Abstract
One of the mechanisms by which malignancies can induce immune suppression is through the production of cytokines that affect the maturation and differentiation of inflammatory cells in the tumor microenvironment. Semaphorin 4D (Sema4D) is a proangiogenic cytokine produced by several malignancies, which has been described in the regulation of the immune system. In the present study, we examined the role of human head and neck squamous cell carcinoma (HNSCC)–secreted Sema4D on myeloid cell differentiation. CD33+ cells cultured in HNSCC cell line–derived conditioned medium differentiated into myeloid derived suppressor cells (MDSC) (CD33+CD11b+HLA-DR−/low). The addition of anti-Sema4D Ab to HNSCC conditioned medium significantly reduced the expansion of the MDSC population. Similarly, knockdown of Sema4D in an HNSCC cell line resulted in a loss of MDSC function as shown by a decrease in the production of the immune-suppressive cytokines arginase-1, TGF-β, and IL-10 by MDSC, concomitant with recovery of T cell proliferation and IFN-γ production following stimulation of CD3/CD28. Importantly, CD33+ myeloid and T cells cultured in conditioned medium of HNSCC cells in which Sema4D was knocked down promoted antitumor inflammatory profile, through recovery of the effector T cells (CD4+T-bet+ and CD8+T-bet+), as well as a decrease in regulatory T cells (CD4+CD25+FOXP3+). We also showed that Sema4D was comparable to GM-CSF in its induction of MDSC. Collectively, this study describes a novel immunosuppressive role for Sema4D in HNSCC through induction of MDSC, and it highlights Sema4D as a therapeutic target for future studies to enhance the antitumorigenic inflammatory response in HNSCC and other epithelial malignancies.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a malignancy of high morbidity and mortality, with 45,780 new cases and 8,650 estimated deaths of oral and pharyngeal cancer estimated to occur in the United States in the year 2015 (1). There is accumulating evidence indicating the immunomodulatory effects of HNSCC by which it can escape and/or suppress the immune system (2–6).
Myeloid-derived suppressor cells (MDSC) have been described in peripheral blood, draining lymphoid tissue, and tumor tissue of several malignancies (5, 7–10). Circulating MDSC correlated with advanced stages of HNSCC (stages III and IV) as well as other carcinomas (8, 10, 11). MDSC represent a key player in immune regulation in the tumor microenvironment. It is generally agreed that they comprise a heterogeneous population of myeloid progenitor cells and immature myeloid cells that have a suppressive function on T cells (12, 13). MDSC described in human malignancies have the phenotype of CD33+, CD11b+, and non–lineage determined with poor Ag presentation abilities (HLA-DR−/low). They can have a progranulocytic phenotype expressing CD66b or CD15 (polymorphonuclear leukocyte–MDSC) or monocytic features expressing CD14 (10, 14, 15). MDSC induce their immune-suppressive effect mainly through production of arginase-1 and inducible NO synthase, which consume extracellular arginine and accordingly suppress T cell activation in an Ag-nonspecific manner in the tumor microenvironment. However, they mediate Ag-specific suppression by NADPH oxidase production of reactive oxygen and nitrogen species, particularly in peripheral lymphoid tissue, as well as by other mechanisms (12, 15–17). In addition to direct T cell suppression, recent evidence suggests a role for MDSC in the expansion of CD4+CD25+FOXP3+ regulatory T cells (Tregs) in the tumor microenvironment through both TGF-β–dependent and independent pathways (11, 18). Although several mechanisms have been described by which tumor cells induce MDSC, the specific pathways by which HNSCC recruit, expand, and activate MDSC remain to be investigated (15, 19, 20).
Tumor cells overexpress several cytokines to manipulate their own microenvironment, among which are multiple semaphorins, which have the potential to act on different stromal cells (18). Semaphorin 4D (Sema4D; CD100) is a transmembrane glycoprotein belonging to the fourth group of the semaphorin family that can also be found in a soluble form following proteolytic cleavage. It was initially identified as an evolutionarily conserved chemorepellent protein that regulates axonal guidance in the developing nervous system (21). Later on, its interactions in other systems were emphasized, including the cardiovascular system and immune system. In the immune system, Sema4D is described as being expressed abundantly on resting T cells and weakly on resting B cells and APCs (22–26). Two opposing roles of Sema4D have been described in the immune system. One role is a proinflammatory response where, for example, in the humoral and cell-mediated immune system, Sema4D acts on B cells and dendritic cells, respectively, promoting proinflammatory cytokines (25–27). Sema4D expressed by T cells and NK cells has also been implicated in their activation through a Sema4D-associated tyrosine kinase (28), and it has been shown to play a role in T cell priming and accordingly in the pathogenesis of autoimmune diseases (29). Alternatively, an anti-inflammatory role of Sema4D in the immune system has also been described. On monocytes and immature dendritic cells, Sema4D can act on plexin C1 and plexin B1, respectively, inhibiting their migration, but not that of mature dendritic cells, which can provide more interaction between immature myeloid cells and T cells (30, 31). Furthermore, in vitro studies have shown that Sema4D can modulate cytokine production by monocytes and dendritic cells, and it can induce a significant increase in the anti-inflammatory cytokine IL-10 and a decrease in the proinflammatory cytokines IL-6, IL-8, and TNF-α (26, 30, 31).
The role of Sema4D produced by tumor cells in inducing tumor angiogenesis, invasiveness, and progression has been demonstrated in several malignancies, both in human and animal models, and correlates with poor prognosis (32–38). Sema4D overexpression has been described in a large number of human HNSCC tumor tissue samples and in a panel of primary and metastatic cell lines (32). In the tumor microenvironment, tumor-associated macrophages were reported to be the main producers of Sema4D in a mouse breast cancer model (39). Taken together, the hypothesis tested in the present study was that the net inflammatory profile orchestrated by Sema4D in the tumor microenvironment is protumorigenic through induction of immune-suppressive cells.
In this study, we investigated whether HNSCC-derived Sema4D plays a role in inducing MDSC and examined its effect on T cell phenotype and function. We found that Sema4D produced by the HNSCC HN6 and HN13 cell lines polarized myeloid cells into an MDSC phenotype, which corresponded with a reduction in T cell proliferation and IFN-γ production. Furthermore, short hairpin RNA (shRNA) inhibition of Sema4D in HN6 resulted in a decrease in the production of the immunosuppressive factors arginase-1, TGF-β, and IL-10 by myeloid cells and the recovery of autologous T cell proliferation, as well as IFN-γ production, specifically leading to an increase in the effector T cell population and decrease in Tregs. Our findings describe HNSCC-associated Sema4D production as one of the mechanisms by which the tumors induce MDSC, which subsequently mediate their immunosuppression effects on T cells.
Materials and Methods
Cell lines and tissue culture
Human normal oral keratinocytes (NOK) and human-derived HNSCC cell lines WSU-HN6 and WSU-HN13 underwent DNA authentication (Johns Hopkins Genetic Resources Core Facility, Baltimore, MD) to ensure consistency in cell identity in comparison with their source (40). SCC-9 was obtained from American Type Culture Collection (Manassas, VA). The WSU-HN4 cell line was a gift from Dr. Silvio Gutkind (National Institute of Dental and Craniofacial Research, Bethesda, MD) (40). All HNSCC cell lines used were derived from primary carcinomas of the tongue. All cell lines were grown and maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B (Sigma-Aldrich, St. Louis, MO) at 37°C in humidified air with 5% CO2. For treatment with conditioned medium, culture medium was collected from confluent HNSCC cells grown in 5 ml DMEM for 24 h. For myeloid and T cell coculture experiments, conditioned medium supplemented with 5% FBS was used.
