Abstract
Objective:
The objective of this study was to evaluate the short-term efficacy and safety of the atypical antipsychotic agent lurasidone in the treatment of schizophrenia.
Methods:
In this phase II, randomized, double-blind, placebo-controlled study, hospitalized adult patients diagnosed with schizophrenia and experiencing an acute exacerbation of psychotic symptoms were randomly assigned to 6 weeks of fixed-dose lurasidone 20 mg/day (n = 71), lurasidone 40 mg/day (n = 67), lurasidone 80 mg/day (n = 71), haloperidol 10 mg/day (n = 72, included to test for assay sensitivity), or placebo (n = 72). Efficacy was assessed using the brief psychiatric rating scale, positive and negative syndrome scale, and clinical global impression-severity. Safety assessments included incidence of adverse events and clinical laboratory measures.
Results:
Numerical improvement was observed from baseline to week 6 (last observation carried forward) on all efficacy measures in all treatment groups; however, no statistically significant differences were noted between any lurasidone group and placebo, or between haloperidol and placebo. The most common adverse events in lurasidone-treated patients, with an incidence of at least 10% (dose groups combined) and greater than placebo, were sedation (15.3%), dyspepsia (13.4%), nausea (13.4%), akathisia (12.4%), and vomiting (10.5%); for haloperidol, the most common adverse events (incidence ⩾ 10% and greater than placebo) were extrapyramidal disorder (20.8%), sedation (19.4%), akathisia (19.4%), dystonia (15.3%), insomnia (13.9%), and somnolence (12.5%). Lurasidone was associated with minimal changes in weight, metabolic parameters, and prolactin levels.
Conclusions:
None of the lurasidone groups separated from placebo in this clinical study of patients with acute schizophrenia. In addition, haloperidol, which was included for assay sensitivity, did not separate from placebo, resulting in a failed study. Possible reasons for the lack of assay sensitivity in this study include the use of multiple active treatment arms and the relatively large placebo response. Consistent with other studies, lurasidone was generally safe and well tolerated, with minimal effects on weight or metabolic parameters.
Keywords: antipsychotic agents, clinical trial, drug therapy, lurasidone, medication effects, schizophrenia
Introduction
Lurasidone is an atypical antipsychotic agent approved for the treatment of schizophrenia in the USA, Canada, Australia, and Europe, and for the treatment of major depressive episodes associated with bipolar I disorder, as monotherapy and as adjunctive therapy with lithium or valproate, in the USA and Canada. Five positive short-term studies demonstrated the safety and efficacy of lurasidone 40–160 mg/day in the treatment of schizophrenia [Loebel et al. 2013a; Meltzer et al. 2011; Nakamura et al. 2009; Nasrallah et al. 2013; Ogasa et al. 2013].
Similar to other atypical antipsychotic agents, lurasidone acts as an antagonist with potent affinity for dopamine D2 and serotonin 2A (5-HT2A) receptors. In addition, lurasidone has a high affinity for 5-HT7 and α2C adrenergic receptors (as an antagonist), moderate affinity at α2A adrenergic receptors (as an antagonist), and a high affinity for 5-HT1A receptors (as a partial agonist). Lurasidone demonstrates no appreciable affinity for histamine H1 receptors or muscarinic M1 receptors [Ishibashi et al. 2010].
The aim of this phase II study was to evaluate the efficacy and safety of lurasidone in patients experiencing an acute exacerbation of schizophrenia; haloperidol was included as an active comparator to confirm assay sensitivity. Two previously reported phase II studies demonstrated significantly greater efficacy for lurasidone compared with placebo [Nakamura et al. 2009; Ogasa et al. 2013].
Methods
This was a phase II, randomized, double-blind, placebo-controlled, 6-week study conducted at 33 sites in the USA between August 2002 and May 2003. The primary objective of the study was to evaluate the efficacy of lurasidone compared with placebo in the treatment of patients experiencing an acute exacerbation of schizophrenia. Secondary objectives included assessment of the efficacy of haloperidol compared with lurasidone and with placebo, the extrapyramidal profile of lurasidone compared with that of haloperidol and placebo, the dose-response relationship of lurasidone across three dose levels, the relationship of serum concentrations of lurasidone to its safety and efficacy, and the relationship of lurasidone exposure and changes in bone turnover.
All patients provided informed consent prior to enrollment. Study procedures were approved by institutional review boards associated with each site, and study conduct was consistent with the International Conference on Harmonization Good Clinical Practice guidelines and with the ethical principles of the Declaration of Helsinki.
Entry criteria
Patients hospitalized for an acute exacerbation of psychotic symptoms were enrolled in the current study. All patients were 18–64 years of age, inclusive, with a Diagnostic and Statistical Manual of Mental Disorders, 4th edition primary diagnosis of schizophrenia of at least 1-year duration. Patients were required to have a baseline brief psychiatric rating scale (BPRS) total score of 42 or higher with a score of 4 or more on at least two items of the positive symptom subscale and a clinical global impression-severity (CGI-S) score of moderate or worse (4 or higher). Patients who demonstrated an improvement of 20% or higher in their BPRS score between screening and baseline were excluded from study participation. Other key exclusion criteria were evidence of a chronic disorder of the central nervous system (other than schizophrenia), clinically significant cardiovascular disease, evidence of a movement disorder, history of substance abuse within the previous 3 months, history of resistance to neuroleptic treatment, or imminent risk of injury to self or others.
