Abstract
Brain-derived neurotrophic factor (BDNF) is important for neuronal survival and regeneration. We investigated the diagnostic and prognostic values of serum BDNF in traumatic brain injury (TBI). We examined serum BDNF in two independent cohorts of TBI cases presenting to the emergency departments (EDs) of the Johns Hopkins Hospital (JHH; n = 76) and San Francisco General Hospital (SFGH, n = 80), and a control group of JHH ED patients without TBI (n = 150). Findings were subsequently validated in the prospective, multi-center Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) Pilot study (n = 159). We investigated the association between BDNF, glial fibrillary acidic protein (GFAP), and ubiquitin C-terminal hydrolase-L1 (UCH-L1) and recovery from TBI at 6 months in the TRACK-TBI Pilot cohort. Incomplete recovery was defined as having either post-concussive syndrome or a Glasgow Outcome Scale Extended score <8 at 6 months. Median day-of-injury BDNF concentrations (ng/mL) were lower among TBI cases (JHH TBI, 17.5 and SFGH TBI, 13.8) than in JHH controls (60.3; p = 0.0001). Among TRACK-TBI Pilot subjects, median BDNF concentrations (ng/mL) were higher in mild (8.3) than in moderate (4.3) or severe TBI (4.0; p = 0.004. In the TRACK-TBI cohort, the 75 (71.4%) subjects with very low BDNF values (i.e., <the 1st percentile for non-TBI controls, <14.2 ng/mL) had higher odds of incomplete recovery than those who did not have very low values (odds ratio, 4.0; 95% confidence interval [CI]: 1.5-11.0). The area under the receiver operator curve for discriminating complete and incomplete recovery was 0.65 (95% CI: 0.52-0.78) for BDNF, 0.61 (95% CI: 0.49-0.73) for GFAP, and 0.55 (95% CI: 0.43-0.66) for UCH-L1. The addition of GFAP/UCH-L1 to BDNF did not improve outcome prediction significantly. Day-of-injury serum BDNF is associated with TBI diagnosis and also provides 6-month prognostic information regarding recovery from TBI. Thus, day-of-injury BDNF values may aid in TBI risk stratification.
Key words: : biomarkers, brain-derived neurotrophic factor, glial fibrillary acidic protein, traumatic brain injury, ubiquitin C-terminal hydrolase-L1
Introduction
Diagnosis of traumatic brain injury (TBI) and early identification of patients at risk for long-term consequences of TBI represents a unique clinical challenge with major public health implications. A number of candidate circulating TBI biomarkers have shown promise for aiding in the diagnosis of TBI and in identifying patients with traumatic abnormalities on head computed tomography (CT) scan.1–4 Importantly, their ability to predict adverse consequences of TBI has been limited. Objective diagnosis and prognosis of TBI will help improve triaging to appropriate medical care at time of injury, guide judicious use of neuroimaging, and inform the development of “return to work or play” guidelines. Additionally, while most patients with mild TBI (mTBI) recover to their pre-injury state within 3 months, a significant minority do not. Prognostic biomarkers that identify patients unlikely to make a full recovery are needed to identify appropriate candidates for clinical trials of novel TBI therapies.5
Brain-derived neurotrophic factor (BDNF), a member of the family of neurotrophic proteins, is a secreted autocrine factor that promotes the development, maintenance, survival, differentiation, and regeneration of neurons.6,7 It is also important for synaptic plasticity and memory processing.8,9 BDNF has been implicated in reducing secondary brain injury, with elevations providing neuroprotection and restoring connectivity after TBI.10–12 However, the diagnostic and prognostic significance of day-of-injury circulating BDNF concentration are not well understood. We conducted a study to establish the association between BDNF and TBI and to determine whether day-of-injury BDNF values are associated with TBI severity and outcomes.
Glial fibrillary acidic protein (GFAP) is an astrocytic protein whose functions include cell communication, mitosis, and maintaining the integrity of the blood–brain barrier (BBB).13 GFAP has excellent specificity for TBI-associated intracranial hemorrhage and focal mass lesions.14,15 Elevated values are associated with increased mortality.1,16
Ubiquitin C-terminal hydrolase-L1 (UCH-L1) is a neuronal protein that is involved in the addition and removal of ubiquitin proteins flagged for metabolism. UCH-L1 is especially elevated in TBI and has been found to be associated with mortality.17–19
Methods
BDNF serum concentrations were determined in duplicate in two independent cohorts of TBI cases presenting to the Johns Hopkins Hospital (JHH) and the San Francisco General Hospital (SFGH) emergency departments (ED), and one control cohort of JHH ED patients presenting for non-TBI complaints. Findings were subsequently validated in the prospective, multi-center Transforming Research and Clinical Knowledge in TBI Pilot (TRACK-TBI) Pilot study.20 We also compared the prognostic value of BDNF to that of two well-studied TBI biomarkers, GFAP and UCH-L1, since these biomarker values were available from a previous study.18 Study protocols were approved by the institutional review boards at participating sites.
Study population
Case cohorts
JHH and SFGH are academic, tertiary care, Level 1 trauma institutions. Patients were eligible for inclusion as TBI cases if they presented to JHH and SFGH ED after experiencing acute blunt head trauma and met the following criteria: age 18 years or older; presented within 24 h of injury; met the American College of Emergency Physicians (ACEP) criteria for obtaining head CT scans in TBI21; received a non-contrast head CT scan as part of their clinical evaluation; and had excess serum sample available in the clinical chemistry lab. Cases met the definition of TBI proposed by the Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements (TBI CDE) for Research on Traumatic Brain Injury and Psychological Health.22 Eligible cases were excluded if they had one of the following prior medical conditions: demyelinating disease, neurodegenerative disease, dementia, stroke, brain tumor, intracranial surgery, or active cancer. TBI cases were selected between November 2012 and September 2013. Since we utilized excess clinical blood samples, informed consent was waived.
Control cohort
Patients included as control subjects were JHH ED patients who were evaluated for suspected acute coronary syndrome,23 had no blunt head trauma in the preceding 7 days, and were deemed to have a non-cardiac condition and discharged home from the ED. Eligible control subjects were excluded if they met any of the exclusion criteria for cases (see above). Control subjects did not receive head CT scans since there was no clinical indication for doing so. Clinical and demographic data were collected via structured patient interviews and a review of the electronic medical record. Subjects were enrolled between January 2012 and February 2013. Written informed consent was obtained from all subjects.
Validation cohort
The TRACK-TBI Pilot study enrolled subjects 16 years and older who presented to SFGH ED, the University of Pittsburgh Medical Center (UPMC) ED, and the University Medical Center Brackenridge (UMCB), Austin, TX, ED with TBI.20 Patients were included in the study if they presented to the ED within 24 h of acute blunt force head trauma and met the ACEP criteria for obtaining a head CT in TBI, as previously described.20 Only subjects from TRACK-TBI Pilot who had serum samples available for testing were included in the present study. Subjects in the validation cohort were enrolled from April 2010 to June 2011, and were distinct from those in the SFGH case cohort. Written informed consent was obtained from all subjects prior to enrollment in the study. Subjects unable to provide consent due to their injury were consented through their legally authorized representative at time of injury, and re-consented if cognitively able during their inpatient stay and/or their follow-up assessment time-point.
