Abstract
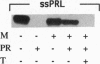
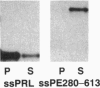
The 37-kDa C-terminal fragment of Pseudomonas exotoxin A (PE; termed PE37 and composed of aa 280-613 of PE) translocates to the cell cytosol to cause cell death. PE37 requires a C-terminal endoplasmic reticulum retention sequence to be cytotoxic, indicating that the toxin may translocate to the cytosol from the endoplasmic reticulum. We show here that the N-terminal region of nascent PE37 can be inserted into the membrane of canine pancreatic microsomes by the preprocecropin signal sequence but then is exported or released from microsomes. The 34 N-terminal amino acids of the toxin fragment are sufficient to arrest translocation and prevent the microsomal accumulation of nascent chains that otherwise are sequestered into microsomes. These data support a role for the N-terminal region of PE37 in the translocation of the toxin from the endoplasmic reticulum to the cytosol in mammalian cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2253–2261. doi: 10.1083/jcb.103.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991 May 24;252(5009):1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. N-Linked glycoprotein assembly. Evidence that oligosaccharide attachment occurs within the lumen of the endoplasmic reticulum. J Biol Chem. 1980 Apr 25;255(8):3600–3604. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. The topological orientation of N,N'-diacetylchitobiosylpyrophosphoryldolichol in artificial and natural membranes. J Biol Chem. 1979 Sep 25;254(18):9237–9246. [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lively M. O., Walsh K. A. Hen oviduct signal peptidase is an integral membrane protein. J Biol Chem. 1983 Aug 10;258(15):9488–9495. [PubMed] [Google Scholar]
- Lukac M., Pier G. B., Collier R. J. Toxoid of Pseudomonas aeruginosa exotoxin A generated by deletion of an active-site residue. Infect Immun. 1988 Dec;56(12):3095–3098. doi: 10.1128/iai.56.12.3095-3098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Chaudhary V. K., Pastan I., FitzGerald D. J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov 25;265(33):20678–20685. [PubMed] [Google Scholar]
- Ogata M., Fryling C. M., Pastan I., FitzGerald D. J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J Biol Chem. 1992 Dec 15;267(35):25396–25401. [PubMed] [Google Scholar]
- Pastan I., Chaudhary V., FitzGerald D. J. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Pastan I., FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991 Nov 22;254(5035):1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Scheele G. Methods for the study of protein translocation across the RER membrane using the reticulocyte lysate translation system and canine pancreatic microsomal membranes. Methods Enzymol. 1983;96:94–111. doi: 10.1016/s0076-6879(83)96011-1. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Gudmundsson G. H., Boman H. G., Zimmermann R. Structural requirements for transport of preprocecropinA and related presecretory proteins into mammalian microsomes. J Biol Chem. 1992 Dec 5;267(34):24328–24332. [PubMed] [Google Scholar]
- Seetharam S., Chaudhary V. K., FitzGerald D., Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991 Sep 15;266(26):17376–17381. [PubMed] [Google Scholar]
- Shelness G. S., Kanwar Y. S., Blobel G. cDNA-derived primary structure of the glycoprotein component of canine microsomal signal peptidase complex. J Biol Chem. 1988 Nov 15;263(32):17063–17070. [PubMed] [Google Scholar]
- Siegall C. B., Chaudhary V. K., FitzGerald D. J., Pastan I. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J Biol Chem. 1989 Aug 25;264(24):14256–14261. [PubMed] [Google Scholar]
- Simon S. M., Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991 May 3;65(3):371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Robbins P. W. Transmembrane organization of protein glycosylation. Mature oligosaccharide-lipid is located on the luminal side of microsomes from Chinese hamster ovary cells. J Biol Chem. 1982 Jun 25;257(12):6796–6801. [PubMed] [Google Scholar]
- Theuer C. P., FitzGerald D. J., Pastan I. A recombinant form of Pseudomonas exotoxin A containing transforming growth factor alpha near its carboxyl terminus for the treatment of bladder cancer. J Urol. 1993 Jun;149(6):1626–1632. doi: 10.1016/s0022-5347(17)36464-9. [DOI] [PubMed] [Google Scholar]
- Theuer C. P., FitzGerald D., Pastan I. A recombinant form of Pseudomonas exotoxin directed at the epidermal growth factor receptor that is cytotoxic without requiring proteolytic processing. J Biol Chem. 1992 Aug 25;267(24):16872–16877. [PubMed] [Google Scholar]
- Walter P., Jackson R. C., Marcus M. M., Lingappa V. R., Blobel G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1795–1799. doi: 10.1073/pnas.76.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Klappa P., Zimmerman R. Protein export in prokaryotes and eukaryotes. Theme with variations. FEBS Lett. 1991 Jul 22;285(2):182–188. doi: 10.1016/0014-5793(91)80800-i. [DOI] [PubMed] [Google Scholar]