Abstract
Background
Selinexor (KPT-330) is an inhibitor of the major nuclear export receptor, exportin 1 (XPO1, also termed chromosome region maintenance 1, CRM1) that has demonstrated activity in preclinical models and clinical activity against several solid and hematological cancers.
Procedures
Selinexor was tested against the Pediatric Preclinical Testing Program (PPTP) in vitro cell line panel at concentrations from 1.0 nM to 10 μM and against the PPTP in vivo xenograft panels administered orally at a dose of 10 mg/kg thrice weekly for 4 weeks.
Results
Selinexor demonstrated cytotoxic activity in vitro, with a median relative IC50 value of 123 nM, (range 13.0 nM to >10 μM). Selinexor induced significant differences in event-free survival (EFS) distribution in 29 of 38 (76%) of the evaluable solid tumor xenografts and in 5 of 8 (63%) of the evaluable ALL xenografts. Objective responses (partial or complete responses, PR/CR) were observed for 4 of 38 solid tumor xenografts including Wilms tumor, medulloblastoma (n=2) and ependymoma models. For the ALL panel, 2 of 8 (25%) xenografts achieved either CR or maintained CR. Two responding xenografts had FBXW7 mutations at R465 and two had SMARCA4 mutations. Selinexor induced p53, p21 and cleaved PARP in several solid tumor models.
Conclusions
Selinexor induced regression against several solid tumor and ALL xenografts and slowed tumor growth in a larger number of models. Pharmacodynamic effects for XPO1 inhibition were noted. Defining the relationship between selinexor systemic exposures in mice and humans will be important in assessing the clinical relevance of these results.
Keywords: Preclinical Testing, Developmental Therapeutics, XPO1 inhibitor
INTRODUCTION
Selinexor (KPT-330) is a Selective Inhibitor of Nuclear Export (SINE) that forms a slowly reversible covalent bond with XPO1 (exportin 1, CRM1) and inhibits its function. XPO1 is a member of the karyopherin-β family of proteins and plays a central role in nuclear export through forming complexes with Ran-GTP and with cargo proteins containing a leucine-rich nuclear export sequence (NES) [1,2]. This complex is transported through the nuclear pore complex (NPC) to the cytoplasm, and in the cytoplasm RanGTP is hydrolyzed to RanGDP releasing XPO1 and the cargo protein, with XPO1 then recirculating to the nucleus. XPO1 is involved in the nuclear export of over 200 NES-containing proteins, with many of them being tumor suppressor proteins and cell cycle regulators [2]. Consistent with a role for XPO1 in cancer, XPO1 is upregulated in a range of hematologic and solid tumors, and overexpression is associated with poor prognosis [3–6]. Because alterations in nuclear-cytoplasmic trafficking may lead to the cytoplasmic mislocalization of proteins involved in cell cycle, cell survival or tumor suppression, forcing their nuclear localization may have potential as a cancer therapeutics strategy [2].
Leptomycin B (elactocin) was the first recognized inhibitor of XPO1. It inhibits the export activities of XPO1 by forming a covalent adduct with cysteine-528 of XPO1 [7–9]. Covalent adducts at cysteine-528 functionally inactivate XPO1 and target it for proteasomal degradation [8] Prior to its identification as an inhibitor of XPO1, leptomycin B had been studied as an anticancer agent and showed in vitro and in vivo activity against a range of cancer cell lines [10,11]. However, leptomycin B produced profound vomiting, anorexia and malaise when administered to patients in a phase 1 clinical trial, which led to the agent being dropped from clinical development [12]. Subsequently, SINE compounds have been developed that also work through formation of a covalent bond to cysteine-528 of XPO1 [13], but that have improved tolerability while maintaining anticancer activity [14].
SINE compounds by inhibiting XPO1 are able to force nuclear retention and activation of multiple proteins involved in cell cycle regulation including p53, FOXO, p21, Rb, p27 and IκB [2]. Preclinical activity for SINE compounds have been documented against adult solid tumors [2], as well as for hematological malignancies including T-cell acute lymphoblastic leukemia (ALL) [15], acute myeloid leukemia (AML) [15,16], multiple myeloma [17,18], and non-Hodgkin lymphoma (NHL) [19,20]. As an example, verdinexor (KPT-335) potently inhibits human and canine lymphoma cell lines with low nanomolar potency, and in a phase 1 study with dose expansion in dogs with lymphoma, partial responses were noted in 4 of 20 treated animals [21]. In a subsequent phase 2 trial of verdinexor for canine lymphoma, objective responses (PR or CR) were noted in 20 of 58 (34%) treated animals [22].
Selinexor is currently in early phase clinical trials in both adult and pediatric malignancies. Phase 1 testing in adults evaluated either twice or thrice weekly dosing, with single agent phase 2 evaluations going forward primarily using the twice-weekly schedule [23,24]. The primary and most prominent adverse events are fatigue, anorexia, nausea and in solid cancer patients also thrombocytopenia. Responses were noted in the phase 1 studies for several diagnoses. For example, for adults with AML approximately 10% of patients achieved a complete response, and a randomized phase 2 study comparing selinexor to physician’s choice of standard treatment is ongoing for adults ≥60 years old with relapsed/refractory AML who are ineligible for intensive chemotherapy and/or transplantation (NCT02088541). In a phase 2 evaluation of selinexor, 37% of patients (19 of 52) with heavily pretreated NHL achieved either a partial or complete response [25].
Because of its novel mechanism of action, selinexor was tested against the entire in vitro and in vivo panels of pediatric tumor models.
MATERIALS AND METHODS
In vitro testing
Testing was performed using DIMSCAN [20], as previously described in a characterized panel of 23 cell lines [26]. Cells were incubated in the presence of selinexor for 96 hours at concentrations from 1.0 nM to 10 μM and analyzed as previously described [27].
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [28]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [29]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Eight to ten mice were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [%hCD45+] cells [ALL xenografts] were determined and responses were determined using three activity measures as previously described [28]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
Selinexor was provided to the Pediatric Preclinical Testing Program by Karyopharm Therapeutics Inc. (Newton, MA), through the Cancer Therapy Evaluation Program (NCI). Selinexor was formulated in 0.56%w/v Pluronic F-68/0.56%w/v Plasdone K-29/32 water-soluble polyvinylpyrrolidone and stored for up to 7 days at 4°C, protected from light. Selinexor was administered orally at 10 mg/kg 3 times per week (Monday/Wednesday/Friday) for 4 consecutive weeks with an additional 2 weeks of observation. Selinexor was provided to each consortium investigator in coded vials for blinded testing.
Pharmacodynamic studies
KT-10 tumors that regress rapidly after treatment with selinexor, were harvested 24 hours after a single dose of drug (10 mg/kg). Other, less responsive tumors, were harvested 2 hours after dose 6 (MWF dosing) at 10 mg/kg/dose. Tumors were processed for immunoblotting as previously described [30]. Immunoblots were probed for p53, p21, PARP and cleaved PARP and XPO1/CRM1. Three control and three treated tumors were analyzed for each xenograft line. GAPDH was used as a loading control. KT-10, KT-11, SK-NEP-1, CHLA258, Rh28 and Rh30 tumors were fixed in 10% buffered formalin and paraffin sections were analyzed by H&E as well with the following antibodies: FOXO1 (#2880, Cell Signaling), IKB (ab32518, Abcam), NFKB (sc-8008, Santa Cruz), pRb-phos (ab76298, Abcam), Mcl-1 (sc-819, Santa Cruz), β-catenin (#610154 BD), ERK-phos (ab32538, Abcam), survivin (ab24479, Abcam), and p53 (sc-126, Santa Cruz).
