Abstract
Traumatic brain injury (TBI) remains a primary cause of death and disability in both civilian and military populations worldwide. There is a critical need for the development of neuroprotective agents that can circumvent damage and provide functional recovery. We previously showed that methylene blue (MB), a U.S. Food and Drug Administration–grandfathered drug with energy-enhancing and antioxidant properties, given 1 and 3 h post-TBI, had neuroprotective effects in rats. This study aimed to further investigate the neuroprotection of delayed MB treatment (24 h postinjury) post-TBI as measured by lesion volume and functional outcomes. Comparisons were made with vehicle and acute MB treatment. Multi-modal magnetic resonance imaging and behavioral studies were performed at 1 and 3 h and 2, 7, and 14 days after an impact to the primary forelimb somatosensory cortex. We found that delaying MB treatment 24 h postinjury still minimized lesion volume and functional deficits, compared to vehicle-treated animals. The data further support the potential for MB as a neuroprotective treatment, especially when medical teatment is not readily available. MB has an excellent safety profile and is clinically approved for other indications. MB clinical trials on TBI can thus be readily explored.
Key words: : behavioral outcomes, CCI, methylene blue, mitochondria, MRI, TBI
Introduction
Traumatic brain injury (TBI) is characterized as a sudden physical impact to the head and is considered a leading cause of death and disability in civilian and military populations.1 In the hours and days after TBI, there is a progression of molecular changes that lead to mitochondrial dysfunction, edema formation, inflammation, blood–brain barrier (BBB) dysfunction, and neuronal degeneration.2,3 Importantly, there are no neuroprotective agents clinically available to counteract the progressive nature of a TBI.
Mitochondrial oxidative damage is well known to precede onset of neuronal damage and loss and is therefore considered an important mediator of secondary brain injury. During injury, a surge in reactive oxygen species (ROS) facilitates a vicious cycle that accelerates mitochondrial damage, excitotoxicity, lipid peroxidation (LPO), and inflammation.4 Thus, mitochondrial targeting strategies in TBI have been increasingly studied given that their maintenance could preserve brain function.5,6 However, the majority of studies using agents that target mitochondria demonstrating neuroprotection have utilized a therapeutic treatment window of less than 1 h post-TBI. Administration of cyclosporine A7 was demonstrated to significantly reduce mitochondrial dysfunction (given 15 min postinjury),8 cortical damage (given 5 min before to immediately after injury),9–11 and cytoskeleton and axonal dysfunction (given as a pretreatment before injury).12,13 Further, dietary supplementation with creatine was also shown to be effective in ameliorating neuronal cell death likely by reducing mitochondrial ROS production and maintaining adenosine triphosphate (ATP) production post-TBI.11 Though these studies provide increasing evidence of the importance of mitochondrial dysfunction and the effects of its failure in secondary brain injury, the potential therapeutic value of pharmacological mitochondrial protection, the time course of intervention needed, and the causes of mitochondrial dysfunction have not yet been fully defined.
Methylene blue (MB), a U.S. Food and Drug Administration–grandfathered drug currently used to treat methemoglobinemia, carbon monoxide poisoning, and cyanide poisoning in humans,14,15 is well established to act on mitochondria by improving energy production and reducing oxidative damage. MB has also recently been shown to reduce neurobehavioral impairment in animal models of optic neuropathy,16,17 Parkinson's disease,15,18 Alzheimer's disease,19–21 and ischemic stroke.22,23 MB has redox recycling properties whereby it acts as an electron cycler and facilitates electron transfer in the mitochondrial electron transport chain by accepting electrons from nicotinamide adenine dinucleotide and transfering them to cytochrome c, bypassing complex I–III.24 We previously showed that low-dose MB (1 mg/kg) administered acutely at 1 and 3 h post-TBI in rats reduces lesion volume, neurological deficits, and neuronal degeneration.25
The aims of this study were to: 1) extend our previous findings to test whether delayed (24 h) MB treatment would show efficacy in reducing lesion and behavioral deficits after an open-skull TBI and 2) determine the effects of MB on magnetic resonance imaging (MRI) parameters (T2, apparent diffusion coefficient [ADC], fractional anisotropy [FA], and cerebral blood flow [CBF]) in vehicle, acute, and delayed MB treatment groups. A controlled cortical impact (CCI) was applied over the left primary forelimb somatosensory cortex (S1FL) in rats. Multi-parametric quantitative MRI measurements (T2, ADC, FA, and CBF) and longitudinal behavioral assessments (foot fault and forelimb asymmetry tests)26–28 were made from 3 h to 14 days post-TBI. Comparisons were made with our previously published vehicle and acute MB treatment groups in terms of lesion volume and behavioral measures.25 Moreover, analysis was also performed on how MB affects T2, ADC, FA, and CBF of vehicle, acute and delayed MB treatment groups.