Serum sample collection and biomarker measurement
For the JHH and SFGH TBI case cohorts, excess serum samples stored in a 4°C refrigerator were retrieved from their respective clinical chemistry laboratory and stored in a − 80°C freezer. These samples were kept at 4°C for variable duration (median of 5 days). Serum samples for JHH control subjects and for TRACK-TBI Pilot subjects were collected, processed and stored in a − 80°C freezer within 2 h of collection, as previously described.23,24 Samples for TRACK-TBI patients were collected within 24 h of injury.13
Samples were randomized and BDNF assayed in batches with an electrochemiluminescent sandwich immunoassay and read with a Sector Imager 2400 (Meso Scale Discovery, Rockville, MD). BDNF assay capture (MAB848) and detection antibodies (MAB648) and assay standard (248BD005) were obtained from R&D Systems (Duoset reagents, Cat. # DY248; Minneapolis, MN). Assays were performed within a single laboratory by staff blinded to clinical outcomes or study cohort. Samples from the different cohorts were shipped to this single academic laboratory. The assay lower limit of detection (LOD) was 0.0125 ng/mL and the lower limit of quantification was 0.5 ng/mL. As specified by the manufacturer, these assay reagents have no overlap with the TrK receptor proteins B-NGF, GDNF, NT-3, and NT-4. Assays were performed in duplicate. A previous study examining the stability of BDNF in blood samples stored at room temperature for 0-24 h, 24-48 h, 48-72 h, or > 72 h revealed an average increase of 1.67 (95% CI: 1.08-2.26) ng/mL per each 24-h period.25 Since BDNF values are high in healthy subjects and low in diseased subjects, we defined low BDNF values as values that are lower than the 1st percentile in JHH non-TBI control subjects. This is analogous to the use of the 99th percentile as the recommended cut-off value in cardiac biomarkers26,27 (values are high in diseased and low in healthy subjects).
GFAP and UCH-L1 were previously measured in TRACK-TBI Pilot in a single laboratory (Banyan Biomarkers, Alachua, FL).15,18 The LOD of GFAP and UCH-L1 were 0.1 ng/mL and 0.03 ng/mL, respectively.
Outcomes
All patients enrolled in TRACK-TBI Pilot received head CT scans at the time of presentation to the ED. Each head CT was de-identified and read by a blinded board-certified neuroradiologist following the recommendations of the TBI-CDE Neuroimaging Working Group.28 Our primary outcome, incomplete recovery at 6 months, was defined as a composite outcome of either post-concussive syndrome (PCS) or Glasgow Outcome Scale Extended (GOSE) score of <8 at 6 months, as these two measures together encompass a wider spectrum of the entire sphere of post-TBI outcomes. We defined PCS as having three or more symptoms on the 6-month Rivermead Post-Concussion Questionnaire29 that were rated as worse than before the injury (score of 2).30 The GOSE categorizes recovery after TBI on a scale of 1–8, where 1 = dead and 8 = upper good recovery. GOSE < 8 signifies incomplete recovery.31 Additionally, head CT findings were classified as traumatic lesion present (this does not include isolated skull fractures) or no traumatic lesion present. TBI severity was classified as mild, moderate, or severe based on the Department of Defense/Department of Veterans Affairs definition (Table 1).32
Table 1.
The Department of Defense/Department of Veterans Affairs Classification of TBI Severity
| Criteria | Mild | Moderate | Severe |
|---|---|---|---|
| Head CT/MRI | Normal | Normal/ abnormal | Normal/ abnormal |
| Loss of consciousness | 0-30 min | > 30 min and < 24 h | > 24 h |
| Alteration of consciousness/mental state | < 24 h | > 24 h | > 24 h |
| Post-traumatic amnesia | < 1 day | > 1 and < 7 days | > 7 days |
| Best Glasgow Coma Scale score within first 24 h | 13–15 | 9–12 | <9 |
TBI, traumatic brain injury; CT, computed tomography; MRI, magnetic resonance imaging.
Statistical analyses
Clinical and demographic data were summarized with descriptive statistics and differences were examined using the Mann-Whitney test (2-groups), the Kruskal-Wallis test (n-groups) and the χ2 test (proportions). We quantified the discriminative ability of BDNF to distinguish between cases and controls, and to distinguish between TBI patients with relevant clinical outcomes and those without using area under the receiver operator curve (AUC). We also constructed logistic regression models to evaluate the association between BDNF values and clinical outcomes. We compared the AUCs of combinations of BDNF, GFAP, and UCH-L1 for discriminating between relevant clinical outcomes, using the method suggested by DeLong and colleagues.33 This is a widely cited and generally accepted method that provides the confidence interval and standard error of the difference between two (or more) correlated AUCs.
To understand the determinants of BDNF in the control population, we constructed univariable and multi-variable linear regression models. Variables included in the models (age, gender, race, blood pressure, history of hypertension, history of depression or schizophrenia)34–37 were selected based on an a priori literature review. A two-tailed p value of < 0.05 was considered statistically significant. Statistical analyses were performed using STATA/MP statistical software version 11.2 (StataCorp, College Station, TX), and RStudio statistical software version 0.97.312 (Boston, MA).
Results
A total of 311 TBI cases were analyzed: 76 cases in the JHH TBI cohort, 76 cases in the SFGH TBI cohort, and 159 cases in TRACK-TBI Pilot, in addition to 150 JHH non-trauma control subjects. Non-trauma control subjects were older and more likely to be female or African-American, compared with TBI cases (Table 2).
Table 2.
Demographic and Clinical Characteristics of Study Population
| JHH non-TBI controls n = 150 | JHH TBI cases n = 76 | SFGH TBI cases n = 76 | TRACK-TBI pilot cases n = 159 | p value | |
|---|---|---|---|---|---|
| Median age in years (IQR) | 54 (47 – 62) | 47 (30 – 56) | 42 (26 – 56) | 41 (25 – 56) | <0.001 |
| Female (%) | 79 (52.7) | 29 (38.2) | 22 (29.0) | 45 (28.3) | <0.001 |
| Race (%) | <0.001 | ||||
| • African-American | 116 (77.3) | 41 (54.0) | 5 (6.6) | 15 (9.5) | |
| • White | 30 (20.0) | 25 (32.9) | 59 (77.6) | 132 (83.5) | |
| • Other | 4 (2.7) | 10 (13.2) | 12 (15.8) | 11 (7.0) | |
| Mechanism of injury (%) | 0.003 | ||||
| • Assault | 19 (25.0) | 13 (17.1) | 23 (14.6) | ||
| • Fall | 26 (34.2) | 23 (30.3) | 50 (31.6) | ||
| • MVC | 21 (27.6) | 11 (14.5) | 51 (32.3) | ||
| • Pedestrian struck | 4 (5.3) | 14 (18.4) | 9 (5.7) | ||
| • Struck by/against | 3 (4.0) | 2 (2.6) | 5 (3.2) | ||
| • Other trauma | 3 (4.0) | 13 (17.1) | 20 (12.7) | ||
| Glasgow Coma Scale (%) | 0.09 | ||||
| • 3-8 | 5 (6.6) | 4 (5.4) | 19 (12.0) | ||
| • 9-12 | 3 (4.0) | 4 (5.4) | 6 (3.8) | ||
| • 13 | 2 (2.6) | 3 (4.0) | 1 (0.6) | ||
| • 14 | 11 (14.5) | 20 (27.0) | 22 (13.8) | ||
| • 15 | 55 (72.4) | 43 (58.1) | 111 (69.8) | ||
| Traumatic intracranial abnormality on head CT (%) | 21 (27.6) | 24 (31.6) | 75 (47.2) | 0.006 |
JHH, Johns Hopkins Hospital; TBI, traumatic brain injury; SFGH, San Francisco General Hospital; TRACK-TBI, Transforming Research and Clinical Knowledge in TBI study; IQR, interquartile range; MVC, motor vehicle collision ; CT, computed tomography.