RESULTS
In vitro testing
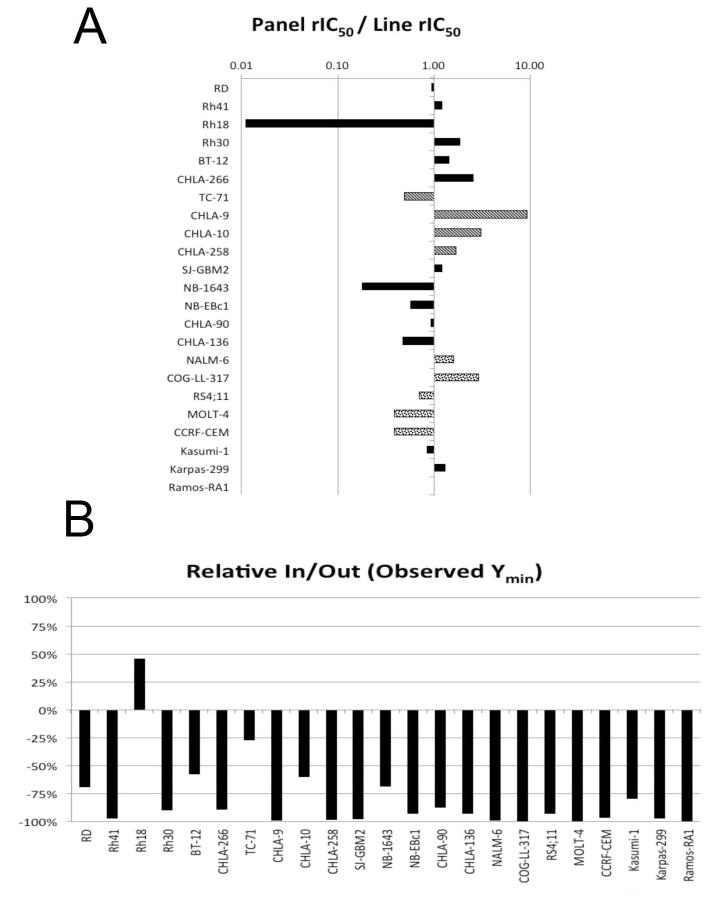
Selinexor was tested against the PPTP’s in vitro cell line panel at concentrations ranging from 1.0 nM to 10.0 μM using the PPTP’s standard 96 hour exposure period. The median relative IC50 (rIC50) value for the cell line panel was 123 nM, with a range from 13 nM (CHLA-9, Ewing sarcoma) to > 10 μM (Rh18 rhabdomyosarcoma). A metric used to compare the relative responsiveness of these cell lines to selinexor is the ratio of the median rIC50 of the entire panel to that of each cell line (Table I and Figure 1A). Higher ratios are indicative of greater sensitivity to selinexor by bars to the right of the midpoint line. As shown in Figure 1A, the cell lines of the Ewing sarcoma panel (bars with stripes) were relatively sensitive to selinexor with 3 of 4 cell lines having rIC50 values less than the median for the entire panel. The ALL cell lines (bars with dots) include 3 lines that were less sensitive than the panel median and 2 that were more sensitive. Both rhabdoid tumor cell lines (BT-12 and CHLA-266) had rIC50 values greater than then panel median. The Relative In/Out (I/O)% values compare the relative difference in final cell number compared with the starting cell number for treated cells and for control cells. Relative I/O% values ranged between 100% (no treatment effect) to −100% (complete cytotoxic effect), with a Relative I/O% value of 0% being observed for a completely effective cytostatic agent. Figure 1B shows that most cell lines had Relative I/O% values between −75% and −100%, consistent with a prominent cytotoxic effect. Rh18 showed clear resistance to selinexor. Both the rIC50 values and the Ymin values were similar for p53 mutated and wild-type cell lines, with no statistically significant differences for either parameter based on p53 status.
Table I.
In vitro activity of selinexor against PPTP cell lines.
| Cell Line | Histotype | rIC50 (μM) | Panel rIC50/ Line rIC50 | Ymin % (Observed) | P53 Status |
|---|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 0.128 | 0.96 | 1.7 | Mutant |
| Rh41 | Rhabdomyosarcoma | 0.100 | 1.22 | 0.6 | Mutant |
| Rh18 | Rhabdomyosarcoma | >10.000 | <0.01 | 69.8 | WT |
| Rh30 | Rhabdomyosarcoma | 0.065 | 1.88 | 1.7 | Mutant |
| BT-12 | Rhabdoid | 0.085 | 1.44 | 3.5 | WT |
| CHLA-266 | Rhabdoid | 0.048 | 2.56 | 2.8 | WT |
| TC-71 | Ewing sarcoma | 0.247 | 0.50 | 0.9 | Mutant |
| CHLA-9 | Ewing sarcoma | 0.013 | 9.32 | 0.0 | WT |
| CHLA-10 | Ewing sarcoma | 0.040 | 3.06 | 2.5 | Mutant |
| CHLA-258 | Ewing sarcoma | 0.073 | 1.68 | 0.5 | WT |
| SJ-GBM2 | Glioblastoma | 0.101 | 1.21 | 0.2 | Mutant |
| NB-1643 | Neuroblastoma | 0.683 | 0.18 | 6.6 | WT |
| NB-EBc1 | Neuroblastoma | 0.214 | 0.57 | 1.6 | WT |
| CHLA-90 | Neuroblastoma | 0.131 | 0.94 | 3.4 | Mutant |
| CHLA-136 | Neuroblastoma | 0.255 | 0.48 | 2.0 | WT |
| NALM-6 | ALL | 0.077 | 1.59 | 0.0 | WT |
| COG-LL-317 | ALL | 0.042 | 2.90 | 0.0 | WT |
| RS4;11 | ALL | 0.174 | 0.71 | 1.1 | WT |
| MOLT-4 | ALL | 0.317 | 0.39 | 0.0 | WT |
| CCRF-CEM (1) | ALL | 0.317 | 0.39 | 0.2 | Mutant |
| Kasumi-1 | AML | 0.143 | 0.86 | 5.8 | Mutant |
| Karpas-299 | ALCL | 0.093 | 1.31 | 0.2 | Mutant |
| Ramos-RA1 | NHL | 0.123 | 1.00 | 0.0 | Mutant |
| Median | 0.123 | 1.00 | 1.1 | ||
| Minimum | 0.013 | <0.01 | 0.0 | ||
| Maximum | >10.000 | 9.32 | 69.8 |
Figure 1.
Selinexor in vitro activity: A. The median rIC50 ratio graph shows the relative rIC50 values for the cell lines of the PPTP panel. Bars with stripes represent Ewing sarcoma cell lines and bars with dots represent ALL cell lines. Each bar represents the ratio of the panel rIC50 to the rIC50 value of the indicated cell line. Bars to the right represent cell lines with higher sensitivity, while bars to the left indicate cell lines with lesser sensitivity; B. Most cell lines have Relative I/O% values between −75% and −100%, consistent with a prominent cytotoxic effect. Rh18 shows clear resistance to selinexor.
In vivo testing
Selinexor was tested against the PPTP solid tumor xenografts using oral dosing on a thrice weekly (M-W-F) for 4 weeks schedule. The total planned treatment and observation period was 6 weeks. Toxicity testing using cohorts of 5 non-tumored animals was performed prior to efficacy testing. Doses of 15 mg/kg and greater were not tolerated with treatment stopped within two weeks of initiation because of excessive toxicity. The 10 mg/kg dose was adequately tolerated with a maximum weight loss of approximately 10% with recovery during continued dosing, and this dose was selected for efficacy testing. Treatment at the 10 mg/kg dose resulted in 4 toxicity related deaths of 450 mice (0.9%), whereas there were no deaths in the control groups (0/441).