Methods
Animal preparation
All animal procedures follow the ARRIVE guidelines and were approved by the institutional animal care and use committee of the University of Texas Health Science Center San Antonio (San Antonio, TX). Induction of TBI was done as previously described.29 Three groups of animals were studied: 1) vehicle treated (N=6); 2) acute MB treated (N=5), where MB was given at 1 and 3 h post-TBI; and 3) delayed MB treated (N=10), where MB was given 24 h post-TBI. Note that the lesion volume and behavioral measures data from vehicle and acute MB treatment groups have been previously published.25 T2, ADC, FA, and CBF in the acute MB treatment group have not been previously published.
Male Sprague-Dawley rats (250–350 g) were anesthetized initially with 5% isoflurane mixed with room air and maintained at 1.2% isoflurane throughout all surgical and imaging procedures. The animal was secured in a stereotaxic frame and an incision was made as posterior (at the level of the cerebellum) from the impact site as possible to prevent artifacts during MRI acquisition, and the periosteum was removed over the impact site. A diameter 5-mm craniotomy was created over the left forelimb primary somatosensory cortex (S1FL; +0.25 mm anterior and 3.5 mm lateral to bregma), exposing the dura matter. The intact dura matter was impacted using a pneumatic controlled cortical impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA) fitted with a Ø 3-mm tip (5.0 m/s, 250 μs dwell time, and 1 mm depth) to mimic a mild focal TBI. After impact, the cranial opening was sealed with bone wax, the scalp sutured closed, and antibiotic ointment applied. Saline was placed under the skin to facilitate removal of air pockets between the scalp and skull to minimize artifacts during MRI acquisition. Buprenex (0.05 mg/kg) was given subcutaneously every 12 h for 3 days for pain.
Magnetic resonance imaging
MRI (Bruker 7-Tesla BioSpec; Bruker Corporation, Billerica, MA) was acquired on the day of the TBI procedure (1–3 h post-TBI), and again on days 2, 7, and 14 post-TBI onset to longitudinally monitor lesion volume. The animal was anesthetized with 1.5% isoflurane and secured in a MRI-compatible stereotaxic holder with ear and tooth bars. A transceiver surface coil of 2 cm in diameter was placed on top of the rat's head. T2-weighted images were acquired using a fast spin-echo sequence with repetition time=3 sec (90-degree flip angle), effective echo time=18, 54, 90, and 126 ms, 8 echo train length, seven 1.0-mm thick coronal images, field of view=2.56×2.56 cm, matrix 96×96 and reconstructed to 128×128, and four transients for signal averaging. Images were coregistered across time points using QuickVol and MRIAnalysisPak software. Lesion volumes were determined as pixels that had T2 values higher than the mean plus 2 standard deviations of the value in the homologous contralesional region.30
Functional assessment
Behavioral assessments were made 1–3 days pre-TBI and again 1, 2, 7, and 14 days post-TBI preceding MRI experiments in the same animals. Behavioral tests were not performed on the day of TBI induction owing to incomplete recovery from anesthetic. Sensorimotor function was assessed using the asymmetry forelimb placement (cylinder) test and foot-fault test.29 Previous behavioral studies have demonstrated that these functional tests have the appropriate sensitivity for this injury model.26–28 Testing was conducted 1–3 days pre-TBI and again 1, 2, 7, and 14 days post-TBI.
Foot-fault test
The foot fault test is utilized as a measure of neurologcal deficits associated with motor impairment and has been used widely to measure deficits after stroke. In this test, the rat is placed on a grid-like surface and allowed to move freely upon it for 5 min. When an animal places the limbs inaccurately on the grid, the limb falls through and is counted as a foot fault.