Separation of myeloid and T cells from human peripheral blood
Mononuclear cell suspension was prepared from peripheral blood of healthy donors obtained from commercial vendors, that is, Biological Specialty (Colmar, PA) or New York Blood Center (New York, NY), using Ficoll-Paque Plus density gradient centrifugation (GE Healthcare Life Sciences, Pittsburgh, PA). CD33+ myeloid cells were separated from the PBMC using MACS CD33 microbeads (catalog no.130-045-501), and HLA-DR+ cells were separated using anti-HLA-DR microbeads (catalog no.130-046-100; Miltenyi Biotec, Auburn, CA). Autologous T cells were enriched by negative selection with the human pan T cell isolation kit (catalog no. 19051; Stemcell Technologies, Vancouver, BC, Canada).
Flow cytometry characterization of MDSC and T cells
MDSC were characterized using multicolor staining fluorochrome-labeled anti-human CD33-PerCP/Cy5.5 (catalog no. 303414), HLA-DR-allophycocyanin (catalog no. 307610), CD11b-PE (catalog no.301306), and CD14-FITC (catalog no. 325604) (BioLegend, San Diego, CA). For T cell analysis, CD3-allophycocyanin/Cy7 (catalog no. 300317) (BioLegend), CD8a-PE-Cy7 (catalog no. 25-0088-42), CD25-allophycocyanin (catalog no. 17-0259-42), FOXP3-PE (catalog no. 12-4776-42), and T-bet–PerCP-Cy5.5 (catalog no. 45-5825-82) (eBioscience, San Diego, CA) were used. For anti–FOXP3-PE and anti–T-bet-PerCP/Cy5.5, fixation and permeabilization buffer (catalog no. 00-8333) eBioscience was used. For T cell proliferation, T cells were stained with CFSE (catalog no. C34554) (Life Technologies, Grand Island, NY), prior to plating. Dynabeads human T-activator CD3/CD28 (Life Technologies) were used at a 1:3 (T cell/bead) ratio after coculture with myeloid cells for at least 4 h. Data were acquired using a BD LSR II flow cytometer, FlowJo software (Tree Star, Ashland, OR), and analyzed using BD FACSDiva software (BD Biosciences, San Jose, CA).
Immunoblot, Abs, and reagents
Cells were harvested in cell lysis buffer (20mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) (catalog no. 9803) (Cell Signaling Technology, Danvers, MA) with the addition of 1× protease inhibitor cOmplete, Mini, EDTA-free (catalog no. 11836170001) (Roche Diagnostics, Indianapolis, IN). Whole-cell lysate was separated using SDS-PAGE. The primary Abs Sema4D (30/CD100, catalog no. 610670) (BD Biosciences/Pharmingen, San Diego, CA), arginase-1 (A-2; sc-365547), GAPDH (8C2; sc-81545), and β-actin (catalog no. sc-2301) (Santa Cruz Biotechnology, Dallas, TX) were used. The secondary Abs used were anti-rabbit IgG (catalog no. sc-2301) and anti-mouse IgG (catalog no. sc-2302) (Santa Cruz Biotechnology). For arginase-1 detection, 15 μg protein was loaded, whereas for Sema4D detection, 70–100 μg was loaded. For detection of Sema4D in the tumor conditioned medium, equal aliquots of the medium or of a concentrate of the medium using Millipore Amicon Ultra centrifugal filter units were loaded after adding the sample buffer. Cell viability was measured using the cell proliferation reagent WST-1 (Roche Diagnostics). The plate was read at 450 nm on a BioTek Epoch microplate spectrophotometer (BioTek, Winooski, VT). The recombinant human Sema4D (sCD100) (catalog no. 310-29) was obtained from (PeproTech, Rocky Hill, NJ). Recombinant human GM-CSF (catalog no. 215-GM-010) (R&D Systems Minneapolis, MN) and IL-6 recombinant human (catalog no. I1395) from (Sigma-Aldrich) were also used. Griess reagent (catalog no. G2930) (Promega, Madison, WI) was used to measure the nitrite concentration as a stable nonvolatile byproduct of NO in the media, read at absorbance of 520 nm using a BioTek Epoch microplate spectrophotometer.
Anti-Sema4D treatment and immune depletion
For target protein inhibition in the conditioned medium, anti-Sema4D (catalog no.610670) (BD Biosciences) was used at a concentration of 10 μg/ml. For immune depletion, immunoprecipitation of Sema4D or GM-CSF was carried out by overnight incubation of the conditioned media with Sema4D (catalog no. ab39710) (Abcam, Cambridge, MA) or GM-CSF (clone BVD2-21C11; catalog no.502301) (BioLegend; 10 μg/ml) mAbs using protein A beads, followed by precipitation.
Sema4D shRNA and lentivirus infections
The lentivirus and shRNA system were a gift of Dr. John R. Basile (University of Maryland, Baltimore) (32). In brief, the shRNA sequences for human Sema4D were obtained from Cold Spring Harbor Laboratory’s RNAi library (RNAi Codex; http:katahdin.cshl.org:9331_homepage_portal_scripts_main2.pl). The oligonucleotides (Invitrogen, Grand Island, NY) used to knockdown Sema4D protein levels were 5′-GGCCTGAGGACCTTGCAGAAGA-3′. The Sema4D shRNA oligonucleotides were cloned into lentiviral expression vector pWPI GW as previously described (32). pWPI (empty) vector was used as negative control. Infections were performed using FuGENE HD transfection reagent (catalog no. E2311; Promega).
ELISA
Human ELISA MAX IFN-γ (catalog no. 430103), TGF-β1 total (catalog no. 436707), IL-10 (catalog no. 430601), and IL-4 (catalog no. 430301) were obtained from BioLegend. Samples were run in triplicates and read using a BioTek Epoch microplate spectrophotometer at 450 nm wavelength.