All 46 tested xenograft models were considered evaluable for efficacy, with one ALL model (ALL-19) having to be repeatd because of excessive numbers of mice developing lymphomas. Complete details of testing are provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
Selinexor induced significant differences in EFS distribution compared to control in 29 of 38 (76%) solid tumor xenografts and in 5 of 8 (63%) ALL xenografts (Table II). For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of > 2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. Selinexor induced tumor growth inhibition meeting criteria for intermediate (n=10) or high (n=1) EFS T/C activity in 11 of 32 (34%) solid tumor xenografts evaluable for this metric. Intermediate or high activity for the EFS T/C metric occurred most frequently in the Wilms tumor panel (2 of 3) and the Ewing sarcoma panel (4 of 5), but was observed for only 1 of 6 rhabdomyosarcoma xenografts, 1 of 6 neuroblastoma xenografts and for no osteosarcoma xenografts (n=6). Intermediate or high EFS T/C activity was also observed for the rhabdoid panel (n=1), medulloblastoma (n=2) and glioblastoma panel (1 of 4). For the ALL panel, 2 of 8 (38%) xenografts met criteria for intermediate or high EFS T/C activity.
Table II.
Summary of in Vivo Activity of selinexor.
| Line | Tumor Type | Estimate of Median Time to Event | P-value | EFST/C | Median RTV/CD45 at End of Study | Tumor Volume T/C | Median Group Response | EFS Activity |
|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | > EP | <0.001 | > 1.8 | 1.5 | 0.29 | PD2 | NE |
| KT-16 | Rhabdoid | 34.8 | <0.001 | 4.1 | >4 | 0.39 | PD2 | Int |
| KT-14 | Rhabdoid | > EP | <0.001 | > 1.7 | 2.3 | 0.48 | PD2 | NE |
| KT-10 | Wilms | > EP | <0.001 | > 4.0 | 0.0 | 0.00 | MCR | High |
| KT-11 | Wilms | 20.2 | 0.072 | 1.3 | >4 | 0.69 | PD1 | Low |
| KT-13 | Wilms | 18.0 | <0.001 | 2.0 | >4 | 0.35 | PD2 | Int |
| SK-NEP-1 | Ewing | 36.2 | <0.001 | 3.9 | >4 | 0.34 | PD2 | Int |
| EW5 | Ewing | 9.9 | 0.083 | 1.1 | >4 | 0.83 | PD1 | Low |
| EW8 | Ewing | 22.1 | <0.001 | 2.7 | >4 | 0.67 | PD2 | Int |
| TC-71 | Ewing | 25.0 | 0.001 | 3.1 | >4 | 0.79 | PD2 | Int |
| CHLA258 | Ewing | 31.8 | <0.001 | 3.3 | >4 | 0.22 | PD2 | Int |
| Rh10 | Alveolar RMS | 34.5 | 0.004 | 1.4 | >4 | 0.55 | PD1 | Low |
| Rh28 | Alveolar RMS | 32.4 | 0.006 | 1.5 | >4 | 0.48 | PD2 | Low |
| Rh30 | Alveolar RMS | 28.4 | <0.001 | 3.2 | >4 | 0.55 | PD2 | Int |
| Rh30R | Alveolar RMS | 24.2 | <0.001 | 1.7 | >4 | 0.42 | PD2 | Low |
| Rh41 | Alveolar RMS | 26.2 | <0.001 | 1.9 | >4 | 0.44 | PD2 | Low |
| Rh18 | Embryonal RMS | 11.1 | <0.001 | 1.6 | >4 | 0.78 | PD2 | Low |
| BT-28 | Medulloblastoma | 30.8 | <0.001 | 3.1 | >4 | 0.69 | PD2 | Int |
| BT-45 | Medulloblastoma | > EP | 0.47 | NE | 0.6 | 0.28 | PR | NE |
| BT-50 | Medulloblastoma | > EP | <0.001 | > 1.2 | 0.5 | 0.17 | CR | NE |
| BT-36 | Ependymoma | > EP | <0.001 | > 1.1 | 1.6 | 0.37 | PD2 | NE |
| BT-41 | Ependymoma | > EP | 1.000 | . | 0.7 | 0.49 | CR | NE |
| GBM2 | Glioblastoma | 28.5 | 0.020 | 2.2 | >4 | 0.62 | PD2 | Int |
| BT-39 | Glioblastoma | 17.4 | 0.165 | 2.0 | >4 | 0.70 | PD2 | Low |
| D645 | Glioblastoma | 11.4 | 0.003 | 2.0 | >4 | 0.44 | PD2 | Low |
| D456 | Glioblastoma | 9.0 | 0.288 | 1.5 | >4 | 0.60 | PD1 | Low |
| NB-SD | Neuroblastoma | 10.5 | 0.300 | 1.1 | >4 | 0.97 | PD1 | Low |
| NB-1771 | Neuroblastoma | 20.8 | <0.001 | 2.0 | >4 | 0.44 | PD2 | Low |
| NB-1691 | Neuroblastoma | 6.6 | 0.165 | 1.2 | >4 | 0.80 | PD1 | Low |
| NB-EBc1 | Neuroblastoma | 7.7 | 0.046 | 1.4 | >4 | 0.64 | PD1 | Low |
| CHLA-79 | Neuroblastoma | 39.1 | <0.001 | 4.3 | >4 | 0.58 | PD2 | Int |
| NB-1643 | Neuroblastoma | 6.7 | 0.010 | 1.2 | >4 | 0.64 | PD1 | Low |
| OS-1 | Osteosarcoma | 30.4 | <0.001 | 1.3 | >4 | 0.57 | PD1 | Low |
| OS-2 | Osteosarcoma | 18.0 | <0.001 | 1.1 | >4 | 0.91 | PD1 | Low |
| OS-17 | Osteosarcoma | 26.5 | 0.003 | 1.4 | >4 | 0.74 | PD1 | Low |
| OS-9 | Osteosarcoma | 30.5 | <0.001 | 1.3 | >4 | 0.73 | PD1 | Low |
| OS-33 | Osteosarcoma | 18.3 | <0.001 | 1.4 | >4 | 0.71 | PD1 | Low |
| OS-31 | Osteosarcoma | 17.6 | 0.315 | 1.0 | >4 | 0.93 | PD1 | Low |
| ALL-2 | ALL B-precursor | 36.8 | 0.003 | 1.9 | >25 | . | PD2 | Low |
| ALL-4 | ALL B-precursor | 6.0 | 0.647 | 1.0 | >25 | . | PD1 | Low |
| ALL-7 | ALL B-precursor | 6.0 | 0.647 | 1.0 | >25 | . | PD1 | Low |
| ALL-8 | ALL T-cell | > EP | <0.001 | > 7.5 | 14.0 | . | CR | Int |
| ALL-17 | ALL B-precursor | 29.8 | 0.001 | 4.0 | >25 | . | PD2 | Int |
| ALL-19 | ALL B-precursor | > EP | 0.001 | > 6.0 | 0.2 | . | MCR | High |
| ALL-31 | T-cell ALL | 14.5 | 0.006 | 1.8 | >25 | . | PD2 | Low |
| MLL-7 | ALL B-precursor | 12.5 | 0.077 | 2.1 | >25 | . | PD1 | Low |
Tumor Volume T/C value: Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤ 0.15; Intermediate activity = T/C ≤ 0.45 but > 0.15; and Low activity = T/C > 0.45.
Objective response measures are described in detail in the Supplemental Response Definitions. PD1 = progressive disease with EFS T/C ≤ 1.5, and PD2 = progressive disease with EFS T/C > 1.5.