Cylinder test
The cylinder test provides a way to evaluate a rodent's spontaneous forelimb use and is commonly used to evaluate motor/sensory behavior. A rat is placed in a Plexiglas cylinder and the rat will actively explore the vertical surfaces by rearing. The number of individual limb placements and simultaneous placements are observed for each limb. Unilateral brain damage will result in an asymmetry in forelimb use.
Statistical analysis
Unpaired t-tests were used to compare T2 lesion volumes between vehicle- and MB-treated groups. Mann-Whitney's U tests were used to compare differences in asymmetrical limb use and the percentage of foot faults between vehicle- and MB-treated animals. Values are presented as mean±standard error of the mean. Statistical significance was set at p<0.05. Multiple regression analysis was performed to determine the correlation between MRI-determined lesion volume and asymmetry scores and foot-fault scores.
Results
Methylene blue improves lesion volumes
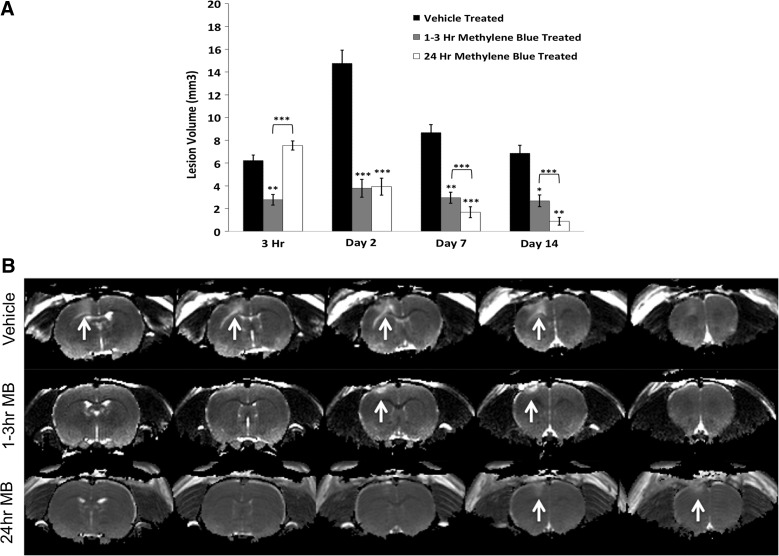
Figure 1A displays T2 lesion volumes for vehicle-, acute MB-, and delayed MB-treated animals at 3 h and 2, 7, and 14 days post-TBI. The delayed MB group had similar lesion volumes at the 3-h time point (given that MB has not yet been delivered in the delayed MB treatment group) compared to the vehicle-treated group, as expected, whereas the acute MB group (post-MB) had reduced lesion volume at this time point. By day 2 post-TBI, the delayed treatment group demonstrated a similar reduction in lesion volume as the acutely treated animals, suggesting that delayed treatment is as effective as acute treatment. The delayed group continued to show significant reductions in lesion volume, surpassing those of the acute treatment group on days 7 and 14. Figure 1B demonstrates representative T2 maps for vehicle-, acute MB-, and delayed MB-treated animals at 2 days post-TBI, with white arrows indicating the lesion. In vehicle-treated animals the lesion spread over four slices, indicated by the hyperintense areas of the cortex, whereas the acute and delayed MB treatment groups only spanned two slices. The data suggest that delayed treatment is more effective at reducing lesion volume than acute treatment.
FIG. 1.
(A) Lesion volumes for MB- and vehicle-treated animals are shown. MB-treated animals were either treated acutely (1–3 h) or subacutely (24 h; n=10 per group,±standard error of the mean). (B) Representative T2 maps for vehicle-, 1- to 3-h MB-, and 24-h MB-treated animals on day 2 after traumatic brain injury. White arrows indicate areas of abnormal T2 indicating the lesion. MB, methylene blue.