Statistical analyis
Student paired t tests were performed as appropriate. All data analysis was presented with custom SD. The p values are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Results
Sema4D expressed by HNSCC cell lines induces CD33+, CD11b+, HLA-DR−/low cells
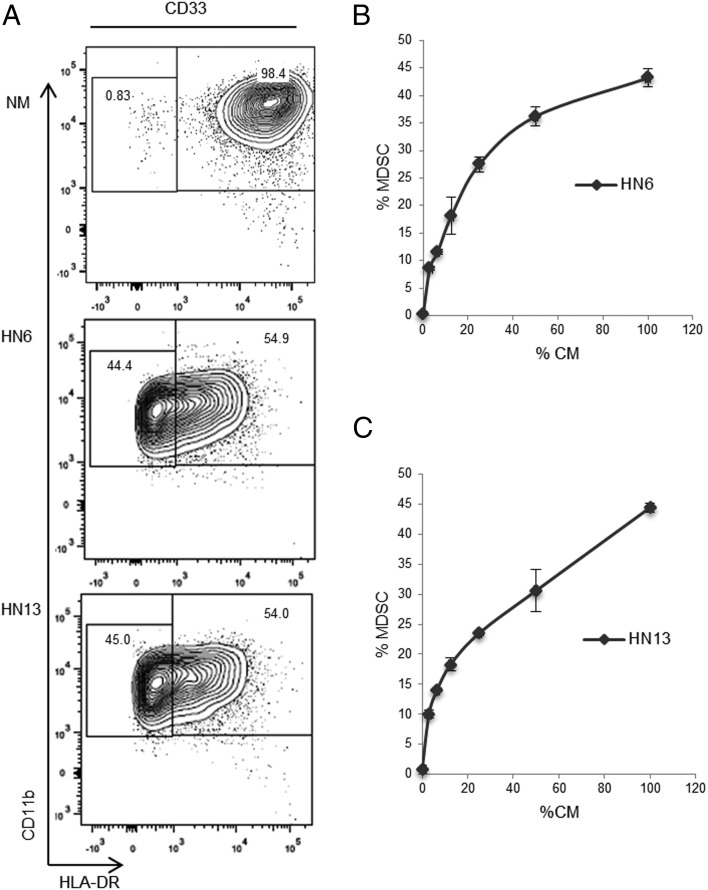
To examine the effects of HNSCC-derived condition medium on myeloid differentiation, total human PBMC or total myeloid cells (CD33+) isolated from PBMC were plated in conditioned medium from HNSCC cell lines HN4, HN6, HN13, and SCC-9. Total PBMC or separated myeloid cells cultured in HNSCC conditioned medium displayed markers characteristic of MDSC, namely CD33+CD11b+HLA-DR−/low compared with cells cultured in normal medium (NM) or NOK conditioned medium. The percentage of MDSC developed from total PBMC was ∼20% (Supplemental Fig. 1A), whereas that generated from CD33+ myeloid cells averaged 30% depending on the HNSCC cell line and density used (Fig. 1A, Supplemental Fig. 1B). Moreover, titration of HN6- and HN13-derived conditioned medium with NM corresponded with a dose-dependent decrease in the MDSC population (Fig. 1B, 1C).
FIGURE 1.
HNSCC conditioned media polarizes myeloid cells toward an MDSC phenotype. (A) CD33+ cells were cultured in NM or HNSCC conditioned media (HN6, HN13) for 72 h and then analyzed by flow cytometry. (B) Dose-dependent induction of MDSC following culture in HNSCC conditioned media. CD33+ cells were cultured in serial dilutions of HN6 conditioned medium (CM) and (C) HN13 CM. Cells were first gated for CD33+ then for CD11b+, followed by a third gate for CD11b+HLA-DR−/low. CD33 cells were isolated using MACS from PBMC separated by centrifugation gradient of peripheral blood. All samples were run in duplicates. CM, conditioned medium.
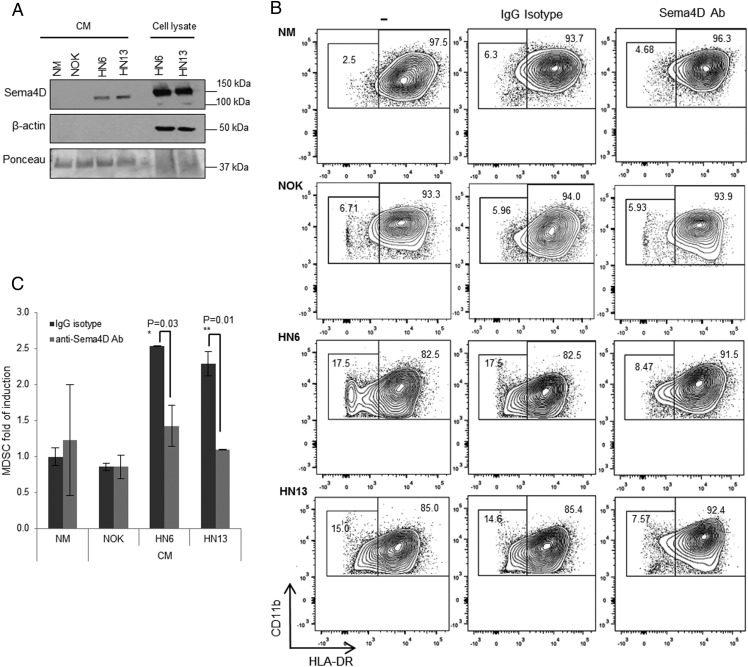
Several groups have recently described an immunomodulatory role for tumor-produced proangiogenic factors (41). Given the immune modulatory role previously described for Sema4D in the immune system (27, 30), we sought to investigate whether the proangiogenic factor Sema4D, secreted by HNSCC (32), plays a role in the induction of CD33+CD11b+HLA-DR−/low myeloid cells. Immunoblotting was performed on conditioned medium from HN6 and HN13 HNSCC cell lines, and their cellular lysates served as controls. We detected the cleaved form of Sema4D in the medium at ∼120 kDa (Fig. 2A) as previously reported (42). We then neutralized Sema4D in the HN4-, HN6-, HN13-, and SCC-9 conditioned medium using anti-Sema4D Ab (clone 30/CD100) and then used the medium to culture myeloid cells. Blocking Sema4D in HNSCC conditioned medium resulted in an ∼50% reduction in the MDSC population compared with cells growing in HNSCC conditioned medium pretreated with the isotype control mAb (Fig. 2B, 2C, Supplemental Fig. 1B).
FIGURE 2.
Sema4D produced by HNSCC plays a role in MDSC induction. (A) Sema4D is secreted by HN6 and HN13 cell lines. Conditioned medium (CM) and total cell lysates from HN6 and HN13 HNSCC cell lines were analyzed by Western blot. Ponceau staining was used as a control for protein loading prior to immunoblotting. β-Actin served as control for cell lysates. (B) Anti-Sema4D Ab treatment downregulates MDSC induction by HNSCC cells. CD33+ cells were cultured in NM, NOK, HN6, and HN13 CM in the presence or absence of anti-Sema4D mAb (10 μg/ml) or isotype control mAb for 72 h. Cells were analyzed by flow cytometry by gating on CD33+CD11b+, then analyzed for CD11b+HLA-DR−/low. The experiment was independently repeated twice. (C) Graphical presentation of two combined experiments showing MDSC reduction (CD33+CD11b+HLA-DR−/low) upon anti-Sema4D Ab treatment of HN6 and HN13 CM. Data were normalized to MDSC in NM treated with IgG isotype. CM, conditioned medium.