Patients remained in the hospital for the first 3 weeks of double-blind treatment; hospitalization could be extended for an additional week at the investigator’s discretion. Patients who had not improved clinically after the fourth week (as determined by investigator judgment, corroborated by a CGI-S score equal to or greater than the patient’s baseline score) were discontinued from the study.
Study medication
Patients who met entry criteria after a screening period of up to 14 days and a 3–4-day washout period were randomized to one of three fixed doses of lurasidone (20 mg/day, 40 mg/day, or 80 mg/day), haloperidol (10 mg/day, to confirm the sensitivity of the study), or placebo in a 1:1:1:1:1 ratio. Study medication consisted of over-encapsulated tablets of lurasidone or haloperidol or matching placebo and was taken in the morning within 30 min after breakfast. Study medication was dispensed weekly (7 days of medication plus 2 extra days in case of scheduling difficulties).
Other psychotropic medications, including antidepressants, mood stabilizers, and other antipsychotic agents, were discontinued during the washout period and prohibited for the duration of the study. Benzodiazepines (lorazepam [⩽ 6 mg/day, ⩽ 4 mg/day, and ⩽ 2 mg/day for days 1–4, days 5–8, and days 9–28 of the double-blind period, respectively, for the inpatient period and ⩽ 4 mg/day for the outpatient status] and temazepam [⩽ 30 mg/day]) for agitation or anxiety, zolpidem (⩽ 10 mg/day) for insomnia, and benztropine (1–2 mg, twice daily) for extrapyramidal symptoms were permitted on an as-needed basis, but not within 8 h of an efficacy assessment.
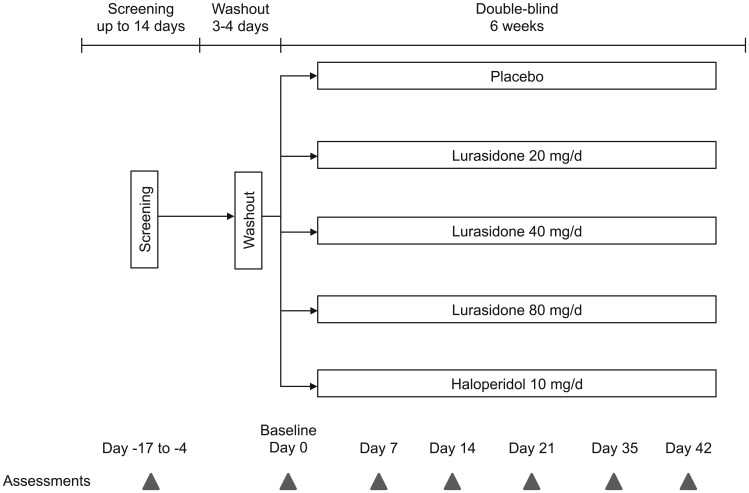
Assessments
Assessments were conducted at screening, baseline, and weekly thereafter for 6 weeks (Figure 1). The primary measure of efficacy was the BPRS [Overall and Gorha, 1962] as derived from the positive and negative syndrome scale (PANSS) [Kay et al. 1987]. Secondary efficacy measures included the PANSS total and subscale scores, CGI-S [Guy, 1976], and Montgomery–Åsberg depression rating scale (MADRS) [Montgomery and Åsberg, 1979]. Safety assessments included incidence of adverse events, clinical laboratory measures, vital signs, electrocardiogram, and bone turnover. Movement disorder symptoms were evaluated using the Simpson–Angus scale (SAS) [Simpson and Angus, 1970], the Barnes akathisia rating scale (BARS) [Barnes, 1989], and the abnormal involuntary movement scale (AIMS) [Guy, 1976].
Figure 1.
Study design.
For patients who discontinued study participation before day 42, final efficacy and safety assessments were completed at an early termination visit, no more than 72 h after the last dose of study medication.
Statistical methods
In order to detect a standardized treatment difference of 0.63 between any lurasidone-dose group and the placebo group with 85% power (two-tailed), an alpha level of 0.019 (i.e. 0.05 adjusted for Dunnett’s test with three comparisons), and an expected attrition rate of 10% before the first post-baseline assessment (day 3), the required sample size was 66 patients per treatment group. This effect size (0.63) was derived from an expected treatment difference between lurasidone and placebo of 6.6 points on the BPRS and a pooled standard deviation of 10.5 – values that were based on a previously completed study of lurasidone in patients with acute schizophrenia [Ogasa et al. 2013], and other previous trials of antipsychotic agents of comparable length and design that used the BPRS to assess efficacy. The intent-to-treat population included all randomized patients who received at least one dose of study medication and had at least one post-baseline efficacy assessment. The safety population consisted of all randomized patients who received at least one dose of study medication.