Methylene blue effects on magnetic resonance imaging measured parameters
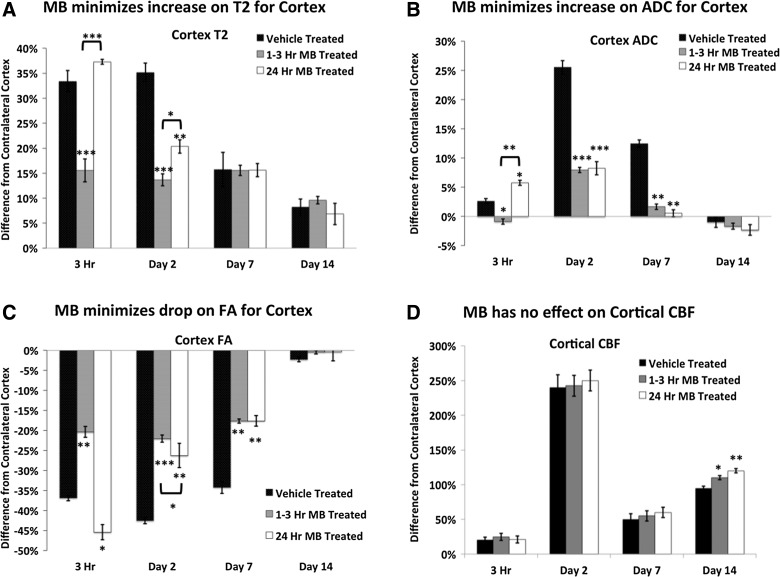
T2, ADC, FA, and CBF percent differences from the contralesional cortex were analyzed using region of interest analysis for vehicle-, acute MB-, and delayed MB-treated groups at 3 h and 2, 7, and 14 days post-TBI.
Vehicle-treated animals had increased T2 values in the cortex by 3 h and remained elevated on day 2 postinjury. T2 values decreased by day 7 and continued to decrease through day 14. Similarly, 3 h postinjury, the delayed MB group did not show significant differences from the vehicle group in T2, as expected. The acute MB group showed a significant decrease in T2 from both the vehicle and delayed MB groups at both the 3-h and 2-day time points. By day 7, all groups had decrased T2 values, which continued to decrease through day 14, with no significant differences detected among the groups (Fig. 2A).
FIG. 2.
Percent differences from the contralesional cortex were calculated for T2, ADC, FA, and cortical CBF in vehicle-, acute (1–3 h), and delayed (24 h) MB-treated groups after traumatic brain injury (n=10 per group,±standard error of the mean). MB, methylene blue; ADC, apparent diffusion coefficient; FA, fractional anisotropy; CBF, cerebral blood flow.
ADC was significantly elevated at 3 h, peaked on day 2, and returned toward normal values on day 14 in vehicle-treated animals (Fig. 2B). In a similar fashion, acute and delayed MB-treated animals had significant elevations in ADC on day 2; however, they did not reach the severity of the vehicle-treated animals. On day 7 postinjury, the MB-treated groups had significantly lower ADC values, compared to vehicle-treated animals. All treatment groups had returned toward normal on day 14, with no significant differences between groups.
In vehicle-treated animals, abnormal FA in the cortex was apparent within 3 h of impact. FA was significantly reduced at 3 h, remained reduced on days 2 and 7, but returned toward normal values on day 14 (Fig. 2B). In contrast, acute MB-treated animals had significantly reduced FA, compared to vehicle-treated animals, at 3 h and on days 2 and 7. The delayed MB-treated group showed significantly reduced FA, similar to vehicle-treated animals. By day 2, MB treatment had minimized FA decrease, similar to the acute MB-treated group. By day 14, all treatment groups had returned toward normal.
Cortical CBF peaked on day 2 in all groups, reduced on day 7, and increased again on day 14. There were no significant differences in the CBF among vehicle, acute, and delayed MB groups until day 14 post-TBI (Fig. 2D). On day 14, acute and delayed MB treatment increased CBF values, compared to vehicle-treated animals.