Sema4D inhibition rescues MDSC-induced T cell suppression
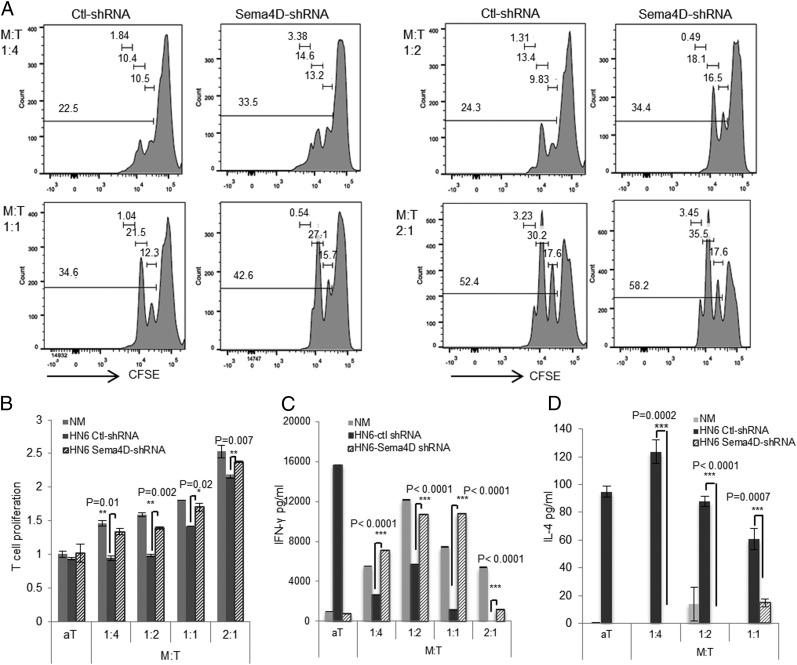
We next sought to ascertain whether HNSCC-derived Sema4D mediated its suppressive effects when myeloid cells were cultured with primary T cells. The induction of HLA-DR−/low cells by HN6 conditioned media was still observed upon coculture of the CD33+ cells with autologous T cells (Supplemental Fig. 2A). To determine whether Sema4D induction of MDSC induces T cell suppression, we constructed Sema4D-shRNA to knock down Sema4D in HN6 cells and accordingly in the conditioned medium (Supplemental Fig. 2B, 2C). Significant recovery of T cell proliferation was observed upon coculture of myeloid and T cells in the HN6 Sema4D-shRNA compared with the control (Ctl)-shRNA medium (Fig. 3A, 3B). T cells grown in HN6 Ctl-shRNA medium were suppressed upon coculture with the myeloid cells, but not when cultured in NM, indicating that the suppression is mediated by the induction of MDSC (Fig. 3B). Notably, the knockdown of Sema4D produced by HN6 resulted in significant recovery of the T cells at different myeloid/T cell ratios (Fig. 3A, 3B). This was paralleled with recovery of IFN-γ production (Fig. 3C) as well as inhibition of IL-4 in the supernatant (Fig. 3D).
FIGURE 3.
Inhibition of Sema4D produced by HN6 rescues MDSC-mediated T cell suppression. (A) Recovery of T cell proliferation following inhibition of Sema4D in HN6 cell line using lentivirus shRNA. T cells stained with CFSE and myeloid cells were added at the indicated ratios (myeloid/T cell [M:T]). Cells were cocultured in conditioned medium (CM) from HN6 Sema4D-shRNA or HN6 Ctl-shRNA and activated by anti-CD3/CD28 microbeads for 72 h. CFSE dilution in T cells was analyzed by FACS. (B) Graphical representation of flow cytometry data shown in (A). Data were normalized to activated T cells cultured alone in NM. The supernatants were collected to assess IFN-γ (C) and IL-4 (D) production using ELISA. Error bars indicate SD of triplicate assays.
HN6-secreted Sema4D induces an immunosuppressive T cell phenotype
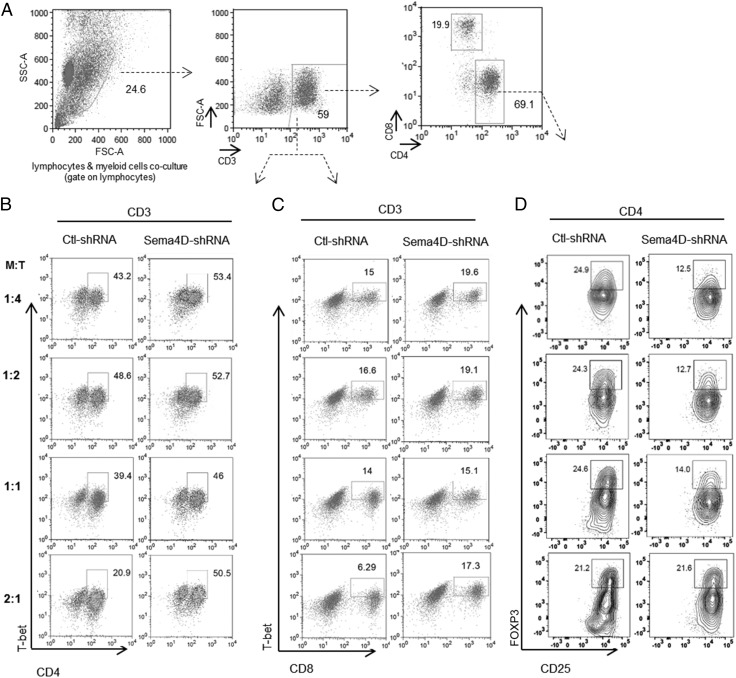
To investigate which T cell subset is rescued following blockade of Sema4D, a phenotypic analysis of the T cells was performed. We found that inhibition of Sema4D restored effector Th1 cells (CD4+T-bet+) and cytotoxic T cells (CD8+T-bet+) and significantly decreased Tregs (CD4+CD25+FOXP3+) at different myeloid/T cell ratios (Fig. 4, Supplemental Fig. 2D–F). These findings indicate that inhibition of Sema4D can rescue effector T cells while suppressing the Treg population in the presence of myeloid cells.
FIGURE 4.
Sema4D produced by HN6 induces an immune-suppressive T cell phenotype. (A) T cells were cocultured with myeloid cells at the indicated ratios, followed by CD3/CD28 activation for 72 h. Then cells were analyzed by flow cytometry. (A) The cells were gated on the T cell subpopulation, followed by a second gate on CD3+ cells. CD3+ cells were then gated on (B) CD4+T-bet+ and (C) CD8+T-bet+. The CD4+ population was gated on CD25+FOXP3+. Representative flow cytometry plots displaying percentage of recovery of (B) CD3+CD4+T-bet+ effector cells, (C) CD3+CD8+T-bet+ effector cells, and (D) decrease in CD4+CD25+FOXP3+ Tregs in HN6 conditioned medium (CM) of Sema4D-shRNA versus Ctl-shRNA.