The primary efficacy outcome, change in BPRS score from baseline to week 6 with the last observation carried forward (LOCF), was analyzed using an analysis of covariance (ANCOVA) model with treatment and center as fixed effects and baseline BPRS score as a covariate. Each lurasidone-dose group (20 mg/day, 40 mg/day, 80 mg/day) and haloperidol 10 mg/day was compared to placebo using Dunnett’s test adjusted for multiplicity. Similar ANCOVA models were used for the secondary efficacy outcomes (PANSS total score, CGI-S, MADRS), without adjustment for multiple comparisons.
Significance testing on the movement disorder measures was performed using one-way analysis of variance without multiplicity adjustment. The incidence of adverse events and changes in weight and laboratory parameters (e.g. cholesterol, glucose, and prolactin) were summarized by treatment group.
Results
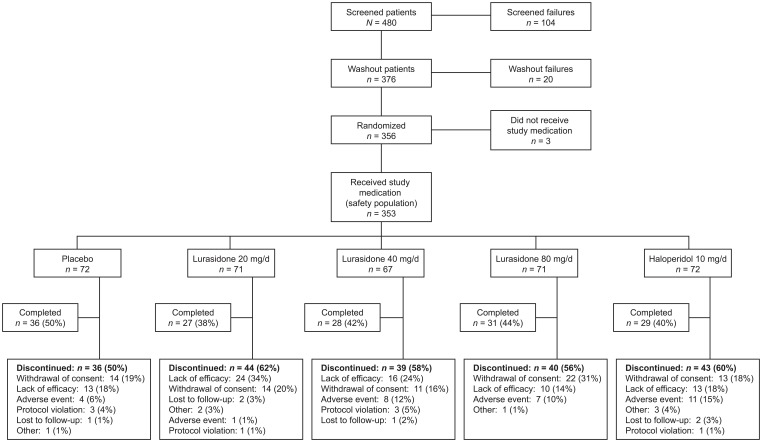
Of the 353 patients who were randomized and received study medication, 151 (42.8%) completed the 6-week study. Patient disposition by treatment group is shown in Figure 2. Rates of study discontinuation due to lack of efficacy were 33.8% for lurasidone 20 mg/day, 23.9% for lurasidone 40 mg/day, 14.1% for lurasidone 80 mg/day, 17.8% for haloperidol, and 18.1% for placebo. Discontinuations attributed to adverse effects occurred in 1.4%, 11.9%, and 9.9% of patients receiving lurasidone 20 mg/day, 40 mg/day, and 80 mg/day, respectively, 15.1% receiving haloperidol 10 mg/day, and 5.6% in the placebo group.
Figure 2.
Patient disposition.
Patient characteristics at baseline were comparable across treatment groups (Table 1). At baseline, mean PANSS total score ranged from 93.1 to 96.5 and mean CGI-S score from 4.7 to 4.8, indicative of moderate to severe psychopathology.
Table 1.
Baseline characteristics of patients randomized to receive lurasidone, haloperidol, or placebo (safety population).
| Characteristic | Lurasidone 20 mg/day(n = 71) | Lurasidone 40 mg/day(n = 67) | Lurasidone 80 mg/day(n = 71) | Haloperidol 10 mg/day(n = 72) | Placebo(n = 72) |
|---|---|---|---|---|---|
| Male, n (%) | 51 (71.8) | 46 (68.7) | 52 (73.2) | 58 (80.6) | 55 (76.4) |
| Race, n (%) | |||||
| White | 38 (53.5) | 34 (50.7) | 29 (40.8) | 34 (47.2) | 41 (56.9) |
| Black | 30 (42.3) | 32 (47.8) | 40 (56.3) | 35 (48.6) | 28 (38.9) |
| Asian | 3 (4.2) | 0 (0) | 1 (1.4) | 1 (1.4) | 1 (1.4) |
| Other | 0 (0) | 1 (1.5) | 1 (1.4) | 2 (2.8) | 2 (2.8) |
| Ethnicity, Hispanic/Latino, n (%) | 6 (8.5) | 4 (6.0) | 5 (7.0) | 5 (6.9) | 3 (4.2) |
| Age (years), mean (SD) | 40.7 (10.5) | 42.0 (10.9) | 42.2 (8.3) | 40.0 (10.5) | 41.0 (9.7) |
| BPRS score, mean (SD) | 55.4 (7.2) | 55.0 (7.4) | 54.5 (7.3) | 56.1 (7.9) | 56.8 (8.3) |
| PANSS total score, mean (SD) | 94.7 (13.3) | 93.4 (15.0) | 93.1 (13.6) | 94.3 (13.6) | 96.5 (15.2) |
| CGI-S score, mean (SD) | 4.7 (0.8) | 4.8 (0.8) | 4.7 (0.8) | 4.8 (0.7) | 4.8 (0.7) |
| MADRS score, mean (SD) | 13.5 (7.3) | 13.1 (7.5) | 13.6 (8.0) | 15.6 (9.6) | 14.7 (8.6) |
BPRS, brief psychiatric rating scale; CGI-S, clinical global impression-severity; MADRS, Montgomery–Åsberg depression rating scale; PANSS, positive and negative syndrome scale; SD, standard deviation.