EFS T/C values = the ratio of the median time to event of the treatment group and the median time to event of the respective control group. High activity requires: a) an EFS T/C > 2; b) a significant difference in EFS distributions, and c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to at treatment initiation. Intermediate activity = criteria a) and b) above, but not having a net reduction in median tumor volume for treated animals at the end of the study. Low activity = EFS T/C < 2.
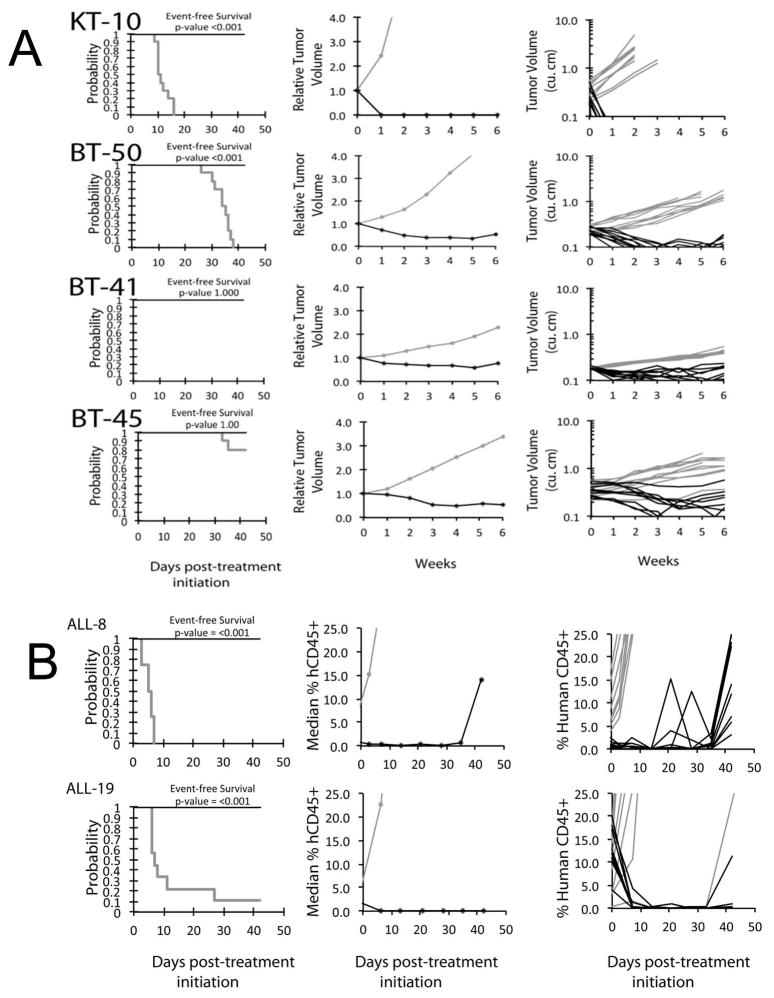
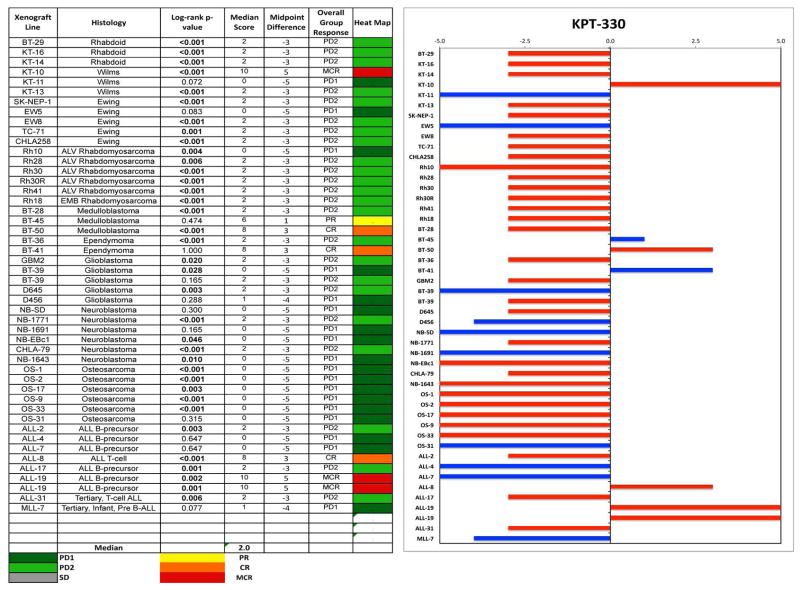
Objective responses were observed in 4 of 38 (11%) solid tumor xenografts, including a maintained complete response (MCR) for the Wilms tumor xenograft KT10, a PR and CR for the medulloblastoma xenografts BT-45 and BT-50, respectively, and a CR for the slow-growing ependymoma xenograft BT-41 (Figure 2A). As many control BT-41 and BT-45 tumors did not reach event status (4-fold increase in tumor size) during the 6 week evaluation period, the treatment groups for these models were not statistically different from controls in terms of EFS distribution. For the ALL panel, 2 of 8 (25%) xenografts achieved either CR (T-cell ALL xenograft, ALL-8) or MCR (B-precursor ALL xenograft, ALL-19) (Figure 2B). The objective response results for both solid tumor and leukemia models are represented using a ‘COMPARE’ format, based on the objective response scoring criteria centered around the midpoint score of 0 that represents stable disease, and in heat-map format (Figure 3).
Figure 2.
Selinexor in vivo objective response activity. A solid tumor and brain tumor models. KT-10 (Wilms tumor). BT-50 (medulloblastoma), BT-41 (ependymoma), and BT-45 (medulloblastoma); B ALL models, ALL-8 (T-cell), ALL-19 (B precursor): Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown. Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
Figure 3.
Left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
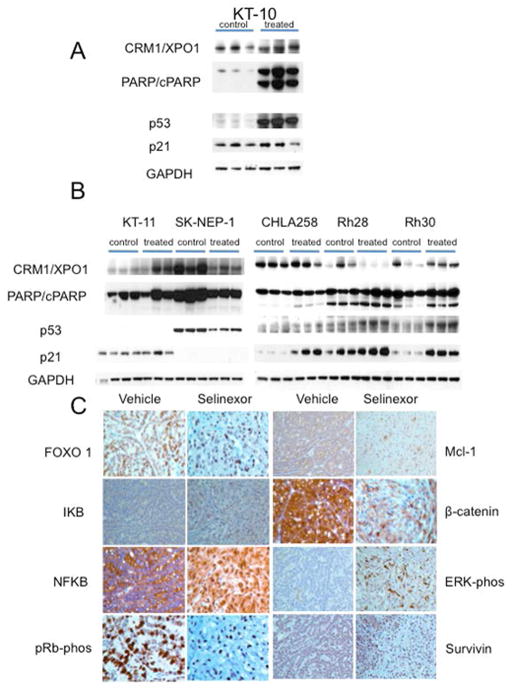
Selinexor induced very rapid regression of KT-10 Wilms tumor xenografts, but was less active against other solid tumors. To determine whether selinexor was inducing pharmacodynamics effects associated with XPO1/CRM1 inhibition (increased p53 and cleavage of PARP), KT-10 tumors were harvested 24 hours after dose 1, whereas the other tumors were harvested 2 hours post dose six. As shown in Figure 4A, selinexor induced a robust increase in p53 in KT-10 xenografts with cleavage of PARP apparent after the first dose of drug, consistent with the rapid response of this tumor to treatment. Of interest, however, was that the basal expression levels of p21 are relatively high and there was very little increase in p21 expression following selinexor treatment. In tumors that responded poorly [KT-11 (PD1) and SKNEP1, CHLA-258, Rh28, Rh30 (all PD2)] a slight increase in p53 was detected only for Rh28 and Rh30 xenografts. However, p21 was induced by selinexor in CHLA-258, Rh28 and Rh30 tumors. PARP cleavage was clearly induced by treatment in Rh30 tumors, but was only slightly increased in Rh28 and CHLA258 xenografts (Figure 4B).