Sema4D enhances GM-CSF plus IL-6–mediated induction of MDSC
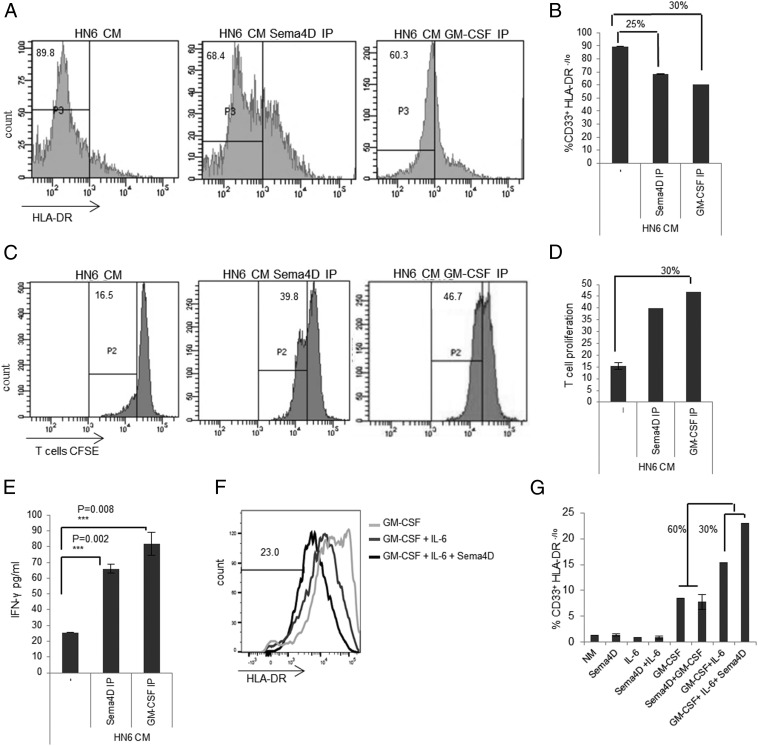
To better assess the role of HNSCC-derived Sema4D on myeloid differentiation, we compared Sema4D with another well-described MDSC inducer, GM-CSF (14). GM-CSF has been shown to be produced by several MDSC-inducing human solid tumors, including HNSCC, and to best induce human CD33+ suppressive cells upon combination with IL-6 (14, 16, 19, 20). In fact, we tested the HN6 HNSCC cell line using ELISA and it showed production of both GM-CSF and IL-6 (data not shown). To compare the effect of tumor-secreted Sema4D on the induction of MDSC to GM-CSF–mediated induction, CD33+ cells were cocultured with T cells in HN6 medium with immune depletion of Sema4D versus immune depletion of GM-CSF. Immune depletion of Sema4D resulted in a significant reduction in the MDSC population that was comparable to that observed upon depletion of GM-CSF in HN6 conditioned medium (Fig. 5A, 5B). The reduction in MDSC corresponded with a significant increase in T cell proliferation (Fig. 5C, 5D) and IFN-γ production (Fig. 5E). These data strongly support a role for Sema4D produced by HN6 cells in the induction of MDSC and its subsequent T cell suppression. To further study the role of Sema4D in conjunction with other tumor-produced cytokines in MDSC induction, we grew CD33+ cells in NM to which the three recombinant proteins (Sema4D, IL-6, and GM-CSF) were added. The three-cytokine induction mixture of Sema4D plus GM-CSF plus IL-6 resulted in a 30% increase in MDSC induction compared with the GM-CSF plus IL-6 mixture. This indicated that Sema4D acts synergistically with GM-CSF plus IL-6 to increase the repertoire of the MDSC in the HNSCC tumor microenvironment (Fig. 5F, 5G).
FIGURE 5.
Sema4D enhances GM-CSF plus IL-6–mediated induction of MDSC. (A) The percentage of CD33+HLA-DR−/low MDSC induced by culture in HN6 conditioned medium (CM) is reduced following Sema4D or GM-CSF immunoprecipitation (IP) HN6 CM. (B) Graph shows comparable decrease in the CD33+HLA-DR−/low population following immune depletion of Sema4D or GM-CSF, compared with the control HN6 CM. (C) HN6 CM was depleted of Sema4D or GM-CSF by IP and then used to culture myeloid cells and T cells, followed by anti-CD3/CD28 stimulation. After 72 h, the cells were analyzed by flow cytometry analysis, and the supernatants were collected for ELISA. CFSE dilution in T cells was analyzed by flow cytometry. (D) Graph shows comparable recovery of T cell proliferation upon immune depletion of Sema4D or GM-CSF in HN6 CM. (E) IFN-γ production following immune depletion of Sema4D is comparable to GM-CSF–depleted CM. (F) Sema4D is synergistic to GM-CSF plus IL-6 in MDSC induction. CD33+ cells separated from PBMC of normal donors were cultured in NM with the recombinant protein GM-CSF alone, GM-CSF plus IL-6, or the cytokine induction mixture of GM-CSF plus IL-6 plus Sema4D. (G) CD33+HLA-DR−/low induction in response to treatment with Sema4D, IL-6, and GM-CSF alone or in combination. For (F) and (G), each of the recombinant proteins was used at a concentration of 10 ng/ml. CM, conditioned medium; IP, immunoprecipitation.
Sema4D promotes the production of immunosuppressive mediators by MDSC
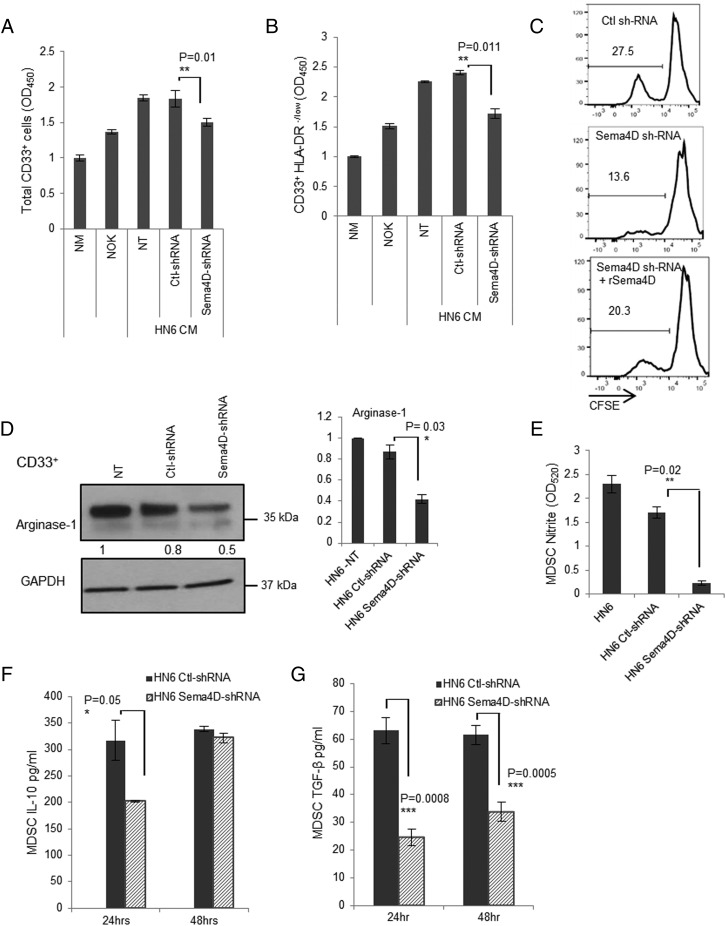
To investigate whether HNSCC-associated Sema4D has an effect on MDSC viability, a WST cell proliferation assay and a CFSE proliferation tracking experiment were performed using CD33+ cells grown in conditioned medium from the HN6 cells in which Sema4D has been knocked down using shRNA. The loss of Sema4D resulted in a significant decrease in the proliferation of total CD33+ cells, as well as that of the CD33+ HLA-DR−/low population compared with conditioned medium from controls (Fig. 6A, 6B). Interestingly, reconstitution of the Sema4D-shRNA HN6 conditioned media with human recombinant Sema4D protein recovered the CD33+HLA-DR−/low proliferation as shown by CFSE analysis (Fig. 6C). Taken together, these findings showed that Sema4D produced by HNSCC plays a significant role in the proliferation and viability of MDSC.
FIGURE 6.