The mean duration of exposure to study medication was 26.7 days, 30.4 days, and 28.5 days for the lurasidone-dose groups of 20 mg/day, 40 mg/day, and 80 mg/day, respectively, compared with 25.3 days in the haloperidol group and 29.2 days in the placebo group. The most commonly used concomitant medications were benzodiazepines (i.e. lorazepam and temazepam), taken by 93.0%, 86.6%, and 83.1% of patients in the lurasidone 20 mg/day, 40 mg/day, and 80 mg/day groups, respectively, 77.8% in the haloperidol group, and 84.7% in the placebo group. Sedative hypnotics (i.e. zolpidem) were used by 25–37% of patients across all groups.
Efficacy
Numerical improvement on the BPRS from baseline to study endpoint (week 6, LOCF) was seen in all treatment groups and in the placebo group; however, there were no statistically significant differences in BPRS change scores between any dose of lurasidone and placebo, or between haloperidol and placebo (Table 2). Similarly, analyses of change from baseline to study endpoint for PANSS total, CGI-S, and MADRS scores showed numerical improvement in all treatment groups with no significant differences for lurasidone or haloperidol versus placebo (Table 2). As both lurasidone and haloperidol failed to separate from placebo on the primary outcome measure and other standard measures of efficacy, the study is considered a failed study and further between-group comparisons are not reported.
Table 2.
Change from baseline to week 6 on efficacy measures (intent-to-treat population).
| Outcome measure | Lurasidone 20 mg/day(n = 71) | Lurasidone 40 mg/day(n = 65) | Lurasidone 80 mg/day(n = 70) | Haloperidol 10 mg/day(n = 72) | Placebo(n = 71) |
|---|---|---|---|---|---|
| BPRS | |||||
| LS mean change (SE) | −5.0 (1.4) | −5.2 (1.4) | −8.0 (1.4) | −9.8 (1.4) | −7.9 (1.4) |
| p value versus placebo | 0.357 | 0.437 | 0.999 | 0.747 | — |
| PANSS | |||||
| LS mean change (SE) | −7.1 (2.3) | −7.2 (2.4) | −13.6 (2.3) | −16.0 (2.3) | −12.3 (2.3) |
| p value versus placebo | 0.109 | 0.126 | 0.694 | 0.252 | — |
| CGI-S | |||||
| LS mean change (SE) | −0.5 (0.1) | −0.4 (0.1) | −0.8 (0.1) | −0.8 (0.1) | −0.7 (0.1) |
| p value versus placebo | 0.179 | 0.128 | 0.595 | 0.463 | — |
| MADRS | |||||
| LS mean change (SE) | −1.3 (0.97) | −1.1 (1.0) | −2.5 (0.98) | −2.7 (0.96) | −1.9 (0.97) |
| p value versus placebo | 0.620 | 0.565 | 0.668 | 0.562 | — |
BPRS, brief psychiatric rating scale; CGI-S, clinical global impression-severity; LS, least squares; MADRS, Montgomery–Åsberg depression rating scale; PANSS, positive and negative syndrome scale; SE, standard error.
Safety
Adverse events
Most adverse events were mild or moderate in severity; adverse events rated as severe did not show a dose relationship with lurasidone and were reported for 11.3%, 29.9%, and 5.6% of patients receiving 20 mg/day, 40 mg/day, and 80 mg/day, respectively, compared with 15.3% of patients receiving placebo and 6.9% receiving haloperidol. The most common adverse events (experienced by 10% or higher of patients, with an incidence greater than placebo) in lurasidone-treated patients were sedation (15.3%), dyspepsia (13.4%), nausea (13.4%), and akathisia (12.4%); for haloperidol, the most common such adverse events were extrapyramidal disorder (20.8%), sedation (19.4%), akathisia (19.4%), dystonia (15.3%), insomnia (13.9%), and somnolence (12.5%) (Table 3). A lurasidone-dose relationship was apparent for akathisia and sedation; incidence was also greater with lurasidone 80 mg/day compared with other doses for nausea, vomiting, somnolence, and fatigue.
Table 3.
Adverse events reported in 10% or higher of patients in any group (safety population).