Figure 4.
A. Pharmacodynamic changes induced by selinexor. A. KT-10 Wilms tumor. Tissue was harvested 24 hr after a single dose of selinexor (10 mg/kg); B. Tumors were harvested 2 hr after dose six of selinexor (10 mg/kg/dose). With the exception of SK-NEP1, all tumors are p53 wild type by sequence analysis. Three control and 3 tumors from treated mice were used for each xenograft line. GAPDH was used as a loading control. B. In vivo treatment with selinexor blocked XPO1 cargos in the nucleus and reduced the expression of signaling proteins that are associated with cell proliferation. Sections from vehicle and selinexor treated KT-10 were analyzed by immunohistochemistry (IHC). Cells from tumors that were treated with selinexor show increased nuclear accumulation of FOXO1, IKB, NFKB, pRb, ERK and Survivin. In addition the IHC slides show reduction in Mcl-1 and β-catenin.
While the Wilms tumor xenograft KT-10 responded better than other sarcomas to the cytotoxic and the cytostatic effects of selinexor, analysis of sections demonstrated effects of response in all the tumor types. Comparison of sections from all the tumors, pre- and post- treatment showed increased areas of necrosis, decreased cell number and increased exposure of tumor stromal cells. Interestingly those were very prominent in Rh28, the alveolar rhabdomyosarcoma tumor that showed poor response at the end of the 4 week in vivo study (Supplemental Figure 1).
Immunohistochemical analysis of sections from the most sensitive tumor, KT-10 (Wilms tumor), demonstrated the pharmacodynamic marker changes that were expected upon XPO1 inhibition (Figure 4C). These included the accumulation of XPO1 protein cargos in the nucleus: FOXO1, IKB, NFKB, pRb, ERK and survivin. As expected, selinexor not only induced nuclear accumulation of pRb, but also reduced its inactive form (i.e. decreased phosphorylated pRb), suggesting reduced proliferation. The treatment also induced the nuclear accumulation of phosphorylated ERK. In addition to effects on XPO1 cargos, selinexor also reduced the expression of the anti-apoptotic protein Mcl-1 and β-catenin that transduce pro survival signals and induce cell cycle promotion.
Analysis of the models that were less sensitive to the cytostatic and cytotoxic effects of selinexor (KT-11, SK-NEP-1, CHLA258, Rh28 and Rh30), showed less pronounced changes in some of the above pharmacodynamics markers for XPO1 inhibition. For example, Rh28 showed partial FOXO3 nuclear accumulation with much cytoplasmic stain. In addition it showed minimal reduction of β-catenin and almost no reduction in Mcl-1 protein (Supplemental Figure 2).
Surprisingly, analysis of the p53 nuclear localization in KT-10, the most selinexor sensitive tumor on the study did not show increased nuclear accumulation following treatment (not shown). KT-10 tumor cells express a non-clinically important p53 mutation (R290H) with wild type transcriptional activity [31], and therefore p53 would be expected to be enriched in the nucleus in the treated cells. However, p53 proteins, either wild type in CHLA258 and Rh28 tumors or mutant in Rh30 tumors were sensitive to XPO1 inhibition and showed nuclear accumulation upon selinexor treatment in these three cases (Supplemental Figure 3). This experiment suggests that p53 nuclear accumulation is a pharmacodynamic marker of XPO1 inhibition only in certain cells types.
The mutation profiles for models with objective responses for which exome sequencing data were available (KT-10, BT-50, ALL-8, and ALL-19) were examined (Supplemental Table II). All mutations were verified and assessed as somatic using a virtual normal subtraction algorithm. Recurring mutations were observed for FBXW7 and SMARCA4. For FBXW7, known oncogenic mutations were identified for both BT-50 (R465H) and ALL-8 (R465C). One additional tested xenograft (ALL-31) had an FBXW7 mutation, but it was in the N-terminal region in the nuclear localization signal domain (c.45_46insCCT; p.15_16TR>TLR). For SMARCA4, the R1189Q mutation, which is predicted to be deleterious by both SIFT and and PolyPhen, was observed in BT-45 and ALL-8. KT-10 has a PALB2 mutation that leads to defective homologous recombination and to sensitivity to PARP inhibition [32].
DISCUSSION
Similar to the natural inhibitors of XPO1 (e.g. leptomycin B), the molecularly modeled SINE selinexor has activity against cancer cells both in vitro and in vivo. However, the more specific inhibitors such as selinexor do not show the profound toxicities of the early compounds. The most common adverse events in the first phase 1 trials of selinexor in adults were thrombocytopenia, nausea, anorexia, and fatigue. Early clinical experience with selinexor has demonstrated that objective responses can be induced at doses and schedules of the agent that are generally well tolerated [23,24,33].
The PPTP in vitro results revealed a broad range of activity against pediatric cancer types. All but two of the cell lines tested had IC50 values between 13-317 nM. Relative to the entire panel, the Ewing sarcoma and rhabdoid tumor cell lines were somewhat more sensitive than the remaining cell lines, but the differences in sensitivity were not large. Across the entire panel, selinexor showed a prominent cytotoxic effect independent of TP53 mutation status, indicating the lack of dependence of the agent on an intact p53 pathway for activity.
At the 10 mg/kg thrice weekly dose selected for study, selinexor showed broad tumor growth inhibitory activity against the solid tumor xenografts, with statistically significant differences in EFS distribution in 76% of xenografts. Growth delay was most pronounced for the Ewing sarcoma xenografts and least apparent for the osteosarcoma xenografts. In terms of objective responses, these were seen in Wilms tumor (one maintained complete response), medulloblastoma, and ependymoma. The responding Wilms tumor xenograft (KT-10) is known to be defective in homologous recombination as a result of biallelic PALB2 mutation [32]. KT-10 and the responding medulloblastoma xenograft BT-45 were the two PPTP solid tumor xenografts that showed objective responses to both the PARP inhibitor talazoparib and to cisplatin [32,34]. For the ALL panel, CRs were noted for 2 of 8 models, consistent with prior preclinical work on ALL and with the clinical activity observed for selinexor against lymphoid malignancies [15,23]. It is possible that even higher levels of activity are achievable with changes to the dosing and schedule of the drug. A twice weekly schedule and caloric supplementation may allow for increased drug exposures while minimizing toxicity.
While most PPTP xenografts failed to show objective responses, several showed pronounced sensitivity to selinexor with CRs observed. These models may be considered “exceptional responders” and, analogous to reports describing exceptional clinical activity for other targeted agents, may show specific genomic lesions that lead to sensitivity [35,36]. Regarding the xenografts exhibiting objective responses, the Wilms tumor xenograft (KT-10) with the most pronounced solid tumor response is known to be defective in homologous recombination as a result of biallelic PALB2 mutation [32]. Evaluations of additional models will be required to determine whether defective homologous recombination in general (or specific to PALB2 loss of function) sensitizes to SINE compounds. The identification of mutated PALB2 in sensitive selinexor tumors is potentially more interesting in light of the recent report that SINE compounds inhibit the expression of DNA damage repair proteins [37]. Finding of activity or minimal activity of selinexor across many sarcoma tumor types together with this connection to DNA damage repair suggest that combination treatment of DNA damage inducing agents with selinexor could potentially be effective treatment in sarcoma tumors, an hypothesis that warrants further preclinical testing.