Association between BDNF and TBI
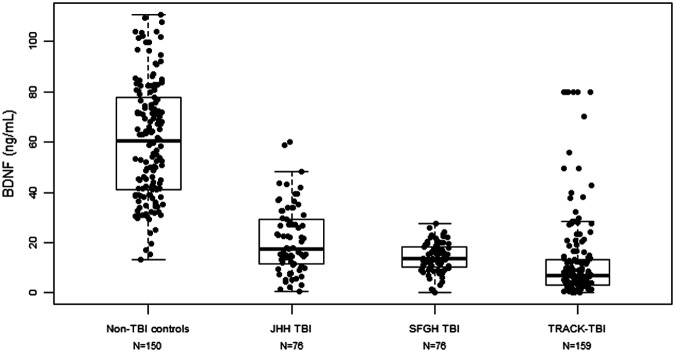
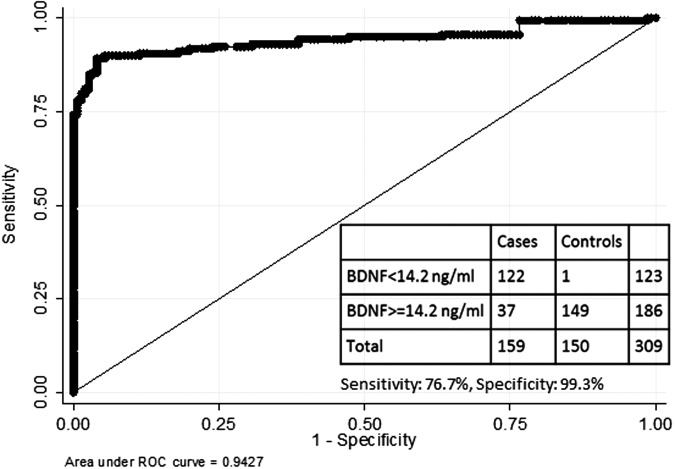
In the initial case-control study, median day-of-injury BDNF values (ng/mL) were lower among TBI cases (17.5; interquartile range [IQR], 11.3-29.6) in JHH TBI group and 13.8 (IQR, 10.1-18.3) in the SFGH group) than in non-TBI controls (60.3; IQR, 41.1-78.2; p = 0.0001). The 1st percentile of BDNF values in JHH non-TBI controls was 14.2 ng/mL. BDNF discriminated between TBI cases (JHH and SFGH) and non-TBI controls with an AUC of 0.96 (95% CI: 0.94-0.98), which is considered excellent accuracy. There was no significant association between duration of storage of serum samples in 4°C and BDNF value among TBI cases (Supplementary Fig. 1; see online supplementary material at www.liebertpub.com). Similarly, in a validation study, median day-of-injury BDNF values (ng/mL) were found to be low among TRACK-TBI Pilot subjects (6.8; IQR, 3.0-13.5). The distribution of BDNF values among the TBI cases and non-TBI control subjects studied is presented in Figure 1. BDNF discriminated between TRACK-TBI Pilot cases and JHH non-TBI controls with an AUC of 0.94 (95% CI: 0.91-0.97; Fig. 2). BDNF values were lower in TRACK-TBI Pilot cases (prospectively collected samples) than in the JHH or SFGH cohorts (excess clinical samples; p < 0.001). BDNF discriminated between JHH non-TBI controls and TRACK-TBI cases classified as mild TBI with an AUC of 0.95 (95% CI: 0.92-0.98).
FIG. 1.
Distribution of brain-derived neurotrophic factor (BDNF) in traumatic brain injury (TBI) and non-TBI cohorts. Graphical distribution of individual BDNF values and the corresponding box plots for Johns Hopkins Hospital (JHH) non-TBI control subjects, JHH TBI cases, San Francisco General Hospital (SFGH) TBI cases and Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) Pilot cases.
FIG. 2.
Receiver operator curve for distinguishing between traumatic brain injury (TBI) cases and controls with brain-derived neurotrophic factor (BDNF). The receiver operator curve for discriminating between Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) Pilot cases and Johns Hopkins Hospital (JHH) controls using BDNF values. The table reports the diagnostic accuracy of using a cut-off value of 14.2 ng/mL for distinguishing between TBI cases and controls.
Association between BDNF and TBI severity
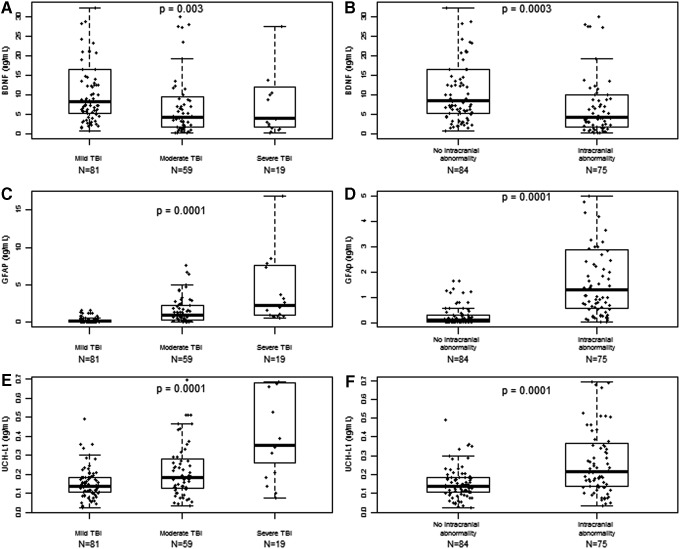
Within the TRACK-TBI Pilot cohort, day-of-injury BDNF values (ng/mL) were higher in mild TBI subjects (8.3; IQR, 5.2-16.5) than in moderate (4.3; IQR, 1.8-10.1) or severe TBI (4.0; IQR, 1.5-13.8; p = 0.003). The JHH and SFGH cohorts did not have sufficient moderate and severe TBI patients to assess BDNF variation with TBI severity (Table 1). Among TRACK-TBI Pilot subjects, median day-of-injury BDNF values (ng/mL) were higher in subjects without intracranial abnormality on head CT (8.4; IQR, 5.2-16.6) than in subjects with intracranial abnormality on head CT (4.2; IQR, 1.8-10.1; p < 0.001; Fig. 3). BDNF discriminated between subjects with and without intracranial abnormality on head CT with an AUC of 0.67 (95% CI: 0.58-0.75). In the JHH cohort, median BDNF (ng/mL) for normal CT and abnormal head CT were 17.8 (IQR, 12.5-30.8) and 16.2 (IQR, 4.8-23.2), respectively (p = 0.13). Whereas in the SFGH cohort, median BDNF (ng/mL) for normal and abnormal head CT were 13.0 (IQR, 9.4-17.1) and 15.1 (IQR, 10.5-21.3), respectively (p = 0.17.