Methylene blue improves behavioral outcomes
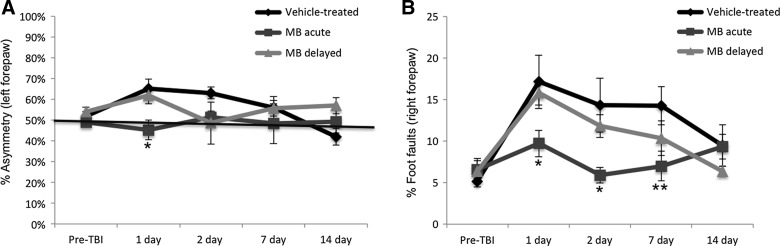
Sensorimotor function was assessed using the forelimb asymmetry (cylinder) and foot-fault tests. Before TBI induction, mean forelimb asymmetry scores were not significantly different between the vehicle-, acute MB-treated, and delayed MB-treated groups (49±2%, 52±2%, and 53%±3%, respectively; p>0.05; Fig. 3A) indicating symmetrical use of the two forelimbs. Data from our previous study demonstrated that, in vehicle-treated animals, forelimb asymmetry scores worsened on days 1 and 2 post-TBI and that acute MB treatment significantly reduced asymetrical use of forelimbs. We extended functional assessment in this study using delayed treatment of MB 24 h post-TBI. In the delayed MB group, forelimb asymmetry scores peaked on day 1. By day 2, asymmetry scores returned to baseline levels in the delayed MB group. By days 7 and 14, scores rose slightly, although they were not significantly different than vehicle- or acute MB-treated animals (Fig. 3A).
FIG. 3.
(A) Line graph demonstrates the averaged percent asymmetry of the left forepaw for vehicle-, acute (at 1 and 3 h), and delayed (at 24 h) MB-treated animals pre- and postinjury on days 1, 2, 7, and 14. *p<0.05, compared to vehicle-treated animals; n=10 per group; average±standard error of the mean (SEM). (B) Line graph demonstrating the percent foot faults of the right forepaw in vehicle-, acute (at 1 and 3 h), and delayed (at 24 h) MB-treated animals preinjury and 1, 2, 7, and 14 days postinjury. *p<0.05 and **p<0.01, compared to vehicle-treated animals; n=10 per group; average±SEM. MB, methylene blue; TBI, traumatic brain injury.
Percentages of foot faults were not statistically different among the vehicle- and acute or delayed MB-treated groups before TBI induction (Fig. 3B). Vehicle-treated animals had significant increases in the number of right forepaw foot faults by day 1 that persisted through day 7 and improved on day 14. In the acute MB group, by contrast, we previously demonstated that right foot faults were only slightly elevated post-TBI, did not reach the severity observed in the vehicle group, and were significantly lower, compared to the vehicle-treated group, on days 1, 2, and 7 post-TBI (p=0.043, 0.018, and 0.0058, respectively). In the delayed MB group, foot faults followed the same pattern as vehicle-treated animals, but were lower at each time point studied (Fig. 3B). The vehicle- and delayed MB-treated groups showed no difference in the number of foot faults on day 1, as expected, given that animals were tested before administration of MB. On days 2, 7, and 14, there was a trend of decreasing foot faults, compared to vehicle-treated animals, but did not reach significance. Together, these data indicate that acute and delayed MB treatment reduces sensorimotor deficits post-TBI.
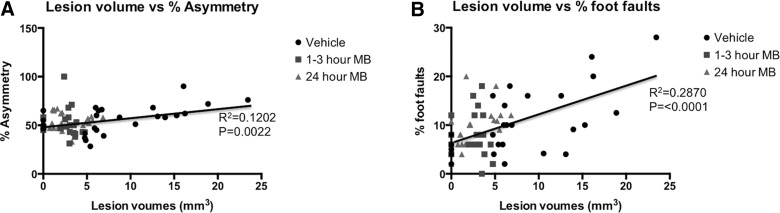
Correlation analysis between T2 MRI lesion volume and forelimb asymmetry scores for all time points is plotted in Figure 4A for vehicle-, 1- to 3-h MB-, and 24-h MB-treated animals. Regression analysis revealed an R2 value of 0.1202 (p=0.0022) for lesion volume and percent asymmetry scores in all groups. Individual regression analysis for each group was also performed between lesion volume and percent asymmetry, wherein vehicle-treated animals yielded a significant correlation with an R2 value of 0.32 (p=0.002; data not shown). MB-treated animals yielded a nonsignificant correlation with an R2 value of 0.009 (p=0.67) in acutely treated animals and an R2 value of 0.0007 (p=0.89) in treatment-delayed animals when comparisons were made between lesion volume to asymmetry scores. Correlation analysis between MRI-determined lesion volume and foot-fault scores for vehicle-treated animals for all time points is plotted in Figure 4B for vehicle-, 1- to 3-h MB-, and 24-h MB-treated animals. Regression analysis revealed an R2 value of 0.2870 (p<0.0001) for lesion volume and percent asymmetry scores in all groups. Individual regression analysis for each group was also performed between lesion volume and percent foot fault, wherein vehicle-treated animals yielded a significant correlation with an R2 value of 0.43 (p=0.0002; data not shown). MB-treated animals yielded a nonsignificant correlation with an R2 value of 0.009 (p=0.88) in acutely treated animals, whereas a significant correlation was found in delayed MB-treated animals with an R2 value of 0.286 (p=0.004) when comparisons were made between lesion volume to foot fault scores. Vasogenic edema and T2 MRI lesion volume were in general agreement with behavioral scores.
