D., Zanchetta J. R., Prince R., Gaich G. A., Reginster J. Y., Hodsman A. B., Eriksen E. F., Ish-Shalom S., Genant H. K., Wang O., and Mitlak B. H. (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 [DOI] [PubMed] [Google Scholar]
- 4. Bellido T., Ali A. A., Plotkin L. I., Fu Q., Gubrij I., Roberson P. K., Weinstein R. S., O'Brien C. A., Manolagas S. C., and Jilka R. L. (2003) Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 278, 50259–50272 [DOI] [PubMed] [Google Scholar]
- 5. Dobnig H., and Turner R. T. (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138, 4607–4612 [DOI] [PubMed] [Google Scholar]
- 6. Iida-Klein A., Zhou H., Lu S. S., Levine L. R., Ducayen-Knowles M., Dempster D. W., Nieves J., and Lindsay R. (2002) Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J. Bone Miner. Res. 17, 808–816 [DOI] [PubMed] [Google Scholar]
- 7. Jilka R. L. (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40, 1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jilka R. L., O'Brien C. A., Ali A. A., Roberson P. K., Weinstein R. S., and Manolagas S. C. (2009) Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone 44, 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jilka R. L., Weinstein R. S., Bellido T., Roberson P., Parfitt A. M., and Manolagas S. C. (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Investig. 104, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim S. W., Pajevic P. D., Selig M., Barry K. J., Yang J. Y., Shin C. S., Baek W. Y., Kim J. E., and Kronenberg H. M. (2012) Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. 27, 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindsay R., Cosman F., Zhou H., Bostrom M. P., Shen V. W., Cruz J. D., Nieves J. W., and Dempster D. W. (2006) A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J. Bone Miner. Res. 21, 366–373 [DOI] [PubMed] [Google Scholar]
- 12. Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., and Wu D. (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280, 19883–19887 [DOI] [PubMed] [Google Scholar]
- 13. Semënov M., Tamai K., and He X. (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 280, 26770–26775 [DOI] [PubMed] [Google Scholar]
- 14. Day T. F., Guo X., Garrett-Beal L., and Yang Y. (2005) Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 15. Hill T. P., Später D., Taketo M. M., Birchmeier W., and Hartmann C. (2005) Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell 8, 727–738 [DOI] [PubMed] [Google Scholar]
- 16. Rodda S. J., and McMahon A. P. (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 [DOI] [PubMed] [Google Scholar]
- 17. Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., Paes-Alves A. F., Hill S., Bueno M., Ramos F. J., Tacconi P., Dikkers F. G., Stratakis C., Lindpaintner K., Vickery B., Foernzler D., and Van Hul W. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 [DOI] [PubMed] [Google Scholar]
- 18. Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., and Mulligan J. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClung M. R., Grauer A., Boonen S., Bolognese M. A., Brown J. P., Diez-Perez A., Langdahl B. L., Reginster J. Y., Zanchetta J. R., Wasserman S. M., Katz L., Maddox J., Yang Y. C., Libanati C., and Bone H. G. (2014) Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 [DOI] [PubMed] [Google Scholar]
- 20. Kedlaya R., Veera S., Horan D. J., Moss R. E., Ayturk U. M., Jacobsen C. M., Bowen M. E., Paszty C., Warman M. L., and Robling A. G. (2013) Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci. Transl. Med. 5, 211ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinder B. P., Eddy M. M., Ominsky M. S., Caird M. S., Marini J. C., and Kozloff K. M. (2013) Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J. Bone Miner. Res. 28, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearman A. T., Chou W. Y., Bergman K. D., Pulumati M. R., and Partridge N. C. (1996) Parathyroid hormone induces c-fos promoter activity in osteoblastic cells through phosphorylated cAMP response element (CRE)-binding protein binding to the major CRE. J. Biol. Chem. 271, 25715–25721 [DOI] [PubMed] [Google Scholar]
- 23. Boguslawski G., Hale L. V., Yu X. P., Miles R. R., Onyia J. E., Santerre R. F., and Chandrasekhar S. (2000) Activation of osteocalcin transcription involves interaction of protein kinase A- and protein kinase C-dependent pathways. J. Biol. Chem. 275, 999–1006 [DOI] [PubMed] [Google Scholar]
- 24. Selvamurugan N., Chou W. Y., Pearman A. T., Pulumati M. R., and Partridge N. C. (1998) Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J. Biol. Chem. 273, 10647–10657 [DOI] [PubMed] [Google Scholar]
- 25. Lee S. K., and Lorenzo J. A. (1999) Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140, 3552–3561 [DOI] [PubMed] [Google Scholar]
- 26. Calvi L. M., Sims N. A., Hunzelman J. L., Knight M. C., Giovannetti A., Saxton J. M., Kronenberg H. M., Baron R., and Schipani E. (2001) Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J. Clin. Investig. 107, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schipani E., Kruse K., and Jüppner H. (1995) A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science 268, 98–100 [DOI] [PubMed] [Google Scholar]
- 28. Hsiao E. C., Boudignon B. M., Chang W. C., Bencsik M., Peng J., Nguyen T. D., Manalac C., Halloran B. P., Conklin B. R., and Nissenson R. A. (2008) Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc. Natl. Acad. Sci. U.S.A. 105, 1209–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T. Jr., Kronenberg H. M., and Segre G. V. (1992) Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. U.S.A. 89, 2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh A. T., Gilchrist A., Voyno-Yasenetskaya T., Radeff-Huang J. M., and Stern P. H. (2005) Gα12/Gα13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology 146, 2171–2175 [DOI] [PubMed] [Google Scholar]