FIG. 3.
Association between biomarkers examined and traumatic brain injury (TBI) severity. Presented are the graphical distribution of individual brain-derived neurotrophic factor (BDNF), glial fibrillary acidic protein (GFAP), and ubiquitin C-terminal hydrolase-L1 (UCH-L1) values in Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) Pilot and the corresponding boxplots according to TBI severity, classified as mild, moderate, or severe; and the presence or absence of traumatic intracranial abnormality on head computed tomography (CT) scan: (A) depicts BDNF versus TBI severity classified as mild moderate or severe; (B) depicts BDNF versus TBI severity classified by CT scan; (C) depicts GFAP versus TBI severity classified as mild, moderate, or severe; (D) depicts GFAP versus TBI severity classified by head CT scan; (E) depicts UCH-L1 versus TBI severity classified as mild, moderate or severe; (F) depicts UCH-L1 versus TBI severity classified by head CT scan. Individual values that were extreme outliers are excluded from the graphical presentation.
Association between BDNF and TBI outcomes
Among the 159 TRACK-TBI Pilot subjects, 94 (59%) had the Rivermead Post-Concussion Questionnaire measured and 111 (69%) had the GOSE score measured at 6 months post-injury. Of those with 6-month outcome measures, 62% (58/94) were determined to have PCS, 70% (78/111) had a GOSE < 8, and 80% (85/106) had either PCS or GOSE < 8. Among the 94 subjects with both PCS and GOSE measures, 51 (54%) had both PCS and GOSE < 8, 21 (22%) had neither PCS nor GOSE < 8, 15 (16%) had GOSE < 8 but no PCS, and seven (7%) had PCS and GOSE = 8. Day-of-injury BDNF values (ng/mL) were not significantly different between subjects with PCS (7.2; IQR, 3.0-12.8) and those without PCS (7.1; IQR, 4.0-21.0), or between subjects with GOSE = 8 (7.9; IQR, 4.0-23.3) and those with GOSE < 8 (7.1; IQR, 2.8-13.0). The 76 (72.4%) TRACK-TBI subjects who had very low BDNF values (i.e., less than the 1st percentile for non-TBI controls [ < 14.2 ng/mL]) had higher odds of incomplete recovery than those without very low BDNF (odds ratio, 4.0; 95% CI: 1.5-11.0). Very low BDNF values were associated with higher odds of incomplete recovery among those with mild TBI (4.9; 95% CI: 1.3-17.9) than those with moderate or severe TBI (2.0; 95% CI: 0.3-12.5).
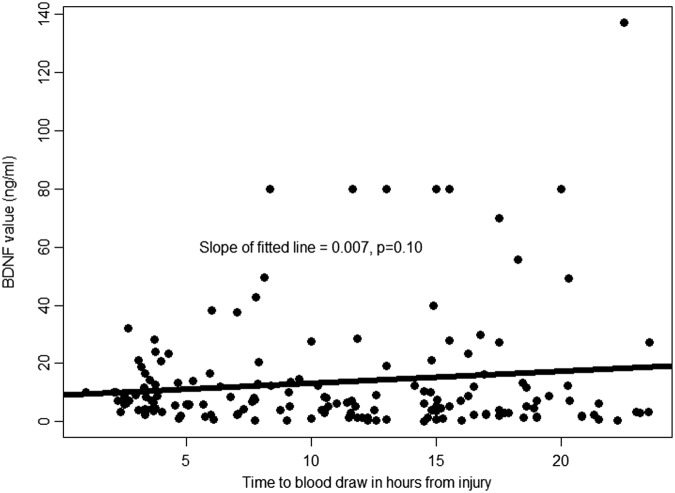
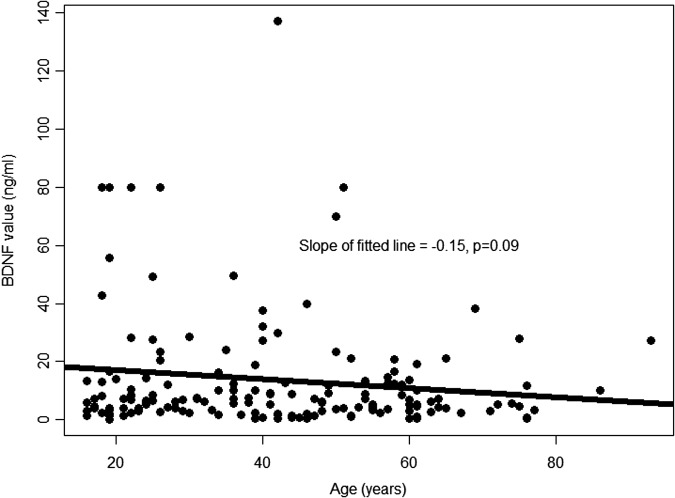
There was a trend toward higher BDNF values as the time interval between injury and serum sampling for BDNF measurement increased (Fig. 4). The trend was similar among those with complete and incomplete recovery. However, this trend did not reach statistical significance (p = 0.10). Similarly, there was a trend toward lower BDNF values with increasing age (Fig. 5). However, this trend was not statistically significant (p = 0.09). After adjustment for age and time between injury and serum sampling for BDNF measurement, very low BDNF (<14.2 ng/mL) remained statistically significantly associated with incomplete recovery (odds ratio, 4.16; 95% CI: 1.48-11.70).
FIG. 4.
Association between brain-derived neurotrophic factor (BDNF) and time from injury to blood sampling. This is a scatter plot of the association between day-of-injury BDNF values and time between injury and blood draw (in hours). The line represents the best fitting linear regression line that summarizes this association.
FIG. 5.
Association between brain-derived neurotrophic factor (BDNF) and age. This is a scatter plot of the association between day-of-injury BDNF values and age (in years). The line represents the best fitting linear regression line that summarizes this association.