FIG. 4.
(A) Multiple regression correlation plots are demonstrated for T2 magnetic resonance imaging (MRI) lesion volume versus percent asymmetry scores in vehicle-, 1- to 3-h MB-treated, and 24-h MB-treated animals, with different symbols indicating data from the different treatment groups. (B) Multiple regression correlation plots are also demonstrated for T2 MRI lesion volume versus percent foot-fault scores in vehicle-, 1- to 3-h MB-treated, and 24-h MB-treated animals, with different symbols indicating data from the different treatment groups. MB, methylene blue.
Discussion
This study demonstrates that delayed MB treatment is also neuroprotective post-TBI using an open-skull, CCI model in rats. The major findings are: 1) Delayed MB treatment was effective in reducing lesion volumes and behavioral deficits on days 2, 7, and 14 compared to vehicle treatment; 2) T2-determined lesion volume peaked on day 2 in vehicle-treated animals and acute MB-treated animals whereas delayed MB lesion volume peaked at 3 h; 3) increased T2 was apparent 3 h post-TBI and was significantly decreased by 3 h in acutely treated MB animals and by day 2 in delayed MB animals, with all groups gradually returning toward normal by day 14; 4) the increase in ADC was significantly reduced by acute and delayed MB treatment on days 2 and 7 and gradually returned toward normal at day 14; 5) CBF measures indicated severe hypoperfusion at 3 h, marked hyperperfusion peaking on day 2, hypoperfusion again on day 7, and a return toward normal on day 14 in all groups, with only a significant increase with acute and delayed MB found on day 14; and 6) acute and delayed MB treatment reduce sensorimotor deficits post-TBI. In sum, we provide further evidence that MB acts as a neuroprotective agent that decreases lesion volume, reduces cytotoxic edema formation, improved MRI defined parameters, and improves functional outcome.
Methylene blue reduces lesion volume
Delayed MB treatment was effective in reducing lesion volumes and behavioral deficits on days 2, 7, and 14, compared to vehicle treatment. The delayed MB group had similar lesion volumes at the 3-h time point (pre-MB) compared to the vehicle group, as expected, whereas the acute MB group at the 3-h time point (post-MB) had reduced lesion volume compared to the vehicle group. These findings suggest that MB has immediate effects. A likely mechanism of neuroprotection is that MB acts as an energy enhancer, sustaining some level of ATP production post-TBI. MB also has antioxidant properties. Thus, MB also likely reduces oxidative damage from excessive oxygen free radicals post-TBI. MB has been shown to decrease ROS production in ischemia/reperfusion injury31 and neuron cell death induced by oxidative stress.32 In addition, MB inhibits rotenone-induced LPO17 and decreases oxidative damage after ischemic-reperfusion injury.33,34 The ability of delayed MB to exert improved lesion volumes, compared to acutely treated animals, may be owing to the time course of oxidative damage that occurs post-TBI. Ansari and colleagues demonstrated, using the same CCI model utilized in this study, that the level of LPO peaks around 24–48 h postinjury, with a concomitant peak decrease in the primary antioxidant, glutathione, at the same time.35 In addition, activity of several antioxidant enzymes are also significantly reduced 24 h post-TBI, including glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase. This suggests that antioxidant defenses are unable to counteract increased production of ROS. Therefore, MB's antioxidant property likely contributes to the observed reduction in vasogenic edema, lesion volume, neuronal cell death, and behavioral deficits in our study when oxidative stress levels are at their highest.