HN6-secreted Sema4D induces production of immune-suppressive mediators by MDSC. (A) Sema4D promotes proliferation of total myeloid cells. Bulk CD33+ cells cultured in HN6 control or Sema4D-shRNA conditioned medium (CM) for 24 h were analyzed using the WST-1 proliferation assay. (B) Sema4D promotes proliferation of CD33+HLA-DR−/low cells. CD33+HLA-DR−/low cells were sorted using magnetic beads and analyzed by WST-1 proliferation assay. All readings were normalized to cells growing in NM. (C) Reconstitution of HN6 Sema4D-shRNA CM with human recombinant Sema4D rescues CD33+HLA-DR−/low MDSC proliferation. CD33+HLA-DR−/low cells labeled with CFSE were analyzed by FACS. (D) Sema4D inhibition in HN6 decreases arginase-1 expression in myeloid cells. CD33+ cells were cultured in nontransfected (NT), Ctl-shRNA, or Sema4D-shRNA HN6 CM for 24 h and then analyzed by Western blot for arginase-1 expression. GAPDH served as a loading control. Densitometric analyses included for Western blot data were determined using ImageJ. (E) Sema4D inhibition in HN6 decreases NO production by myeloid cells. HN6 Sema4D-shRNA CM inhibits nitrite production by myeloid cells. Total CD33+ cells were cultured in CM from HN6 Ctl-shRNA or HN6 Sema4D-shRNA for 72 h, and then the media were collected to detect the nitrite concentration using Griess reagent. (F) CD33+ cells were cultured in HN6 Sema4D-shRNA or Ctl-shRNA CM, and cytokine production was assessed by ELISA. Knockdown of Sema4D results in a decrease in IL-10 production by CD33+ cells. (G) A reduction in Sema4D results in a concomitant decrease in TGF-β production by CD33+ cells. Data are shown with background levels of cytokine subtracted to demonstrate myeloid cell–specific cytokine production. CM, conditioned medium; NT, nontransfected.
The myeloid cells mediate their suppressive effects by various mechanisms. Among these, arginase-1 and NO play a major role in the MDSC suppressive effect. To investigate whether Sema4D produced by HNSCC-HN6 can affect arginase-1 production, CD33+-enriched myeloid cells were grown in conditioned media from Sema4D-shRNA HN6 and then checked for arginase-1 expression. A significant reduction in arginase-1 production by the CD33+ cells was observed when grown in Sema4D-shRNA HN6 conditioned media (Fig. 6D). To check for the effect of Sema4D knockdown on NO production by myeloid cells, CD33+ cells were grown in HN6 Sema4D-shRNA– versus Ctl-shRNA conditioned media, and then the media were collected after 72 h to check for nitrite concentration. Interestingly, there was a significant reduction in nitrite concentration produced by the myeloid cells in the media upon Sema4D knockdown in HN6 (Fig. 6E).
MDSC can also indirectly induce a suppressive effect through production of other immune-suppressive cytokines such as IL-10 and expansion of Tregs in a TGF-β–dependent pathway (11, 18). These cytokines were assessed by ELISA, specifically to measure IL-10 and TGF-β produced by MDSC (43). Interestingly, there was a significant reduction in both IL-10 and TGF-β production by CD33+ cells upon inhibition of Sema4D in HN6 (Fig. 6F, 6G).
Discussion
Understanding the specific mechanisms underlying tumor-mediated immunosuppression is important for the development of effective antitumor immunotherapy (44). Myeloid progenitor cells have been shown to mediate T cell suppression in patients with HNSCC (8, 45, 46), and even inducing differentiation of these immature myeloid cells can indirectly activate tumor-infiltrating T cells (47). The link between angiogenesis and immune suppression has been emphasized recently to play a crucial role in tumor development, progression, and metastases. In this study, we investigated Sema4D, a cytokine expressed by several epithelial malignancies and known to induce tumor angiogenesis, for its role in induction of MDSC, in the tumor microenvironment of HNSCC (24, 32, 33). We show Sema4D produced by HNSCC-induced MDSC and its immune-suppressive cytokines, with subsequent suppression of T cell proliferation and IFN-γ production. These findings are supported by others, who reported that Sema4D acts on immature myeloid cells and dendritic cells by inhibiting their migration and inducing significant increases in the immune-suppressive profile (26, 30, 31). They are also in good agreement with a recent report showing that Sema4D modulates the inflammatory cytokine milieu in a murine colon and mammary carcinoma model (48).
Several clinical trials showed that advanced cancers can be controlled by immunotherapy. However, these therapies are often compromised by the immune-suppressive tumor microenvironment (49, 50). Interestingly, the composition of T cells reported in human tumor-infiltrating lymphocytes in a cohort of colorectal cancer liver metastases and ovarian cancer indicated that whereas the CD8+ T cells showed signs of proliferation and activation, still there was no tumor rejection (50). This was attributed to several factors, including, but not limited to, presence of MDSC and Tregs. In this context, our data show promising results, in which inhibition of Sema4D in HN6 cells cocultured at several ratios of myeloid/T cells resulted in enhanced IFN-γ production (Fig. 3C) and effector CD4 and CD8 T cells (Fig. 4B, 4C), concomitant with a significant decrease in both Tregs (Fig. 4D) and MDSC (Fig. 5A).
Our data indicate that inhibition of Sema4D produced by the tumor cells may recover T cell activation to Ag-nonspecific stimuli and avoid T cell anergy. This would be of importance in adoptive immunotherapy in which the transferred activated T cells are Ag specific, yet the presence of MDSC in the tumor microenvironment might induce anergy (51). This is further supported by others showing that although Sema4D knockout mice display severe impairments in the Ag priming of T cells, interestingly, Sema4D-deficient T cells still retained the ability to respond normally to either mitogens or anti-CD3 stimulation, and, more importantly, the development of dendritic cells is not defective in cells lacking Sema4D (25, 52).
Intriguingly, the opposing cellular responses played by Sema4D in the immune system (53), either as an anti-inflammatory or a proinflammatory cytokine, seem to be dependent on several factors, including, but not restricted to, its concentration, the specific cell type present in the microenvironment, and its stage of maturation upon which Sema4D acts (27, 30, 37, 54). Also, note that in the studies showing the immunosuppressive effect and inhibition of myeloid cell migration, Sema4D recombinant protein was effective at <10 ng/ml and ranged up to 80 ng/ml (30, 31), and the concentrations of Sema4D used to show its proangiogenic effect in tumor microenvironment were in the range of 400 ng/ml (55). In contrast, concentrations used to show increased activation of the immune system, induction of proinflammatory cytokines, and maturation and activation of dendritic cells were in the micromolar range of soluble Sema4D (4–20 μg/ml), suggesting that the Sema4D mechanism of action postulated in other pathological conditions, such as autoimmune-mediated diseases (52, 56), may differ from those in the tumor microenvironment, depending on its concentration and presence of tumor-educated cells in the microenvironment.
There is evidence for the involvement of multiple pathways by which malignancies induce MDSC: several cytokines, including IL-6, cyclooxygenase 2–generated PGE2, GM-CSF, vascular endothelial growth factor, IL-10, matrix metalloproteinases, and others (7, 12, 57–59), acting through several cellular receptors on myeloid cells and stimulating transcription factors that involve STAT3, STAT6, STAT1, NF-kβ, HIF1-α, C/EBPβ have been described. Our data do provide evidence that Sema4D acts synergistically with GM-CSF and IL-6 (Fig. 5F, 5G) to increase the repertoire of MDSC in the tumor microenvironment and accordingly the downstream effectors arginase-1, NO, TGF-β, and IL-10. We also did detect Sema4D receptors CD72 and plexin-B1 on myeloid cells treated with recombinant Sema4D, as well as plexin-C1, a Sema7A receptor (unpublished observations); however, we are currently investigating the exact receptor and underlying pathway by which Sema4D may activate downstream transcription factors and induce the effector cytokines in MDSC (18, 60–62).