| Adverse event, n (%) | Lurasidone 20 mg/day(n = 71) | Lurasidone 40 mg/day(n = 67) | Lurasidone 80 mg/day(n = 71) | Haloperidol 10 mg/day(n = 72) | Placebo (n = 72) |
|---|---|---|---|---|---|
| At least one adverse event | 53 (74.6) | 57 (85.1) | 56 (78.9) | 63 (87.5) | 57 (79.2) |
| Headache | 16 (22.5) | 15 (22.4) | 16 (22.5) | 14 (19.4) | 23 (31.9) |
| Sedation | 7 (9.9) | 11 (16.4) | 14 (19.7) | 14 (19.4) | 6 (8.3) |
| Dyspepsia | 10 (14.1) | 8 (11.9) | 10 (14.1) | 7 (9.7) | 9 (12.5) |
| Nausea | 8 (11.3) | 7 (10.4) | 13 (18.3) | 4 (5.6) | 8 (11.1) |
| Akathisia | 4 (5.6) | 9 (13.4) | 13 (18.3) | 14 (19.4) | 7 (9.7) |
| Vomiting | 5 (7.0) | 5 (7.5) | 12 (16.9) | 4 (5.6) | 5 (6.9) |
| Somnolence | 4 (5.6) | 4 (6.0) | 8 (11.3) | 9 (12.5) | 4 (5.6) |
| Fatigue | 5 (7.0) | 2 (3.0) | 9 (12.7) | 6 (8.3) | 3 (4.2) |
| Agitation | 6 (8.5) | 7 (10.4) | 2 (2.8) | 2 (2.8) | 3 (4.2) |
| Insomnia | 5 (7.0) | 5 (7.5) | 4 (5.6) | 10 (13.9) | 4 (5.6) |
| Extrapyramidal disorder | 2 (2.8) | 4 (6.0) | 4 (5.6) | 15 (20.8) | 3 (4.2) |
| Constipation | 2 (2.8) | 1 (1.5) | 6 (8.5) | 3 (4.2) | 9 (12.5) |
| Dystonia | 0 (0) | 2 (3.0) | 2 (2.8) | 11 (15.3) | 1 (1.4) |
Extrapyramidal symptoms and akathisia
On the movement-disorder measures, mean change scores were small and generally similar for each dose of lurasidone and placebo (Table 4); in the haloperidol group mean change was modest but demonstrated significant worsening on all movement-disorder measures at week 6 (LOCF) relative to placebo (p < 0.001 for AIMS and BARS; p < 0.01 for the SAS). Benztropine was administered for extrapyramidal symptoms to 9.9%, 14.9%, and 25.4% of patients treated with lurasidone 20 mg/day, 40 mg/day, and 80 mg/day, respectively, 43.1% of patients treated with haloperidol, and 13.9% of patients receiving placebo. Discontinuation because of extrapyramidal symptoms or akathisia was more common with haloperidol (9.7% of patients) than with lurasidone (1 patient [0.5%] across all dose groups) or placebo (0.0%).
Table 4.
Changes in movement-disorder scale scores at week 6 (last observation carried forward) and use of anti-Parkinson medication (safety population).
| Measure | Lurasidone 20 mg/day(n = 71) | Lurasidone 40 mg/day(n = 67) | Lurasidone 80 mg/day(n = 71) | Haloperidol 10 mg/d(n = 72) | Placebo(n = 72) |
|---|---|---|---|---|---|
| AIMS | |||||
| Baseline, mean (SD) | 1.3 (2.7) | 1.6 (3.6) | 0.9 (2.4) | 0.8 (2.4) | 1.7 (3.5) |
| Mean (SD) change | 0.6 (2.8) | 0.3 (3.2) | 0.0 (1.6) | 1.6 (4.0)** | −0.1 (2.6) |
| BARS | |||||
| Baseline, mean (SD) | 1.0 (2.0) | 1.0 (2.0) | 0.7 (2.0) | 0.9 (1.7) | 1.1 (2.0) |
| Mean (SD) change | −0.2 (2.5) | 0.3 (2.3) | 0.3 (2.4) | 1.5 (3.6)** | −0.1 (1.9) |
| SAS | |||||
| Baseline, mean (SD) | 0.5 (1.2) | 0.8 (1.6) | 0.3 (1.0) | 0.5 (1.1) | 0.6 (1.1) |
| Mean (SD) change | 0.5 (1.9) | −0.2 (1.4) | 0.2 (1.6) | 1.2 (3.3)* | 0.2 (1.9) |
| Benztropine use, n (%) | 7 (9.9) | 10 (14.9) | 18 (25.4) | 31 (43.1) | 10 (13.9) |
p < 0.01 versus placebo, **p < 0.001 versus placebo.
AIMS, abnormal involuntary movement scale; BARS, Barnes akathisia rating scale; SAS, Simpson–Angus scale; SD, standard deviation.
Weight and laboratory parameters
Changes in weight and key laboratory parameters are summarized by treatment group in Table 5. From baseline to week 6 (LOCF), mean weight was essentially unchanged across treatment groups, except for a mean increase of 0.9 kg in the lurasidone 80 mg/day group. A weight gain of more than 7% was experienced by 1.5%, 3.0%, and 5.7% of patients receiving lurasidone 20 mg/day, 40 mg/day, and 80 mg/day, respectively, compared with 4.3% of haloperidol-treated patients and 2.9% receiving placebo. Median total cholesterol and triglyceride levels were decreased at the study endpoint for all treatment groups. Median changes in prolactin levels were small and not clinically significant in the lurasidone groups, whereas notable changes in prolactin levels were observed in the haloperidol group (median change from baseline to LOCF: +8.4 ng/ml for men, +27.6 ng/ml for women).
Table 5.