Performance of GFAP and UCH-L1, compared with BDNF
A comparison of TRACK-TBI Pilot GFAP and UCH-L1 values with BDNF assayed on the same samples showed that GFAP, BDNF and UCH-L1 discriminated between subjects with traumatic abnormalities on head CT and those without, with AUCs of 0.88 (95% CI: 0.83-0.93) for GFAP, 0.70 (95% CI: 0.62-0.79) for UCH-L1, and 0.67 (95% CI: 0.58-0.75) for BDNF. They also discriminated between subjects with complete recovery from TBI and those without, with AUCs of 0.65 (95% CI: 0.52-0.78) for BDNF, 0.61 (95% CI: 0.49-0.73) for GFAP, and 0.55 (95% CI: 0.43-0.66) for UCH-L1 at 6 months. A comparison of the discriminative abilities of the biomarkers examined is presented in Table 3. There was no minimal correlation between BDNF and GFAP values (r = −0.11; p = 0.16) and between BDNF and UCH-L1 values (r = 0.07; p = 0.36), suggesting that they may be associated with different pathways of injury. To determine whether combining biomarkers resulted in improved discrimination of complete versus incomplete recovery, we used combinations of two biomarkers, instead of all three biomarkers, since only 21 subjects had complete recovery (10 events per predictor variable is required for adequate statistical power).38 Addition of GFAP to BDNF did not improve the discrimination of complete versus incomplete recovery (AUC was 0.66 instead of 0.65; p = 0.76). Similarly, addition of UCH-L1 to BDNF did not improve the discrimination of complete versus incomplete recovery (AUC was 0.66 instead of 0.65; p = 0.55).
Table 3.
Discriminative Ability of Different Biomarkers for Relevant TBI Outcomes as Measured by the Area Under the Receiver Operator Curve (AUC) and the Corresponding 95% Confidence Interval
| Outcome | GFAP | UCH-L1 | BDNF |
|---|---|---|---|
| GOSE score < 8 | 0.61 (0.50-0.71) | 0.55 (0.44-0.66) | 0.56 (0.44-0.68) |
| Post-concussive syndrome (PCS) | 0.56 (0.44-0.68) | 0.52 (0.40-0.64) | 0.55 (0.43-0.68) |
| Composite (GOSE score < 8 or PCS) | 0.61 (0.49-0.73) | 0.55 (0.43-0.66) | 0.65 (0.52-0.78) |
| Intracranial abnormality on head CT | 0.88 (0.83-0.93) | 0.70 (0.62-0.79) | 0.67 (0.58-0.75) |
TBI, traumatic brain injury; GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase-L1; BDNF, brain-derived neurotrophic factor; GOSE, Glasgow Outcome Scale Extended; CT, computed tomography.
Predictors of BDNF values in non-TBI control subjects
Among non-TBI controls, after adjustment for age, gender, race, hypertension, diabetes, history of psychiatric illness, and mean arterial pressure, only gender and mean arterial pressure remained independent predictors of BDNF among non-TBI controls (Table 4). Median BDNF levels (ng/mL) were greater in females (69.1; IQR, 41.4-82.4; n = 79) than in males (52.7; IQR, 38.7-71.8; n = 71; p = 0.049). However, there were no gender differences in BDNF levels within the TBI cohorts examined. Among non-TBI controls, BDNF values increased with increasing mean arterial pressure. However, there was no statistically significant association between BDNF and blood pressure within the TBI cohorts examined.
Table 4.
Determinants of BDNF in the Control Population (n = 150)
| Unadjusted regression co-efficient (95% CI) | Adjusted regression co-efficient (95% CI) | p Value for adjusted regression co-efficient | |
|---|---|---|---|
| Age in years | -0.1 (-0.4 to 0.3) | -0.2 (-0.5 to 0.2) | 0.38 |
| Gender | |||
| • Female | Reference | Reference | 0.04 |
| • Male | -7.2 (-15.4 to 1.0) | -8.8 (-17.0 to -0.6) | |
| Race | |||
| • African-American | Reference | Reference | |
| • Caucasian | -9.6 (-19.8 to 0.7) | -8.8 (-18.8 to 1.3) | 0.09 |
| • Other | 0.2 (-25.3 to 25.7) | 0.6 (-24.8 to 26.0) | 0.96 |
| Mean arterial pressure per 10 mm Hg | 2.8 (0.7 to 4.9) | 3.0 (0.9 to 5.1) | < 0.01 |
| History of hypertension | -0.0 (-9.1 to 9.0) | -0.3 (-9.7 to 9.1) | 0.95 |
| History of depression or schizophrenia | -1.2 (-12.7 to 10.2) | -3.3 (-14.6 to 8.0) | 0.57 |
BDNF, brain-derived neurotrophic factor; CI, confidence interval.
Discussion
We report the diagnostic value of day-of-injury circulating BDNF for TBI, and its ability to be prognostic for identifying subjects likely to have persistent TBI-related sequelae at 6 months. Further, we have determined that BDNF has a higher prognostic value among mild TBI subjects than moderate/severe TBI subjects. The dysregulation of BDNF in TBI has been examined with equivocal findings by a number of studies using animal models of TBI.10 In the majority of these studies, BDNF mRNA expression was measured in brain tissue, with reports of upregulation of BDNF mRNA in the hippocampus and cerebral cortex.39–41 However, other studies have suggested reduced secretion of brain BDNF protein after TBI, with subsequent increased secretion following experimental TBI treatment.42 Few studies have measured circulating BDNF in human TBI subjects. Two small pediatric studies reported no differences in plasma BDNF levels between human TBI cases and non-trauma controls.43,44 However, control subjects in these studies had abnormal neurologic status (obstructive hydrocephalus undergoing elective surgery,43 and subjects undergoing lumbar puncture for suspected meningitis44).
Another study measuring BDNF in Olympic boxers and healthy controls also reported no differences in plasma BDNF.45 However this study measured BDNF in plasma samples obtained 1–6 days after a bout and the release and clearance kinetics of BDNF in humans is not known. Further, Buonora and colleagues recently reported higher plasma BDNF levels in TBI cases, compared with controls.46 Our findings and study design are most similar to results reported by Kalish and Phillips.47 These investigators measured BDNF in serum samples obtained from 30 TBI patients and reported decreasing BDNF with increasing severity of TBI. Our study has demonstrated in three separate TBI cohorts that circulating levels of BDNF are lower in TBI cases, compared with non-trauma controls.
BDNF is limited in its ability to distinguish between TBI subjects with and without intracranial abnormalities. This may be due to the fact that structural proteins (such as GFAP) are more likely to have a strong association with radiographic changes in TBI than secreted proteins. However, secreted proteins may reflect both primary and secondary brain injury and therefore may have a stronger association with long-term outcomes. Our findings demonstrate that BDNF has higher prognostic value in mTBI subjects, compared with moderate or severe TBI patients. Therefore, BDNF holds promise for improving clinical prognostication of outcomes in TBI patients who have no intracranial abnormalities on head CT scans.
BDNF is an appealing candidate biomarker for detecting TBI for numerous reasons. First, our study results demonstrate a very strong association between BDNF and TBI, yielding excellent discriminative ability of 0.94-0.95 (as measured by the c-statistic). Second, our findings were replicated across three different TBI cohorts. Third, we have demonstrated an association between BDNF and TBI severity and an association between BDNF and TBI outcome. Finally, the association between TBI and BDNF is biologically plausible and has been demonstrated in diverse TBI models including animal models.10
BDNF is the most abundantly expressed brain neurotrophin48 and as a secreted protein, can be readily and reliably measured in serum using well established immuno-assay techniques, identifying it as a non-necrosis brain injury biomarker. This distinguishes BDNF from other protein-based biomarkers that are structural components of neurons and glial cells—for example, GFAP (an astro-glial intermediate filament cytoskeletal protein), S100B (an intracellular calcium binding protein), UCHL1 (a ubiquitin ligase localized to the neuronal soma), neurofilaments (cytoskeletal components of axons), cleaved tau (intracellular microtubule-associated proteins), and myelin basic proteins (a component of myelin), among others.49 In order for structural proteins to be found in high abundance in circulation, sufficient cellular necrosis and damage to the BBB is required. However, BDNF does not require cellular necrosis or damage to the BBB to be observed in circulation.50 Further, this allows BDNF to be more abundant in circulation than structural proteins, increasing assay sensitivity.