MDSC have proangiogenic activities that can mediate refractoriness to antiangiogenic therapies (49, 63, 64). Our data suggest an important role for Sema4D in the continuous positive feedback mechanism between MDSC induction and angiogenesis that is to an extent comparable to GM-CSF and reminiscent of the angiogenic role ascribed to vascular endothelial growth factor in the tumor microenvironment (41). Of particular note is the ability of MDSC to differentiate further into the immune-suppressive M2 macrophage, which has also been reported to produce Sema4D (39). This has significant implications for tumor resistance to antiangiogenic and immune therapy (41, 63–66) and indicates that Sema4D inhibition might be required to disrupt this positive feedback loop between angiogenesis and immune suppression.
MDSC represent a cell population that contributes to tumor immune suppression. It responds to a variety of proinflammatory and anti-inflammatory stimuli, which drive their recruitment and activation. Understanding how the inflammatory milieu favors immune escape through the accumulation of MDSC could be very useful to improve the efficacy of cancer immunotherapy (67). In addition to the well-described proangiogenic role of Sema4D in the tumor microenvironment, in the present study we describe, to our knowledge for the first time, its immunosuppressive effect through the induction of MDSC. This novel role described for Sema4D may open new avenues for understanding and reversing tumor-associated immunosuppressive mechanisms, with the ultimate goal of increasing T cell activation and enhancing antitumorigenic immune responses in HNSCC, either as a component of immune-based therapy or as a complement to conventional treatment regimens.
Supplementary Material
Acknowledgments
We acknowledge Dr. John R. Basile, Department of Oncology and Diagnostic Sciences, School of Dentistry (University of Maryland, Baltimore), for providing the lentivirus shRNA system for Sema4D knockdown.
This work was supported by School of Dentistry, University of Maryland, Baltimore Grant 00130654 (to R.H.Y.).
The online version of this article contains supplemental material.
- Ctl
- control
- HNSCC
- head and neck squamous cell carcinoma
- MDSC
- myeloid-derived suppressor cell
- NM
- normal medium
- NOK
- normal oral keratinocyte
- Sema4D
- semaphorin 4D
- shRNA
- short hairpin RNA
- Treg
- regulatory T cell.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Siegel R. L., Miller K. D., Jemal A. 2015. Cancer statistics, 2015. CA Cancer J. Clin. 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Hadden J. W., Endicott J., Baekey P., Skipper P., Hadden E. M. 1994. Interleukins and contrasuppression induce immune regression of head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 120: 395–403. [DOI] [PubMed] [Google Scholar]
- 3.Katz A. E. 1993. Update on immunology of head and neck cancer. Med. Clin. North Am. 77: 625–631. [DOI] [PubMed] [Google Scholar]
- 4.Vlock D. R. 1991. Immunobiologic aspects of head and neck cancer. Clinical and laboratory correlates. Hematol. Oncol. Clin. North Am. 5: 797–820. [PubMed] [Google Scholar]
- 5.Russell S. M., Lechner M. G., Gong L., Megiel C., Liebertz D. J., Masood R., Correa A. J., Han J., Puri R. K., Sinha U. K., Epstein A. L. 2011. USC-HN2, a new model cell line for recurrent oral cavity squamous cell carcinoma with immunosuppressive characteristics. Oral Oncol. 47: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gildener-Leapman N., Ferris R. L., Bauman J. E. 2013. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol. 49: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechner M. G., Megiel C., Russell S. M., Bingham B., Arger N., Woo T., Epstein A. L. 2011. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J. Transl. Med. 9: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasquez-Dunddel D., Pan F., Zeng Q., Gorbounov M., Albesiano E., Fu J., Blosser R. L., Tam A. J., Bruno T., Zhang H., et al. 2013. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Invest. 123: 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabitass R. F., Annels N. E., Stocken D. D., Pandha H. A., Middleton G. W. 2011. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 60: 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipazzi P., Huber V., Rivoltini L. 2012. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol. Immunother. 61: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuk-Pavlović S., Bulur P. A., Lin Y., Qin R., Szumlanski C. L., Zhao X., Dietz A. B. 2010. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate 70: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich D. I., Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talmadge J. E., Gabrilovich D. I. 2013. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13: 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechner M. G., Liebertz D. J., Epstein A. L. 2010. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185: 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V. 2012. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12: 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condamine T., Gabrilovich D. I. 2011. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corzo C. A., Condamine T., Lu L., Cotter M. J., Youn J. I., Cheng P., Cho H. I., Celis E., Quiceno D. G., Padhya T., et al. 2010. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207: 2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capparuccia L., Tamagnone L. 2009. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment—two sides of a coin. J. Cell Sci. 122: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 19.Pak A. S., Wright M. A., Matthews J. P., Collins S. L., Petruzzelli G. J., Young M. R. 1995. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34+ cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1: 95–103. [PubMed] [Google Scholar]
- 20.Young M. R., Wright M. A., Lozano Y., Prechel M. M., Benefield J., Leonetti J. P., Collins S. L., Petruzzelli G. J. 1997. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int. J. Cancer 74: 69–74. [DOI] [PubMed] [Google Scholar]
- 21.Kolodkin A. L., Matthes D. J., Goodman C. S. 1993. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75: 1389–1399. [DOI] [PubMed] [Google Scholar]
- 22.Delaire S., Elhabazi A., Bensussan A., Boumsell L. 1998. CD100 is a leukocyte semaphorin. Cell. Mol. Life Sci. 54: 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth L., Koncina E., Satkauskas S., Crémel G., Aunis D., Bagnard D. 2009. The many faces of semaphorins: from development to pathology. Cell. Mol. Life Sci. 66: 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bismuth G., Boumsell L. 2002. Controlling the immune system through semaphorins. Sci. STKE 2002: re4. [DOI] [PubMed] [Google Scholar]
- 25.Shi W., Kumanogoh A., Watanabe C., Uchida J., Wang X., Yasui T., Yukawa K., Ikawa M., Okabe M., Parnes J. R., et al. 2000. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity 13: 633–642. [DOI] [PubMed] [Google Scholar]
- 26.Tamagnone L., Comoglio P. M. 2004. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 5: 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida I., Kumanogoh A., Suzuki K., Akahani S., Noda K., Kikutani H. 2003. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int. Immunol. 15: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 28.Elhabazi A., Lang V., Hérold C., Freeman G. J., Bensussan A., Boumsell L., Bismuth G. 1997. The human semaphorin-like leukocyte cell surface molecule CD100 associates with a serine kinase activity. J. Biol. Chem. 272: 23515–23520. [DOI] [PubMed] [Google Scholar]
- 29.Okuno T., Nakatsuji Y., Moriya M., Takamatsu H., Nojima S., Takegahara N., Toyofuku T., Nakagawa Y., Kang S., Friedel R. H., et al. 2010. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 184: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 30.Chabbert-de Ponnat I., Marie-Cardine A., Pasterkamp R. J., Schiavon V., Tamagnone L., Thomasset N., Bensussan A., Boumsell L. 2005. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int. Immunol. 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 31.Delaire S., Billard C., Tordjman R., Chédotal A., Elhabazi A., Bensussan A., Boumsell L. 2001. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J. Immunol. 166: 4348–4354. [DOI] [PubMed] [Google Scholar]