Changes in weight and laboratory parameters at week 6 (last observation carried forward, safety population).
| Measure | Lurasidone 20 mg/day
(n = 71) |
Lurasidone 40 mg/day
(n = 67) |
Lurasidone 80 mg/day
(n = 71) |
Haloperidol 10 mg/day
(n = 72) |
Placebo
(n = 72) |
|---|---|---|---|---|---|
| Weight (kg), mean (SD) | |||||
| Baseline
Change |
83.8 (20.3)
−0.2 (2.6) |
87.2 (21.2)
0.0 (3.6) |
91.0 (21.5)
0.9 (4.2) |
88.4 (20.2)
0.1 (3.1) |
84.8 (22.9)
0.1 (3.0) |
| > 7% weight gain, n (%) | 1 (1.5) | 2 (3.0) | 4 (5.7) | 3 (4.3) | 2 (2.9) |
| Total cholesterol, mmol/l | |||||
| Baseline mean (SD)
Median change |
5.2 (1.3)
−0.3 |
5.3 (1.0)
−0.5 |
5.4 (1.2)
−0.3 |
5.2 (1.5)
−0.2 |
5.1 (1.0)
−0.3 |
| Triglycerides, mmol/l | |||||
| Baseline mean (SD)
Median change |
2.1 (1.5)
−0.1 |
2.0 (1.1)
−0.1 |
2.3 (1.7)
−0.2 |
2.1 (1.8)
−0.1 |
2.0 (1.4)
−0.1 |
| Glucose, mmol/L | |||||
| Baseline mean (SD)
Median change |
5.7 (1.3)
−0.1 |
5.3 (0.9)
0.1 |
5.7 (1.9)
0.1 |
5.4 (1.1)
0.1 |
5.5 (1.4)
−0.2 |
| Prolactin, ng/ml, men | |||||
| Baseline mean (SD)
Median change |
11.8 (8.2)
−1.1 |
14.7 (12.0)
0.1 |
11.5 (8.9)
1.1 |
11.3 (6.3)
8.4 |
11.8 (8.3)
−2.1 |
| Prolactin, ng/ml, women | |||||
| Baseline mean (SD)
Median change |
13.3 (10.2)
−0.7 |
23.9 (21.4)
−1.3 |
15.9 (11.4)
−1.0 |
22.5 (29.5)
27.6 |
21.2 (11.9)
−7.9 |
SD, standard deviation.
Other safety assessments
There were no clinically significant treatment-emergent changes in physical examination findings or vital signs. One patient experienced an increase in the QTc interval of more than 60 ms from baseline, which occurred 1 h after the first dose of study medication (lurasidone 80 mg/day), but did not recur. Measures of bone turnover were comparable among treatment groups.
Discussion
Lurasidone has been approved for the treatment of schizophrenia in the USA in the dose range of 40–160 mg/day on the basis of five short-term efficacy studies, with subsequent approvals in Canada, Australia, and Europe. In the study reported here, lurasidone did not separate from placebo on the primary (BPRS) or secondary (PANSS, CGI-S, MADRS) efficacy measures. This was the second phase II study conducted during the lurasidone clinical-development program for schizophrenia; two other phase II studies were positive [Nakamura et al. 2009; Ogasa et al. 2013]; three subsequent phase III studies also yielded positive efficacy results [Loebel et al. 2013a; Meltzer et al. 2011; Nasrallah et al. 2013]. Haloperidol, which was included in this study at a standard dose (10 mg/day) as an active comparator to test assay sensitivity, also showed no significant differences compared with placebo on the outcome measures. Thus, this trial is considered a failed study and no inferences or conclusions about efficacy can be made based on these data.
In terms of the safety profile, the results of this study are generally comparable to other short-term [Loebel et al. 2013a; Meltzer et al. 2011; Nakamura et al. 2009; Nasrallah et al. 2013; Ogasa et al. 2013] and long-term [Citrome et al. 2012, 2014; Loebel et al. 2013b; Stahl et al. 2013] studies in the lurasidone clinical-development program, including minimal changes in weight and metabolic parameters. The increase in prolactin levels and incidence of extrapyramidal symptoms with haloperidol are consistent with its known safety profile [Maguire, 2002].
The present study included a low dose of lurasidone, 20 mg/day, which subsequently has been confirmed to be a subtherapeutic dose for treatment of patients with schizophrenia [Loebel et al. 2014]. Lurasidone 40 mg/day and 80 mg/day are within the approved dose range for lurasidone in the treatment of schizophrenia (40–160 mg/day), and have clearly demonstrated efficacy in the treatment of acute schizophrenia in other short-term studies [Loebel et al. 2013a; Meltzer et al. 2011; Nakamura et al. 2009; Ogasa et al. 2013]. Haloperidol was used as the active comparator to determine assay sensitivity because it was, at the time the study protocol was written, regarded as the gold standard treatment for patients with schizophrenia [Adams et al. 2013]. The dose utilized in the present study, 10 mg/day, is clearly within the therapeutic dose range for haloperidol [Adams et al. 2013; Davis and Chen, 2004]. In addition, because the BPRS is heavily weighted to assess positive symptoms, it was expected to be sensitive to the effects of haloperidol treatment on symptom improvement.