The exact mechanisms underlining the dysregulation of BDNF in TBI are not yet well understood. Although some studies implicate BDNF in neuroprotection following injuries,51,52 other studies suggest it contributes to neurodegenerative events that occur following injury.53,54 It also has been suggested that BDNF ameliorates the impact of secondary brain damage by modifying BDNF-induced gene expression.10 Following TBI and acute disconnection of brain circuitry, there is an attempt at reorganization and reconnection of brain circuits. BDNF promotes synaptic plasticity and restoration during the brain circuitry “reconnection” phase. We have found that post-TBI BDNF levels behave unlike the majority of candidate biomarkers of TBI. Lower BDNF values are associated with worse prognosis, whereas with other TBI biomarkers, lower values are typically associated with better prognosis,4 with the exception of microtubule-associated protein 2, a dendritic marker, which has higher values at 6 months after injury in severe TBI subjects with improved outcomes.3 We postulate that during the acute phase of TBI, the formation of new neuronal circuits might not be advisable, and therefore there may be no need for increased production of neurotrophic factors. However, it is possible that the initial decrease in circulating BDNF during the acute phase of trauma (as seen in our study) is potentially followed by a subsequent increase, especially during the sub-acute/chronic phases of TBI. Understanding the temporal variations in BDNF expression will be an important first step towards further elucidating the biological functions of BDNF in TBI and recovery. It is also possible that since decreased BDNF levels are found in patients with anxiety,55 major depressive disorder,56 and schizophrenia,57 low BDNF values on the day of injury identifies subjects at risk for these conditions (whether previously recognized or otherwise) and predisposes this population to incomplete recovery.
Although circulating BDNF may originate from the hippocampus, cerebral cortex, and basal forebrain,58 it also may be derived from other cellular sources, including platelets,59,60 smooth muscle cells,35,61 and vascular endothelial cells.62 This supports BDNF's role as a promoter of neuronal growth and survival both in the central and peripheral nervous system. However, it is unclear whether circulating BDNF values measured in this study are representative of central nervous system values. Prior studies suggest that BDNF crosses the BBB bi-directionally.63 Further, it has been reported that serum and cortical BDNF values are strongly correlated.64 Irrespective of the exact source(s) of circulating BDNF, our finding that circulating BDNF values are suppressed in TBI and that low BDNF values are associated with poor recovery suggest that BDNF deserves further evaluation as a potential biomarker of TBI and TBI recovery.
BDNF has the potential to become a surrogate marker of successful TBI treatment. In a study examining dietary omega-3 fatty acid supplementation in TBI, rats with decreased brain BDNF following mild fluid percussion injury had normalized brain BDNF levels and improved learning ability following 4 weeks of dietary supplementation with omega-3 fatty acids.42 Similarly, rats exposed to delayed exercise (2–3 weeks after injury) had increases in BDNF and improved cognitive performance, compared with rats exposed to early (0-6 days) exercise.65 In our study, low BDNF levels were associated with incomplete recovery at 6 months in individuals with TBI. Further studies are needed to validate this finding and to determine how well longitudinal BDNF values reflect recovery and clinical improvement post-TBI and the BDNF pathway as a therapeutic target.
Decreased circulating BDNF levels have been implicated in other non-TBI conditions including anxiety,55 major depressive disorder,56 schizophrenia,57 and Alzheimer's disease.66 However, these studies did not account for other potential confounders, such as age and gender. In our study, although control subjects with a history of a psychiatric disorder had lower median BDNF values than those without a history of a psychiatric disorder, this difference was not statistically significant. Additionally, after adjustment for age, gender, race, hypertension, diabetes and mean arterial pressure, history of psychiatric disorder was not an independent predictor of BDNF levels, whereas mean arterial pressure and gender were independent predictors of BDNF in control subjects. However, our findings regarding gender suggest that gender-specific cut-offs may be important in determining the reference values of BDNF. Since BDNF values increase during exercise, it is possible that in the case of sports-related concussions, increases in BDNF from exercise may mask a concussion-related decrease. Additional studies are needed to investigate BDNF levels in sports-related concussions.67
Limitations
Our study has a number of limitations. First, storage procedures for serum samples for JHH and SFGH TBI cases and JHH non-trauma controls were different. However, since our findings were reproduced in the TRACK-TBI Pilot cohort, it is unlikely that this discrepancy had an important influence on our study result. Further, BDNF increases with increased duration of storage at room temperature,25 and that may explain why BDNF values in the JHH and SFGH cohorts are higher than BDNF in TRACK-TBI Pilot.
Additionally, the demographic distribution of our TBI cases was different from that of the non-TBI controls. However, the diagnostic accuracy of BDNF for discriminating between TBI cases and controls did not vary significantly after adjustment for potential confounders. Another major limitation is that the JHH controls had not been exposed to trauma. Since a common clinical challenge is to determine if TBI is present in patients who have been involved in automobile accidents, falls, or blast exposures, an important control group would be individuals exposed to orthopedic or systemic trauma but not head injury. Efforts to collect these “other injury” controls are under way.
In our validation cohort, the prevalence of traumatic intracranial abnormalities on head CT scan was much higher (47.2% of TRACK-TBI Pilot cases studied) than that reported in studies that are more representative of the population of ED patients evaluated for TBI.68,69 Thus, examining the validity of our findings in cohorts that are more representative of ED patients evaluated for TBI will be important.
Conclusion
Serum BDNF discriminates between TBI cases and non-trauma controls with excellent diagnostic accuracy. Additionally, lower BDNF values are associated with incomplete recovery after TBI, and may be especially useful in identifying mild TBI patients who are likely to remain symptomatic at 6 months after injury.
Supplementary Material
Contributor Information
Collaborators: the TRACK-TBI investigators including
Author Disclosure Statement
Under a licensing agreement between ImmunArray and the Johns Hopkins University, Drs. Everett, Korley, and Van Eyk are entitled to royalties on an invention described in this article.
This study was supported in part by Grant Numbers RC2NS069409, U01NS086090 from the National Institute of Neurological Disorders and Stroke (NINDS), W81XWH-13-1-0441 from the Department of Defense (DoD) United States Army Medical Research Acquisition Activity, and Contract Number HHSN268201000032C from the National Heart, Lung, and Blood Institute (NHLBI). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NINDS, DoD, NHLBI, or the National Institute of Health.