The only two models tested that harbor FBXW7 “hot spot” mutations showed objective responses. These hot spot mutations occur in the WD40 substrate-binding domain of FBXW7 [38]. FBXW7 encodes three transcripts (α, β, and γ) that result from alternative splicing, with the transcripts showing distinctive cellular localization [39]. The FBXW7α isoform localizes to the nucleus and is responsible for targeting a number of growth/survival promoting factors for degradation [38,40,41]. FBXW7 with hot spot mutations acts in a dominant-negative fashion, such that when mutations are heterozygous (as for BT-50 and ALL-8) they exert a marked effect on levels of FBXW7 target proteins as a result of their inhibition of wildtype FBXW7 in heterodimers [38]. SINE compounds have been shown to enhance nuclear localization of FBXW7 in pancreatic cancer cells [42], and it is plausible that in xenografts with FBXW7 hot spot mutations they are able to elevate the level of active wildtype homodimers sufficiently so that target oncogenic proteins are degraded leading to anticancer activity. ALL-31, which did not respond to selinexor, also harbors an FBXW7 genomic alteration: a 3-base insertion in the N-terminal region that is known to disrupt the nuclear localization signal of FBXW7 and that has previously been reported in prostate cancer and Wilms tumor [38,43]. A consequence of this mutation is the cytoplasmic, rather than nuclear, localization of FBXW7α [38,44]. Hence, the cellular effects and response to SINE compounds for cancer cells with this mutation are likely distinctive from the effects observed for cells with FBXW7 mutations in the WD40 substrate-binding domain. Further testing will be required to define the relationship between FBXW7 hot spot mutations and sensitivity to SINE compounds.
A second gene that was recurringly mutated among responsive xenografts was SMARCA4, a gene that encodes an ATP-dependent catalytic subunit of SWI-SNF complexes [45]. SMARCA4 mutations were present in a medulloblastoma xenograft (BT-45) and in a T-cell ALL xenograft (ALL-8). Germline mutations in SMARCA4 predispose to rhabdoid tumor and to small cell carcinoma of the ovary, hypercalcemic type (malignant rhabdoid tumor of the ovary) [45]. Somatic mutations of SMARCA4 have been reported at relatively low frequency in a number of cancers, including medulloblastoma, mantle cell lymphoma, Burkitt lymphoma, T-cell ALL, and others [45–48].
Pharmacodynamic changes induced by selinexor were examined in six xenografts including the KT-10 Wilms tumor that showed rapid regression. Pharmacodynamic effects were documented using the 10 mg/kg dose, with protein levels of p53 and p21 increased in some, but not all, tumors; similarly, PARP cleavage was induced in three of the studied tumors. This varied response underlines the broad range of effects that result from nuclear export inhibition. Given the number of proteins shuttled out of the nucleus by XPO1, it is reasonable to speculate that multiple pathways will be up- or down-regulated by selinexor. While nuclear localization of XPO1 cargos are good pharmacodynamic markers, demonstrating drug bioavailability, some of these markers may serve as markers of response. In the most sensitive KT-10 tumors, FOXO1, IKB, NFKB, pRb, ERK and survivin were locked in the nuclei of selinexor treated tumor cells. This included phosphorylated ERK, which is associated with growth suppression signals [49]. Some of these markers revealed the cytostatic and cytotoxic effects of selinexor, showing reduction in phosphorylated pRb and decrease of the anti-apoptotic protein Mcl-1 as well as the proto-oncogene β-catenin. Interestingly, selinexor non-sensitive sarcoma tumors showed less significant changes in these pharmacodynamics markers. Since these observations are seen one to two weeks after treatment initiation, they may potentially serve as early predictive markers of response. Unlike the above markers, p53 nuclear localization serves as pharmacodynamic marker only in some sarcomas.
As with other agents, the ability for these murine data to translate accurately to the clinic depends upon drug exposures being similar in the models at doses causing tumor regressions to exposures achieved in patients. Mice in our experiments were dosed with an oral suspension of 10 mg/kg selinexor (KPT-330) three times a week. At this dose and regimen, selinexor was shown to be efficacious and well tolerated in various preclinical cancer models including those reported in this manuscript. With a single oral administration of selinexor at 10 mg/kg (30 mg/m2) in CD1 mouse, an AUCinf of 9530 hr*ng/mL and a Cmax of 1,240 ng/mL (~2.8 μM) were observed (Karyopharm Therapeutics, unpublished data). Thus, the exposure to drug in CD1 mice is similar to that determined in patients treated with 65 mg/m2 [50]. Poor response of a certain xenograft models in this study to selinexor is probably not because of poor drug penetration, but more likely due to the genetic background of the specific cancer models, as discussed above. Evidence for this is the nuclear localization of either p53 or other XPO1 cargos observed in all the tumors following selinexor treatment, irrespective of response. This nuclear localization is a good marker for drug exposure. Thus, as with other targeted therapies, inhibition of ‘target’ does not necessarily correlate with cellular outcome. MDM2 inhibitors [51] or kinase inhibitors being other examples [52–54].
These data support the further development of selinexor for pediatric malignancies. Defining the relationship between selinexor systemic exposures in mice and humans will be important in better assessing the clinical relevance of these results. The first pediatric phase 1 trial for lymphoid malignancies is open (NCT02091245), and a phase 1 trial for children with solid tumors is under development. Additional data from adult clinical trials and further preclinical testing are needed to clearly define a responder hypothesis and to discover rational drug combinations.
Supplementary Material
Supplemental Figure 1. Cytostatic and cytotoxic effects induced by selinexor. Sections from vehicle and selinexor treated KT-10 and KT-11 (Wilms tumors), SK-NEP-1 and CHLA258 (Ewing tumors), Rh28 and Rh30 (Alveolar RMS tumors) xenografts were stained by H&E. All treated tumors, show different levels of decrease number of cells, areas of necrosis and areas of exposed stroma.
Supplemental Figure 2. Effects of selinexor on nuclear localization of XPO1 cargos and expression of cell signaling proteins are weaker in tumors proven to show poor treatment response. Sections from vehicle and selinexor treated Rh28 were analyzed by IHC for the expression of FOXO1, β-catenin and Mcl-1.
Supplemental Figure 3. In vivo treatment with selinexor induces nuclear p53 accumulation also in tumors that respond poorly to selinexor. Sections from vehicle and selinexor treated Rh28, Rh30 and CHLA258 were analyzed by IHC. Cells from tumors that were treated with selinexor show increased nuclear accumulation of p53.
Efficacy of Selinixor (KPT-330) against PPTP Xenograft Models
Verified mutations in responding xenografts.
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used selinexor supplied by Karyopharm Therapeutics. In addition to the authors this paper represents work contributed by the following: Sherry Ansher, Catherine A. Billups, Jennifer Richmond, Joshua Courtright, Kathryn Evans, Edward Favours, Henry S. Friedman, Danuta Gasinski, Nicholas Pettit, Melissa Sammons, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and the Sydney Children’s Hospitals Network.
Footnotes
Conflict of interest statement: Drs Yosef Landesman, and Sharon Shacham are employees of Karyopharm Therapeutics Inc. The other authors consider that there are no actual or perceived conflicts of interest.