Despite efforts to conduct well-designed and well-executed clinical trials, failed studies are not uncommon in schizophrenia research [Laughren, 2001]. For example, there are recently published studies in which quetiapine [Cutler et al. 2010] and olanzapine [Bugarski-Kirola et al. 2014; Kinon et al. 2011], administered at therapeutic doses, did not separate from placebo on standard efficacy measures.
A key study design factor that may have reduced the ability to detect drug–placebo differences in the present study is the number of treatment arms. In an analysis of the schizophrenia research literature from 1997 to 2008, studies with multiple active treatment arms and fewer than 25% of patients randomized to placebo were less likely to detect drug–placebo differences compared with studies with a greater proportion of patients receiving placebo [Mallinckrodt et al. 2010]. The placebo response on the BPRS in the present study, with five total treatment arms and 20% of patients randomized to placebo, was a reduction of 7.9 points (Table 2), which is comparable to 5.0- point, 5.2-point, and 8.0-point reductions for the 20 mg/day, 40 mg/day, and 80 mg/day lurasidone groups, respectively, and a 9.8-point reduction for the haloperidol group. The 6-week completion rate of 50% was highest in the placebo group, which is consistent with the unexpected high placebo response rate in this study. In two similar phase II studies of lurasidone conducted during the same time period, the proportion of patients randomized to placebo was 33–50%, and the BPRS score reduction was 3.8–4.2 points in the placebo group [Nakamura et al. 2009; Ogasa et al. 2013]. Investigator and patient expectations (expectation bias) have been proposed as putative mediators of this phenomenon [Mallinckrodt et al. 2010].
In the present study, the treatment response in the haloperidol group was smaller than has generally been observed in other short-term studies of schizophrenia that used the BPRS [Adams et al. 2013; Beasley et al. 1996; McCue et al. 2006]. Similarly, adjusted mean BPRS change scores in the lurasidone-treatment groups were generally smaller than those in the other phase II studies of lurasidone for schizophrenia [Nakamura et al. 2009; Ogasa et al. 2013].
The sample size in the present study (approximately 70 patients per arm) may not have provided adequate statistical power to detect a treatment effect. Sample size was based on drug–placebo differences (with an effect size of 0.63) in the previously conducted phase II study of lurasidone [Ogasa et al. 2013], which showed a very robust treatment effect; however, this effect size is higher than that typically obtained in studies of atypical antipsychotic agents [Leucht et al. 2009]. The high discontinuation rate in the present study may have further reduced the statistical power to detect treatment differences. However, in the absence of other positive findings, the lurasidone dose-response relationship with study discontinuation due to lack of efficacy (34% for 20 mg/day, 24% for 40 mg/day, and 14% for 80 mg/day; Figure 2) may be interpreted as a potential signal of lurasidone efficacy.
The reasons for the failure of haloperidol and lurasidone to separate from placebo in this study cannot be determined with certainty, and likely reflect a combination of factors including one or more of those discussed above. An in-depth internal analysis of study conduct did not identify any clinical operations issues potentially responsible for the failure of this study, although the influence of operational factors (e.g. insufficient rater training or failure to apply rigorously patient selection criteria) cannot be excluded.
Conclusion
Both lurasidone and haloperidol failed to separate from placebo in this 6-week, randomized, double-blind study of patients with acute schizophrenia, which included haloperidol to assess the assay sensitivity of the study. Given the failure of both lurasidone and haloperidol to confirm assay sensitivity, no conclusions can be made from this study regarding the efficacy of lurasidone in the treatment of acute schizophrenia. As in other studies, lurasidone was generally safe and well tolerated, with minimal effects on weight or metabolic parameters. Consistent with the known safety profile of haloperidol, prolactin levels were moderately increased. In addition, the incidence of adverse events related to extrapyramidal symptoms was greater with haloperidol than with lurasidone or placebo.
Acknowledgments
Nancy Holland, PhD, Synchrony Medical Communications, LLC, provided medical writing and editorial assistance for this manuscript under the direction of the authors.
Footnotes
Funding: Financial support for writing and editorial assistance from Synchrony Medical Communications, LLC, was provided by Sunovion Pharmaceuticals Inc., Marlborough, MA, USA. Clinical research was sponsored by Sumitomo Pharmaceuticals America, Ltd (now Sunovion Pharmaceuticals Inc.). The sponsor was involved in the study design, collection, and analysis of data. The interpretation of results, and the decision to submit this manuscript for publication in Therapeutic Advances in Psychopharmacology, was made by the authors independently.
Conflict of interest statement: SGP received grant support, funding, and honoraria and served as a consultant for the following companies that conducted scientific or medical research and/or marketed medications related to psychiatric and neurodegenerative disorders: Alkermes, Eli Lilly and Company, FORUM Pharmaceuticals, Genentech, Janssen, Lundbeck, Merck & Co., Inc., Novartis, Otsuka Pharmaceutical Co., Ltd, Roche, Sunovion Pharmaceuticals Inc., Takeda Pharmaceutical Company Ltd, Toyama Chemical Co., and VANDA Pharmaceuticals. TK is an employee of Sumitomo Dainippon Pharma Co., Ltd. JG is an employee of Sunovion Pharmaceuticals Inc.