- 32.Basile J. R., Castilho R. M., Williams V. P., Gutkind J. S. 2006. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc. Natl. Acad. Sci. USA 103: 9017–9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H., Yang Y. H., Binmadi N. O., Proia P., Basile J. R. 2012. The hypoxia-inducible factor-responsive proteins semaphorin 4D and vascular endothelial growth factor promote tumor growth and angiogenesis in oral squamous cell carcinoma. Exp. Cell Res. 318: 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H., Binmadi N. O., Yang Y. H., Proia P., Basile J. R. 2012. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis 15: 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Mu L., Wang J., Guo X., Zheng S., Shan K., Jing C., Li L. 2014. [Correlation and clinical significance of expressions of HIF-1α and Sema4D in colorectal carcinoma tissues]. Zhonghua Wei Chang Wai Ke Za Zhi 17: 388–392. [PubMed] [Google Scholar]
- 36.Liu H., Yang Y., Xiao J., Yang S., Liu Y., Kang W., Li X., Zhang F. 2014. Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients. Microvasc. Res. 93: 1–8. [DOI] [PubMed] [Google Scholar]
- 37.Ch’ng E. S., Kumanogoh A. 2010. Roles of Sema4D and Plexin-B1 in tumor progression. Mol. Cancer 9: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Zhang L., Pan Y., Ren X., Hao Q. 2012. Over-expression of semaphorin4D, hypoxia-inducible factor-1α and vascular endothelial growth factor is related to poor prognosis in ovarian epithelial cancer. Int. J. Mol. Sci. 13: 13264–13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierra J. R., Corso S., Caione L., Cepero V., Conrotto P., Cignetti A., Piacibello W., Kumanogoh A., Kikutani H., Comoglio P. M., et al. 2008. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J. Exp. Med. 205: 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin D., Abba M. C., Molinolo A. A., Vitale-Cross L., Wang Z., Zaida M., Delic N. C., Samuels Y., Lyons J. G., Gutkind J. S. 2014. The head and neck cancer cell oncogenome: a platform for the development of precision molecular therapies. Oncotarget 5: 8906–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chouaib S., Messai Y., Couve S., Escudier B., Hasmim M., Noman M. Z. 2012. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front. Immunol. 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumanogoh A., Kikutani H. 2003. Immune semaphorins: a new area of semaphorin research. J. Cell Sci. 116: 3463–3470. [DOI] [PubMed] [Google Scholar]
- 43.Cuenca A. G., Delano M. J., Kelly-Scumpia K. M., Moreno C., Scumpia P. O., Laface D. M., Heyworth P. G., Efron P. A., Moldawer L. L. 2011. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 17: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong C. C., Kao J., Sikora A. G. 2012. Recognizing and reversing the immunosuppressive tumor microenvironment of head and neck cancer. Immunol. Res. 54: 266–274. [DOI] [PubMed] [Google Scholar]
- 45.Young M. R., Lathers D. M. 1999. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int. J. Immunopharmacol. 21: 241–252. [DOI] [PubMed] [Google Scholar]
- 46.Chikamatsu K., Sakakura K., Toyoda M., Takahashi K., Yamamoto T., Masuyama K. 2012. Immunosuppressive activity of CD14+ HLA-DR− cells in squamous cell carcinoma of the head and neck. Cancer Sci. 103: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young M. R., Wright M. A., Pandit R. 1997. Myeloid differentiation treatment to diminish the presence of immune-suppressive CD34+ cells within human head and neck squamous cell carcinomas. J. Immunol. 159: 990–996. [PubMed] [Google Scholar]
- 48.Evans E. E., Jonason A. S., Jr., Bussler H., Torno S., Veeraraghavan J., Reilly C., Doherty M. A., Seils J., Winter L. A., Mallow C., et al. 2015. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol. Res. 3: 689–701. [DOI] [PubMed] [Google Scholar]
- 49.Tartour E., Pere H., Maillere B., Terme M., Merillon N., Taieb J., Sandoval F., Quintin-Colonna F., Lacerda K., Karadimou A., et al. 2011. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 30: 83–95. [DOI] [PubMed] [Google Scholar]
- 50.Kovacsovics-Bankowski M., Chisholm L., Vercellini J., Tucker C. G., Montler R., Haley D., Newell P., Ma J., Tseng P., Wolf R., et al. 2014. Detailed characterization of tumor infiltrating lymphocytes in two distinct human solid malignancies show phenotypic similarities. J. Immunother. Cancer 2: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.June C. H. 2007. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 117: 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumanogoh A., Suzuki K., Ch’ng E., Watanabe C., Marukawa S., Takegahara N., Ishida I., Sato T., Habu S., Yoshida K., et al. 2002. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J. Immunol. 169: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 53.Kataoka T. R., Kumanogoh A., Hirata M., Moriyoshi K., Ueshima C., Kawahara M., Tsuruyama T., Haga H. 2013. CD72 regulates the growth of KIT-mutated leukemia cell line Kasumi-1. Sci. Rep. 3: 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumanogoh A., Marukawa S., Suzuki K., Takegahara N., Watanabe C., Ch’ng E., Ishida I., Fujimura H., Sakoda S., Yoshida K., Kikutani H. 2002. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419: 629–633. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y. H., Zhou H., Binmadi N. O., Proia P., Basile J. R. 2011. Plexin-B1 activates NF-κB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS One 6: e25826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Li M., O’Sullivan K. M., Jones L. K., Semple T., Kumanogoh A., Kikutani H., Holdsworth S. R., Kitching A. R. 2006. CD100 enhances dendritic cell and CD4+ cell activation leading to pathogenetic humoral responses and immune complex glomerulonephritis. J. Immunol. 177: 3406–3412. [DOI] [PubMed] [Google Scholar]
- 57.Kusmartsev S., Gabrilovich D. I. 2006. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 25: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiang X., Poliakov A., Liu C., Liu Y., Deng Z. B., Wang J., Cheng Z., Shah S. V., Wang G. J., Zhang L., et al. 2009. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 124: 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas G. R., Chen Z., Leukinova E., Van Waes C., Wen J. 2004. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: restoration by interferon gamma. Cancer Immunol. Immunother. 53: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piva M. R., DE Souza L. B., Martins-Filho P. R., Soares R. C., DE Santana Santos T., DE Souza Andrade E. S. 2011. Role of inflammation in oral carcinogenesis (part I): Histological grading of malignancy using a binary system. Oncol. Lett. 2: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piva M. R., DE Souza L. B., Martins-Filho P. R., Nonaka C. F., DE Santana Santos T., DE Souza Andrade E. S., Piva D. 2013. Role of inflammation in oral carcinogenesis (Part II): CD8, FOXP3, TNF-α, TGF-β and NF-κB expression. Oncol. Lett. 5: 1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Granziero L., Circosta P., Scielzo C., Frisaldi E., Stella S., Geuna M., Giordano S., Ghia P., Caligaris-Cappio F. 2003. CD100/plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 101: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 63.Finke J., Ko J., Rini B., Rayman P., Ireland J., Cohen P. 2011. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 11: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szala S., Mitrus I., Sochanik A. 2010. Can inhibition of angiogenesis and stimulation of immune response be combined into a more effective antitumor therapy? Cancer Immunol. Immunother. 59: 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strauss L., Volland D., Guerrero A., Reichert T. 2005. [Antiangiogenic and anti-immunosuppressive therapeutic strategies in human head and neck squamous cell carcinoma (HNSCC)]. Mund Kiefer Gesichtschir. 9: 273–281. [DOI] [PubMed] [Google Scholar]
- 66.Strauss L., Volland D., Kunkel M., Reichert T. E. 2005. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med. Sci. Monit. 11: BR280–BR292. [PubMed] [Google Scholar]
- 67.Dolcetti L., Marigo I., Mantelli B., Peranzoni E., Zanovello P., Bronte V. 2008. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 267: 216–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.