References
- 1.Mosammaparast N, Pemberton LF. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends in cell biology. 2004;14(10):547–556. doi: 10.1016/j.tcb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Seminars in cancer biology. 2014;27C:74–86. doi: 10.1016/j.semcancer.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clinical and investigative medicine Medecine clinique et experimentale. 2009;32(6):E315. [PubMed] [Google Scholar]
- 4.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65(1):153–159. doi: 10.1227/01.NEU.0000348550.47441.4B. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- 5.van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, Birrer MJ, Leaner VD. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. International journal of cancer Journal international du cancer. 2009;124(8):1829–1840. doi: 10.1002/ijc.24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, Sun YJ, Tang LN, Zheng SE. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncology reports. 2009;21(1):229–235. [PubMed] [Google Scholar]
- 7.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Carrasco YP, Hu Y, Guo X, Mirzaei H, Macmillan J, Chook YM. Nuclear export inhibition through covalent conjugation and hydrolysis of Leptomycin B by CRM1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(4):1303–1308. doi: 10.1073/pnas.1217203110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chemistry & biology. 1997;4(2):139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts BJ, Hamelehle KL, Sebolt JS, Leopold WR. In vivo and in vitro anticancer activity of the structurally novel and highly potent antibiotic CI-940 and its hydroxy analog (PD 114,721) Cancer chemotherapy and pharmacology. 1986;16(2):95–101. doi: 10.1007/BF00256156. [DOI] [PubMed] [Google Scholar]
- 11.Leopold WR, Shillis JL, Mertus AE, Nelson JM, Roberts BJ, Jackson RC. Anticancer activity of the structurally novel antibiotic Cl-920 and its analogues. Cancer research. 1984;44(5):1928–1932. [PubMed] [Google Scholar]
- 12.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. British journal of cancer. 1996;74(4):648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neggers JE, Vercruysse T, Jacquemyn M, Vanstreels E, Baloglu E, Shacham S, Crochiere M, Landesman Y, Daelemans D. Identifying Drug-Target Selectivity of Small-Molecule CRM1/XPO1 Inhibitors by CRISPR/Cas9 Genome Editing. Chemistry & biology. 2015;22(1):107–116. doi: 10.1016/j.chembiol.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Kalid O, Toledo Warshaviak D, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. Journal of computer-aided molecular design. 2012;26(11):1217–1228. doi: 10.1007/s10822-012-9611-9. [DOI] [PubMed] [Google Scholar]
- 15.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, Christie AL, McCauley D, Rodig SJ, Kauffman M, Shacham S, Stone R, Letai A, Kung AL, Thomas Look A. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. British journal of haematology. 2013;161(1):117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, Walker A, Klisovic R, Blum W, Caligiuri M, Croce CM, Marcucci G, Garzon R. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120(9):1765–1773. doi: 10.1182/blood-2012-04-423160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA, Senapedis W, Saint-Martin JR, Kashyap T, Shacham S, Kauffman M, Gu Y, Wu L, Ghobrial I, Zhan F, Kung AL, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155–165. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, Tiedemann RE, Palmer SE, Garbitt VM, McCauley D, Kauffman M, Shacham S, Chesi M, Bergsagel PL, Stewart AK. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013;27(12):2357–2365. doi: 10.1038/leu.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, Wang M, Tamayo AT, Shacham S, Kauffman M, Lee J, Zhang L, Ou Z, Li C, Sun L, Ford RJ, Pham LV. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Experimental hematology. 2013;41(1):67–78. e64. doi: 10.1016/j.exphem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Azmi AS, Al-Katib A, Aboukameel A, McCauley D, Kauffman M, Shacham S, Mohammad RM. Selective inhibitors of nuclear export for the treatment of non-Hodgkin’s lymphomas. Haematologica. 2013;98(7):1098–1106. doi: 10.3324/haematol.2012.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, Wilson H, Jensen K, Ito D, Modiano JF, Bear MD, Pennell ML, Saint-Martin JR, McCauley D, Kauffman M, Shacham S. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PloS one. 2014;9(2):e87585. doi: 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London CA, Bernabe LF, Barnard S, Kisserberth W, Borgatti A, Henson M, Wilson-Robles H, Jensen K, Ito D, Modiano JF, Bear MD, Pennell ML, Saint-Martin JR, McCauley D, Kauffman M, Shacham S. Evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 (verdinexor) in spontaneous canine cancer: Results of phase I and phase II clinical trials. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5–9; San Diego, CA. Philadelphia (PA): AACR; 2014. p. Abstr #3809. [Google Scholar]
- 23.Gutierrez M, Goy A, Byrd JC, Flynn JM, Sorensen M, Brown P, Gabrail NY, Savona M, Flinn I, Baz RC, Shah BD, Stone RM, Jacobsen E, Kukreti V, Tiedemann RE, Rashal T, Mirza MR, Shacham S, Kauffman M, Kuruvilla J. A phase 1 dose-escalation study of the oral selective inhibitor of nuclear export (SINE) KPT-330 (selinexor) in patients (pts) with heavily pretreated non-Hodgkin lymphoma (NHL) J Clin Oncol. 2014;32(5s suppl):abstr 8518. [Google Scholar]
- 24.Mau-Soerensen M, Razak AR, Shields AF, Gabrail NY, Gerecitano JF, Shacham S, Lassen UN, Rashal T, Cooksey J, Landesman Y, Pond GR, Oza AM, Kauffman M, Siu LL, Bedard PL, Mahaseth H, Mirza MR, Mahipal A. Safety and antitumor activity of selinexor (KPT-330), a first-in-class, oral XPO1 selective inhibitor of nuclear export: A phase I study expanded with colon cancer cohort. J Clin Oncol. 2014;32(5s suppl):abstr 2537. [Google Scholar]
- 25.Kuruvilla J, Byrd JC, Flynn JM, Garzon R, Porcu P, Wagner-Johnston ND, Savoie ML, Stone RM, Jacobsen ED, Mau-Sorensen M, Brown P, Baz RC, Shal B, Flinn I, Gabrail NY, Kukreti V, Tiedemann RE, Landesman Y, Klebanov B, Schacham E, et al. The Oral Selective Inhibitor of Nuclear Export (SINE) Selinexor (KPT-330) Demonstrates Broad and Durable Clinical Activity in Relapsed / Refractory Non Hodgkin’s Lymphoma (NHL) Blood (ASH Annual Meeting Abstracts) 2014:Abstr #396. [Google Scholar]
- 26.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 27.Kang MH, Smith MA, Morton CL, Keshelava N, Houghton PJ, Reynolds CP. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2011;56(2):239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, Liu T, Billups CA, Khan J, Ansher S, Zhang J, Smith MA. The Pediatric Preclinical Testing Program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 29.Liem NL, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD, MacKenzie KL, Lock RB. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 30.Carol H, Gorlick R, Kolb EA, Morton CL, Manesh DM, Keir ST, Reynolds CP, Kang MH, Maris JM, Wozniak A, Hickson I, Lyalin D, Kurmasheva RT, Houghton PJ, Smith MA, Lock R. Initial testing (stage 1) of the histone deacetylase inhibitor, quisinostat (JNJ-26481585), by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2014;61:245–252. doi: 10.1002/pbc.24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soussi T, Leroy B, Taschner PE. Recommendations for analyzing and reporting TP53 gene variants in the high-throughput sequencing era. Human mutation. 2014;35(6):766–778. doi: 10.1002/humu.22561. [DOI] [PubMed] [Google Scholar]