Contributor Information
Steven G. Potkin, Department of Psychiatry and Human Behavior, University of California–Irvine, 5251 California Avenue, Ste 240, Irvine, CA 92617, USA.
Tatsuya Kimura, Drug Development Division, Sumitomo Dainippon Pharma Co., Ltd, Tokyo, Japan.
John Guarino, Global Development Administration, Sunovion Pharmaceuticals Inc., Fort Lee, NJ, USA.
References
- Adams C., Bergman H., Irving C., Lawrie S. (2013) Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev 11: CD003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T. (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672–676. [DOI] [PubMed] [Google Scholar]
- Beasley C., Jr, Tollefson G., Tran P., Satterlee W., Sanger T., Hamilton S. (1996) Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 14: 111–123. [DOI] [PubMed] [Google Scholar]
- Bugarski-Kirola D., Wang A., Abi-Saab D., Blattler T. (2014) A phase II/III trial of bitopertin monotherapy compared with placebo in patients with an acute exacerbation of schizophrenia – results from the CandleLyte study. Eur Neuropsychopharmacol 24: 1024–1036. [DOI] [PubMed] [Google Scholar]
- Citrome L., Cucchiaro J., Sarma K., Phillips D., Silva R., Tsuchiya S., et al. (2012) Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 27: 165–176. [DOI] [PubMed] [Google Scholar]
- Citrome L., Weiden P., McEvoy J., Correll C., Cucchiaro J., Hsu J., et al. (2014) Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr 19: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A., Tran-Johnson T., Kalali A., Astrom M., Brecher M., Meulien D. (2010) A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull 43: 37–69. [PubMed] [Google Scholar]
- Davis J., Chen N. (2004) Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 24: 192–208. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU Assessment Manual for Psychopharmacology (revised), (ADM) 76–338 edn. Rockville, MD: National Institute of Mental Health. [Google Scholar]
- Ishibashi T., Horisawa T., Tokuda K., Ishiyama T., Ogasa M., Tagashira R., et al. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334: 171–181. [DOI] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kinon B., Zhang L., Millen B., Osuntokun O., Williams J., Kollack-Walker S., et al. (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol 31: 349–355. [DOI] [PubMed] [Google Scholar]
- Laughren T. (2001) The scientific and ethical basis for placebo-controlled trials in depression and schizophrenia: an FDA perspective. Eur Psychiatry 16: 418–423. [DOI] [PubMed] [Google Scholar]
- Leucht S., Arbter D., Engel R., Kissling W., Davis J. (2009) How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14: 429–447. [DOI] [PubMed] [Google Scholar]
- Loebel A., Cucchiaro J., Sarma K., Xu L., Hsu C., Kalali A., et al. (2013a) Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 145: 101–109. [DOI] [PubMed] [Google Scholar]
- Loebel A., Cucchiaro J., Xu J., Sarma K., Pikalov A., Kane J. (2013b) Effectiveness of lurasidone versus quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res 147: 95–102. [DOI] [PubMed] [Google Scholar]
- Loebel A., Silva R., Goldman R., Watabe K., Cucchiaro J., Kane J. (2014) Optimizing treatment with lurasidone in patients with schizophrenia: results of a randomized, double-blind, placebo-controlled trial (OPTIMIZE trial). Neuropsychopharmacology 39: S476–S477. [Google Scholar]
- Maguire G. (2002) Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J Clin Psychiatry 63(Suppl. 4): 56–62. [PubMed] [Google Scholar]
- Mallinckrodt C., Zhang L., Prucka W., Millen B. (2010) Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol Bull 43: 53–72. [PubMed] [Google Scholar]
- McCue R., Waheed R., Urcuyo L., Orendain G., Joseph M., Charles R., et al. (2006) Comparative effectiveness of second-generation antipsychotics and haloperidol in acute schizophrenia. Br J Psychiatry 189: 433–440. [DOI] [PubMed] [Google Scholar]
- Meltzer H., Cucchiaro J., Silva R., Ogasa M., Phillips D., Xu J., et al. (2011) Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study.Am J Psychiatry 168: 957–967. [DOI] [PubMed] [Google Scholar]
- Montgomery S., Åsberg M. (1979) A new depression scale designed to be sensitive to change.Br J Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ogasa M., Guarino J., Phillips D., Severs J., Cucchiaro J., et al. (2009) Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 70: 829–836. [DOI] [PubMed] [Google Scholar]
- Nasrallah H., Silva R., Phillips D., Cucchiaro J., Hsu J., Xu J., et al. (2013) Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res 47: 670–677. [DOI] [PubMed] [Google Scholar]
- Ogasa M., Kimura T., Nakamura M., Guarino J. (2013) Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl) 225: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J., Gorha D. (1962) The brief psychiatric rating scale. Psychol Rep 799–812. [Google Scholar]
- Simpson G., Angus J. (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19. [DOI] [PubMed] [Google Scholar]
- Stahl S., Cucchiaro J., Simonelli D., Hsu J., Pikalov A., Loebel A. (2013) Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study.J Clin Psychiatry 74: 507–515. [DOI] [PubMed] [Google Scholar]