- 31. Guo J., Liu M., Yang D., Bouxsein M. L., Thomas C. C., Schipani E., Bringhurst F. R., and Kronenberg H. M. (2010) Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology 151, 3502–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gesty-Palmer D., Chen M., Reiter E., Ahn S., Nelson C. D., Wang S., Eckhardt A. E., Cowan C. L., Spurney R. F., Luttrell L. M., and Lefkowitz R. J. (2006) Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 281, 10856–10864 [DOI] [PubMed] [Google Scholar]
- 33. Sinha P., Aarnisalo P., Chubb R., Ono N., Fulzele K., Selig M., Saeed H., Chen M., Weinstein L. S., Pajevic P. D., Kronenberg H. M., and Wu J. Y. (2014) Loss of Gsα early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J. Bone Miner. Res. 29, 2414–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J. Y., Purton L. E., Rodda S. J., Chen M., Weinstein L. S., McMahon A. P., Scadden D. T., and Kronenberg H. M. (2008) Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 105, 16976–16981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J. Y., Aarnisalo P., Bastepe M., Sinha P., Fulzele K., Selig M. K., Chen M., Poulton I. J., Purton L. E., Sims N. A., Weinstein L. S., and Kronenberg H. M. (2011) Gsα enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J. Clin. Investig. 121, 3492–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsiao E. C., Boudignon B. M., Halloran B. P., Nissenson R. A., and Conklin B. R. (2010) Gs G protein-coupled receptor signaling in osteoblasts elicits age-dependent effects on bone formation. J. Bone Miner. Res. 25, 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 38. Muzumdar M. D., Tasic B., Miyamichi K., Li L., and Luo L. (2007) A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 [DOI] [PubMed] [Google Scholar]
- 39. Guo J., Chung U. I., Kondo H., Bringhurst F. R., and Kronenberg H. M. (2002) The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Dev. Cell 3, 183–194 [DOI] [PubMed] [Google Scholar]
- 40. Dobreva G., Chahrour M., Dautzenberg M., Chirivella L., Kanzler B., Fariñas I., Karsenty G., and Grosschedl R. (2006) SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 125, 971–986 [DOI] [PubMed] [Google Scholar]
- 41. Bouxsein M. L., Boyd S. K., Christiansen B. A., Guldberg R. E., Jepsen K. J., and Müller R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 42. Sims N. A., Brennan K., Spaliviero J., Handelsman D. J., and Seibel M. J. (2006) Perinatal testosterone surge is required for normal adult bone size but not for normal bone remodeling. Am. J. Physiol. Endocrinol. Metab. 290, E456–E462 [DOI] [PubMed] [Google Scholar]
- 43. Bastepe M., Weinstein L. S., Ogata N., Kawaguchi H., Jüppner H., Kronenberg H. M., and Chung U. I. (2004) Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc. Natl. Acad. Sci. U.S.A. 101, 14794–14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenner L., Hoebertz A., Beil F. T., Keon N., Karreth F., Eferl R., Scheuch H., Szremska A., Amling M., Schorpp-Kistner M., Angel P., and Wagner E. F. (2004) Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J. Cell Biol. 164, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Semerad C. L., Christopher M. J., Liu F., Short B., Simmons P. J., Winkler I., Levesque J. P., Chappel J., Ross F. P., and Link D. C. (2005) G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 106, 3020–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., and Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 [DOI] [PubMed] [Google Scholar]
- 47. Yang D., Guo J., Divieti P., Shioda T., and Bringhurst F. R. (2008) CBP/p300-interacting protein CITED1 modulates parathyroid hormone regulation of osteoblastic differentiation. Endocrinology 149, 1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song L., Liu M., Ono N., Bringhurst F. R., Kronenberg H. M., and Guo J. (2012) Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J. Bone Miner. Res. 27, 2344–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zanotti S., and Canalis E. (2014) Notch1 and Notch2 expression in osteoblast precursors regulates femoral microarchitecture. Bone 62, 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong H. K., Chong J. L., Song W., Song E. J., Jyawook A. A., Schook A. C., Ko C. H., and Takahashi J. S. (2007) Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet. 3, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen J., Shi Y., Regan J., Karuppaiah K., Ornitz D. M., and Long F. (2014) Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One 9, e85161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davey R. A., Clarke M. V., Sastra S., Skinner J. P., Chiang C., Anderson P. H., and Zajac J. D. (2012) Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res. 21, 885–893 [DOI] [PubMed] [Google Scholar]
- 53. Ishizuya T., Yokose S., Hori M., Noda T., Suda T., Yoshiki S., and Yamaguchi A. (1997) Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J. Clin. Investig. 99, 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Partridge N. C., Kemp B. E., Livesey S. A., and Martin T. J. (1982) Activity ratio measurements reflect intracellular activation of adenosine 3′,5′-monophosphate-dependent protein kinase in osteoblasts. Endocrinology 111, 178–183 [DOI] [PubMed] [Google Scholar]
- 55. Ferrari S. L., Pierroz D. D., Glatt V., Goddard D. S., Bianchi E. N., Lin F. T., Manen D., and Bouxsein M. L. (2005) Bone response to intermittent parathyroid hormone is altered in mice null for β-arrestin2. Endocrinology 146, 1854–1862 [DOI] [PubMed] [Google Scholar]
- 56. Gesty-Palmer D., Flannery P., Yuan L., Corsino L., Spurney R., Lefkowitz R. J., and Luttrell L. M. (2009) A β-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci. Transl. Med. 1, 1ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]