- 32.Smith MA, Hampton OA, Reynolds CP, Kang MH, Maris JM, Gorlick R, Kolb EA, Lock RB, Carol H, Keir ST, Wu J, Kurmasheva RT, Wheeler DA, Houghton PJ. Initial testing (Stage 1) of the PARP inhibitor BMN 673 by the Pediatric Preclinical Testing Program: PALB2 mutation predicts exceptional in vivo response to BMN 673. Pediatr Blood Cancer. 2015;62:91–98. doi: 10.1002/pbc.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martignetti J, Razak AR, Chen Y, Gabrail NY, Gerecitano JF, Camacho C, Pereira E, Dottino PR, Shacham S, McCauley D, Rashal T, Saint-Martin JR, Shacham E, Vincett D, Kauffman M, Mirza MR, Sorensen M. Preclinical and early clinical activity of the oral selective inhibitor of nuclear export (SINE) exportin 1 (XPO1) antagonist KPT-330 (Selinexor) in patients (pts) with platinum-resistant/refractory ovarian cancer (OvCa) J Clin Oncol. 2014;32(5s suppl):abstr 5522. [Google Scholar]
- 34.Tajbakhsh M, Houghton PJ, Morton CL, Kolb EA, Gorlick R, Maris JM, Keir ST, Wu J, Reynolds CP, Smith MA, Lock RB. Initial testing of cisplatin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(5):992–1000. doi: 10.1002/pbc.21263. [DOI] [PubMed] [Google Scholar]
- 35.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, Pirun M, Sander C, Socci ND, Ostrovnaya I, Viale A, Heguy A, Peng L, Chan TA, Bochner B, Bajorin DF, Berger MF, Taylor BS, Solit DB. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagle N, Grabiner BC, Van Allen EM, Hodis E, Jacobus S, Supko JG, Stewart M, Choueiri TK, Gandhi L, Cleary JM, Elfiky AA, Taplin ME, Stack EC, Signoretti S, Loda M, Shapiro GI, Sabatini DM, Lander ES, Gabriel SB, Kantoff PW, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer discovery. 2014;4(5):546–553. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashyap T, Crochiere M, Friedlander S, Klebanov B, Senadepis W, Baloglu E, del Alamo D, Tamir S, Rashal T, McCauley D, Carlson R, Kauffman M, Shacham S, Landesman Y. Selective inhibitors of nuclear export (SINE) block the expression of DNA damage repair proteins and sensitize cancer cells to DNA damage therapeutic agents. Eur J Cancer. 2014;50 (Suppl 6):83, Abstr #247. [Google Scholar]
- 38.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer research. 2007;67(19):9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 39.Spruck CH, Strohmaier H, Sangfelt O, Muller HM, Hubalek M, Muller-Holzner E, Marth C, Widschwendter M, Reed SI. hCDC4 gene mutations in endometrial cancer. Cancer research. 2002;62(16):4535–4539. [PubMed] [Google Scholar]
- 40.Busino L, Millman SE, Pagano M. SCF-mediated degradation of p100 (NF-kappaB2): mechanisms and relevance in multiple myeloma. Science signaling. 2012;5(253):pt14. doi: 10.1126/scisignal.2003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nature reviews Cancer. 2014;14(4):233–247. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao J, Azmi AS, Aboukameel A, Kauffman M, Shacham S, Abou-Samra AB, Mohammad RM. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget. 2014;5(11):3444–3454. doi: 10.18632/oncotarget.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams RD, Al-Saadi R, Chagtai T, Popov S, Messahel B, Sebire N, Gessler M, Wegert J, Graf N, Leuschner I, Hubank M, Jones C, Vujanic G, Pritchard-Jones K. Subtype-specific FBXW7 mutation and MYCN copy number gain in Wilms’ tumor. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(7):2036–2045. doi: 10.1158/1078-0432.CCR-09-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durgan J, Parker PJ. Regulation of the tumour suppressor Fbw7alpha by PKC-dependent phosphorylation and cancer-associated mutations. The Biochemical journal. 2010;432(1):77–87. doi: 10.1042/BJ20100799. [DOI] [PubMed] [Google Scholar]
- 45.Biegel JA, Busse TM, Weissman BE. SWI/SNF chromatin remodeling complexes and cancer. American journal of medical genetics Part C, Seminars in medical genetics. 2014;166C(3):350–366. doi: 10.1002/ajmg.c.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huether R, Dong L, Chen X, Wu G, Parker M, Wei L, Ma J, Edmonson MN, Hedlund EK, Rusch MC, Shurtleff SA, Mulder HL, Boggs K, Vadordaria B, Cheng J, Yergeau D, Song G, Becksfort J, Lemmon G, Weber C, et al. The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nature communications. 2014;5:3630. doi: 10.1038/ncomms4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, Lugar PL, Rizzieri DA, Lagoo AS, Bernal-Mizrachi L, Mann KP, Flowers CR, Naresh KN, Evens AM, Chadburn A, Gordon LI, et al. The genetic landscape of mutations in Burkitt lymphoma. Nature genetics. 2012;44(12):1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Jima D, Moffitt AB, Liu Q, Czader M, Hsi ED, Fedoriw Y, Dunphy CH, Richards KL, Gill JI, Sun Z, Love C, Scotland P, Lock E, Levy S, Hsu DS, Dunson D, Dave SS. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood. 2014;123(19):2988–2996. doi: 10.1182/blood-2013-07-517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pathria G, Wagner C, Wagner SN. Inhibition of CRM1-mediated nucleocytoplasmic transport: triggering human melanoma cell apoptosis by perturbing multiple cellular pathways. The Journal of investigative dermatology. 2012;132(12):2780–2790. doi: 10.1038/jid.2012.233. [DOI] [PubMed] [Google Scholar]
- 50.Crochiere M, Klebanov B, Baloglu E, Kalid O, Kashyap T, Senapedis W, del Alamo D, Tamir S, McCauley D, Carlson R, Kauffman M, Shachum S, Landesman J. Quantification of Exportin-1 (XPO1) Occupancy by Selective Inhibitor of Nuclear Export/SINE Compounds. Proc EORTCNCIAACR. 2014;26:064. [Google Scholar]
- 51.Carol H, Reynolds CP, Kang MH, Keir ST, Maris JM, Gorlick R, Kolb EA, Billups CA, Geier B, Kurmasheva RT, Houghton PJ, Smith MA, Lock RB. Initial testing of the MDM2 inhibitor RG7112 by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2013;60(4):633–641. doi: 10.1002/pbc.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Neale G, Keir ST, Carol H, Lock R, Phelps D, Kang MH, Reynolds CP, Maris JM, Billups C, Smith MA. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55(4):668–677. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houghton PJ, Gorlick R, Kolb EA, Lock R, Carol H, Morton CL, Keir ST, Reynolds CP, Kang MH, Phelps D, Maris JM, Billups C, Smith MA. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;58(2):191–199. doi: 10.1002/pbc.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorlick R, Kolb EA, Houghton PJ, Morton CL, Phelps D, Schaiquevich P, Stewart C, Keir ST, Lock R, Carol H, Reynolds CP, Maris JM, Wu J, Smith MA. Initial testing (stage 1) of lapatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53(4):594–598. doi: 10.1002/pbc.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cytostatic and cytotoxic effects induced by selinexor. Sections from vehicle and selinexor treated KT-10 and KT-11 (Wilms tumors), SK-NEP-1 and CHLA258 (Ewing tumors), Rh28 and Rh30 (Alveolar RMS tumors) xenografts were stained by H&E. All treated tumors, show different levels of decrease number of cells, areas of necrosis and areas of exposed stroma.
Supplemental Figure 2. Effects of selinexor on nuclear localization of XPO1 cargos and expression of cell signaling proteins are weaker in tumors proven to show poor treatment response. Sections from vehicle and selinexor treated Rh28 were analyzed by IHC for the expression of FOXO1, β-catenin and Mcl-1.
Supplemental Figure 3. In vivo treatment with selinexor induces nuclear p53 accumulation also in tumors that respond poorly to selinexor. Sections from vehicle and selinexor treated Rh28, Rh30 and CHLA258 were analyzed by IHC. Cells from tumors that were treated with selinexor show increased nuclear accumulation of p53.
Efficacy of Selinixor (KPT-330) against PPTP Xenograft Models
Verified mutations in responding xenografts.