References
- 1.Papa L., Lewis L. M., Falk J. L., Zhang Z., Silvestri S., Giordano P., Brophy G. M., Demery J. A., Dixit N. K., Ferguson I., Liu M. C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C. S., Tortella F. C., Hayes R. L., and Wang K. K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mondello S., Jeromin A., Buki A., Bullock R., Czeiter E., Kovacs N., Barzo P., Schmid K., Tortella F., Wang K. K., and Hayes R. L. (2012). Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J. Neurotrauma 29, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondello S., Gabrielli A., Catani S., D'Ippolito M., Jeromin A., Ciaramella A., Bossu P., Schmid K., Tortella F., Wang K. K., Hayes R. L., and Formisano R. (2012). Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Inj. 26, 1629–1635 [DOI] [PubMed] [Google Scholar]
- 4.Shahim P., Tegner Y., Wilson D. H., Randall J., Skillback T., Pazooki D., Kallberg B., Blennow K., and Zetterberg H. (2014). Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 71, 684–692 [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Arrastia R., Kochanek P. M., Bergold P., Kenney K., Marx C. E., Grimes C. J., Loh L. T., Adam L. T., Oskvig D., Curley K. C., and Salzer W. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen-Cory S., Kidane A. H., Shirkey N. J., and Marshak S. (2010). Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 70, 271–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang E. J. and Reichardt L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso M., Vianna M. R., Depino A. M., Mello E.S., Pereira P., Szapiro G., Viola H., Pitossi F., Izquierdo I., and Medina J. H. (2002). BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus 12, 551–560 [DOI] [PubMed] [Google Scholar]
- 9.Bekinschtein P., Cammarota M., Izquierdo I., and Medina J. H. (2008). BDNF and memory formation and storage. Neuroscientist 14, 147–156 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan G. B., Vasterling J. J., and Vedak P. C. (2010). Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 21, 427–437 [DOI] [PubMed] [Google Scholar]
- 11.Sofroniew M. V., Howe C. L., and Mobley W. C. (2001). Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24, 1217–1281 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z., Chen H., Zhang K., Yang H., Liu J., and Huang Q. (2003). Protective effect of nerve growth factor on neurons after traumatic brain injury. J. Basic Clin. Physiol. Pharmacol. 14, 217–224 [DOI] [PubMed] [Google Scholar]
- 13.Hol E. M. and Pekny M. (2015). Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell. Biol. 32, 121–130 [DOI] [PubMed] [Google Scholar]
- 14.Vos P. E., Jacobs B., Andriessen T. M., Lamers K. J., Borm G. F., Beems T., Edwards M., Rosmalen C. F., and Vissers J. L. (2010). GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 15.Okonkwo D. O., Yue J. K., Puccio A. M., Panczykowski D. M., Inoue T., McMahon P. J., Sorani M. D., Yuh E. L., Lingsma H. F., Maas A. I., Valadka A. B., and Manley G. T. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelinka L. E., Kroepfl A., Schmidhammer R., Krenn M., Buchinger W., Redl H., and Raabe A. (2004). Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J. Trauma 57, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 17.Mondello S., Linnet A., Buki A., Robicsek S., Gabrielli A., Tepas J., Papa L., Brophy G. M., Tortella F., Hayes R. L., and Wang K. K. (2012). Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 70, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Arrastia R., Wang K. K., Papa L., Sorani M. D., Yue J. K., Puccio A. M., McMahon P. J., Inoue T., Yuh E. L., Lingsma H. F., Maas A. I., Valadka A. B., Okonkwo D. O., and Manley G. T. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papa L., Akinyi L., Liu M. C., Pineda J. A., Tepas J. J., 3rd., Oli M. W., Zheng W., Robinson G., Robicsek S. A., Gabrielli A., Heaton S. C., Hannay H. J., Demery J. A., Brophy G. M., Layon J., Robertson C. S., Hayes R. L., and Wang K. K. (2010). Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue J. K., Vassar M. J., Lingsma H. F., Cooper S. R., Okonkwo D. O., Valadka A. B., Gordon W. A., Maas A. I., Mukherjee P., Yuh E. L., Puccio A. M., Schnyer D. M., and Manley G. T. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagoda A. S., Bazarian J. J., Bruns J. J., Jr, Cantrill S. V., Gean A. D., Howard P. K., Ghajar J., Riggio S., Wright D. W., Wears R. L., Bakshy A., Burgess P., Wald M. M., and Whitson R.R.; American College of Emergency Physicians; Centers for Disease Control and Prevention. (2008). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 22.Menon D. K., Schwab K., Wright D. W., and Maas A. I. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1637–1640 [DOI] [PubMed] [Google Scholar]
- 23.Korley F. K., Schulman S. P., Sokoll L. J., DeFilippis A. P., Stolbach A. I., Bayram J. D., Saheed M. O., Omron R., Fernandez C., Lwin A., Cai S. S., Post W. S., and Jaffe A. S. (2014). Troponin elevations only detected with a high-sensitivity assay: clinical correlations and prognostic significance. Acad. Emerg. Med. 21, 727–735 [DOI] [PubMed] [Google Scholar]
- 24.Manley G. T., Diaz-Arrastia R., Brophy M., Engel D., Goodman C., Gwinn K., Veenstra T. D., Ling G., Ottens A. K., Tortella F., and Hayes R. L. (2010). Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 91, 1667–1672 [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez S., Bembea M., Everett A., and Schwartz J. (2014). Impact of delayed blood sample processing on brain injury biomarker stability [Abstract 533]. Crit. Care Med. 42(12 Suppl), A1488 [Google Scholar]
- 26.Apple F. S., Quist H. E., Doyle P. J., Otto A. P., and Murakami M. M. (2003). Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin. Chem. 49, 1331–1336 [DOI] [PubMed] [Google Scholar]
- 27.Apple F. S., Parvin C. A., Buechler K. F., Christenson R. H., Wu A. H., and Jaffe A. S. (2005). Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin. Chem. 51, 2198–2200 [DOI] [PubMed] [Google Scholar]
- 28.Duhaime A. C., Gean A. D., Haacke E. M., Hicks R., Wintermark M., Mukherjee P., Brody D., Latour L., and Riedy G. (2010). Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1661–1666 [DOI] [PubMed] [Google Scholar]
- 29.King N. S., Crawford S., Wenden F. J., Moss N. E., and Wade D. T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 30.Babcock L., Byczkowski T., Wade S. L., Ho M., Mookerjee S., and Bazarian J. J. (2013). Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 167, 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin H. S., Boake C., Song J., Mccauley S., Contant C., Diaz-Marchan P., Brundage S., Goodman H., and Kotrla K. J. (2001). Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J. Neurotrauma 18, 575–584 [DOI] [PubMed] [Google Scholar]
- 32.O'Neil M. E., Carlson K., Storzbach D., Brenner L., Freeman M., Quinones A., Motu'apuaka M., Ensley M., and Kansagara D. (2013). Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review [Internet]. Washington (DC): Department of Veterans Affairs (US). VA Evidence-based Synthesis Program Reports [PubMed]
- 33.DeLong E. R., DeLong D. M., and Clarke-Pearson D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 [PubMed] [Google Scholar]
- 34.Golden E., Emiliano A., Maudsley S., Windham B. G., Carlson O. D., Egan J. M., Driscoll I., Ferrucci L., Martin B., and Mattson M. P. (2010). Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore Longitudinal Study of Aging. PLoS One 5, e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., and Virchow J. C. (2005). The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 26, 115–123 [DOI] [PubMed] [Google Scholar]
- 36.Nieto R., Kukuljan M., and Silva H. (2013). BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front. Psychiatry 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidalgo T. M., Morales-Quezada J. L., Muzy G. S., Chiavetta N. M., Mendonca M. E., Santana M. V., Goncalves O. F., Brunoni A. R., and Fregni F. (2013). Biological markers in noninvasive brain stimulation trials in major depressive disorder: a systematic review. J. ECT 30, 47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steyerberg E. W., Eijkemans M. J., and Habbema J. D. (1999). Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J. Clin. Epidemiol. 52, 935–942 [DOI] [PubMed] [Google Scholar]
- 39.Oyesiku N. M., Evans C. O., Houston S., Darrell R. S., Smith J. S., Fulop Z. L., Dixon C. E., and Stein D. G. (1999). Regional changes in the expression of neurotrophic factors and their receptors following acute traumatic brain injury in the adult rat brain. Brain Res. 833, 161–172 [DOI] [PubMed] [Google Scholar]
- 40.Felderhoff-Mueser U., Sifringer M., Pesditschek S., Kuckuck H., Moysich A., Bittigau P., and Ikonomidou C. (2002). Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol. Dis. 11, 231–245 [DOI] [PubMed] [Google Scholar]
- 41.Yang K., Perez-Polo J. R., Mu X. S., Yan H. Q., Xue J. J., Iwamoto Y., Liu S. J., Dixon C. E., and Hayes R. L. (1996). Increased expression of brain-derived neurotrophic factor but not neurotrophin-3 mRNA in rat brain after cortical impact injury. J. Neurosci. Res. 44, 157–164 [DOI] [PubMed] [Google Scholar]
- 42.Wu A., Ying Z., and Gomez-Pinilla F. (2004). Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 21, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 43.Chiaretti A., Piastra M., Polidori G., Di Rocco C., Caresta E., Antonelli A., Amendola T., and Aloe L. (2003). Correlation between neurotrophic factor expression and outcome of children with severe traumatic brain injury. Intensive Care Med. 29, 1329–1338 [DOI] [PubMed] [Google Scholar]
- 44.Chiaretti A., Antonelli A., Riccardi R., Genovese O., Pezzotti P., Di Rocco C., Tortorolo L., and Piedimonte G. (2008). Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 12, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neselius S., Brisby H., Theodorsson A., Blennow K., Zetterberg H., and Marcusson J. (2012). CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One 7, e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buonora J. E., Yarnell A. M., Lazarus R. C., Mousseau M., Latour L. L., Rizoli S. B., Baker A. J., Rhind S. G., Diaz-Arrastia R., and Mueller G. P. (2015). Multivariate analysis of traumatic brain injury: development of an assessment score. Front. Neurol. 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalish H. and Phillips T. M. (2010). Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878, 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofer M., Pagliusi S. R., Hohn A., Leibrock J., and Barde Y. A. (1990). Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J 9, 2459–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zetterberg H., Smith D. H., and Blennow K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radka S. F., Holst P. A., Fritsche M., and Altar C. A. (1996). Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 709, 122–301 [DOI] [PubMed] [Google Scholar]
- 51.Shulga A., Thomas-Crusells J., Sigl T., Blaesse A., Mestres P., Meyer M., Yan Q., Kaila K., Saarma M., Rivera C., and Giehl K. M. (2008). Posttraumatic GABA(A)-mediated Ca2 + ]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J. Neurosci. 28, 6996–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D. H. and Zhao X. (2005). BDNF protects neurons following injury by modulation of caspase activity. Neurocrit. Care 3, 71–76 [DOI] [PubMed] [Google Scholar]
- 53.Rudge J. S., Mather P. E., Pasnikowski E. M., Cai N., Corcoran T., Acheson A., Anderson K., Lindsay R. M., and Wiegand S. J. (1998). Endogenous BDNF protein is increased in adult rat hippocampus after a kainic acid induced excitotoxic insult but exogenous BDNF is not neuroprotective. Exp. Neurol. 149, 398–410 [DOI] [PubMed] [Google Scholar]
- 54.Koh J. Y., Gwag B. J., Lobner D., and Choi D. W. (1995). Potentiated necrosis of cultured cortical neurons by neurotrophins. Science 268, 573–575 [DOI] [PubMed] [Google Scholar]
- 55.Suliman S., Hemmings S. M., and Seedat S. (2013). Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front. Integr. Neurosci. 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunoni A. R., Lopes M., and Fregni F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 11, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 57.Green M. J., Matheson S. L., Shepherd A., Weickert C. S., and Carr V. J. (2011). Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatry 16, 960–972 [DOI] [PubMed] [Google Scholar]
- 58.Yamada K. and Nabeshima T. (2003). Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 91, 267–270 [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto H. and Gurney M. E. (1990). Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 10, 3469–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujimura H., Altar C. A., Chen R., Nakamura T., Nakahashi T., Kambayashi J., Sun B., and Tandon N. N. (2002). Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 87, 728–734 [PubMed] [Google Scholar]
- 61.Lommatzsch M., Braun A., Mannsfeldt A., Botchkarev V. A., Botchkareva N. V., Paus R., Fischer A., Lewin G. R., and Renz H. (1999). Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am. J. Pathol. 155, 1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helan M., Aravamudan B., Hartman W. R., Thompson M. A., Johnson B. D., Pabelick C. M., and Prakash Y. S. (2014). BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J. Mol. Cell. Cardiol. 68, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan W., Banks W. A., Fasold M. B., Bluth J., and Kastin A. J. (1998). Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 64.Karege F., Schwald M., and Cisse M. (2002). Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 328, 261–264 [DOI] [PubMed] [Google Scholar]
- 65.Griesbach G. S., Hovda D. A., Molteni R., Wu A., and Gomez-Pinilla F. (2004). Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125, 129–139 [DOI] [PubMed] [Google Scholar]
- 66.Nagahara A. H., Merrill D. A., Coppola G., Tsukada S., Schroeder B. E., Shaked G. M., Wang L., Blesch A., Kim A., Conner J. M., Rockenstein E., Chao M. V., Koo E. H., Geschwind D., Masliah E., Chiba A. A., and Tuszynski M. H. (2009). Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat. Med. 15, 331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Griesbach G. S., Hovda D. A., Molteni R., Wu A., and Gomez-Pinilla F. (2004). Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125, 129–139 [DOI] [PubMed] [Google Scholar]
- 68.Korley F. K., Morton M. J., Hill P. M., Mundangepfupfu T., Zhou T., Mohareb A. M., and Rothman R. E. (2013). Agreement between routine emergency department care and clinical decision support recommended care in patients evaluated for mild traumatic brain injury. Acad. Emerg. Med. 20, 463–469 [DOI] [PubMed] [Google Scholar]
- 69.Papa L., Stiell I. G., Clement C. M., Pawlowicz A., Wolfram A., Braga C., Draviam S., and Wells G. A. (2012). Performance of the Canadian CT Head Rule and the New Orleans Criteria for predicting any traumatic intracranial injury on computed tomography in a United States Level I trauma center. Acad. Emerg. Med. 19, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.