Magnetic resonance imaging characterization of traumatic brain injury
T2
We previously demonstrated that T2 and diffusion MRI parameters are sensitive to both hyper- and subacute changes in a rat model of mild TBI without treatment.36 This study compared the quantitative analysis of multimodal MRI parameters of both acute and delayed MB treatments (which have not been previously published) to those from our previous multi-modal MRI study of vehicle-treated animals.36 Increased T2 values were apparent 3 h post-TBI in both vehicle- and delayed MB-treated animals, as expected, and suggest increased water that likely indicates edema formation within the impacted area. There are a number of cellular mediators released post-TBI that likely contribute to increased brain edema, including glutamate, lactate, nitric oxide, calcium, potassium, and hydrogen, among others. TBI-induced disruption of the BBB is also an important mediator of increased edema formation, especially during the acute phase postinjury. However, significantly decreased T2 was detected by 3 h in acutely treated MB animals and by 48 h in the delayed MB treatment group, suggesting that MB alleviates edema formation. The mechanisms underlying MB effects on edema formation are not well understood. However, our findings have been corroborated by Fenn and colleagues, who recently demonstrated that an acute single dose of MB caused a reduction in cerebral edema within 24 h postinjury using the fluid percussion model of TBI.37 The researchers also found a reduction in neuroinflammation, which could also contribute to the decrease in edema formation.
Diffusion
Whereas cytotoxic edema has been described as the predominant form of edema postinjury, vasogenic edema is known to cause increased intracranial pressure (ICP) and secondary ischemic events.38,39 In our model of TBI, we detected heterogeneous ADC decreases and increases around the impacted area during the hyperacute phase (3 h), indicating a combination of cytotoxic and vasogenic edema, respectively. Further, the time course of edema formation within the ipsilesional impact area began as early as 1–3 h postinjury, peaked at 48 h, and began to disipate by day 7, correlating with behavioral recovery.36 Both acute and delayed MB treatment resulted in significant elevations of ADC on day 2; however, they did not reach the severity of the vehicle-treated animals. Subsequently, MB-treated animals had significantly reduced ADC values, compared to vehicle-treated animals. These data suggest that MB has important effects on reducing vasogenic edema formation, even when given in a delayed manner (24 h postinjury). MB has previously been shown to have hemodynamic effects in models of pulmonary hypertenstion by reducing edema formation, likely through inhibiting cyclooxygenase products of arachidonic acid,40 and suggests that MB has important in vivo effects on edema formation. The data for acute MB treatment are supported by a study by Fenn and colleagues, who demonstrated that immediate injection of MB reduces cerebral edema using the wet-dry weight method after fluid percussion injury in mice.41 However, there are no other studies that have assessed this phenonmenon following delayed treatment of MB in TBI.
Diffusion tensor imaging parameters, such as FA, have been increasingly used to investigate axonal injury in mild-to-severe TBI.42,43 Post-TBI, there is a reduction in brain white matter FA that may be associated with changes in parenchymal structure44–46; however, it is not fully understood. The potential parenchymal changes associated with TBI include edema formation effects, axonal degeneration, and fiber disruption. The present study is in line with previous studies that have demonstrated a reduction in FA post-TBI, which peaked 2 days postinjury and steadily returned toward normal. Acute and delayed MB administration reduced abnormalities in FA, compared to vehicle, treated animals, suggesting that MB reduces axonal injury. The mechanisms of this protection may be owing to reduction in oxidative stress; however, there is no direct evidence to support this theory in the literature. One study has shown that treating with Edaravone, a free radical scavenger, resulted in suppressed oxidative stress and axonal injury.47 The researchers suggest that the protective function of Edaravone may be mediated, in part, by down-regulation of neuronal nitric oxide synthase (NOS) and inducible NOS (iNOS) with a concomitant increase in iNOS and its free radical scavenging ability. Owing to the similarity in the potential mechanisms of MB as a free radical scavenger, MB could be limiting axonal injury by reducing the level of free radicals; however, additional studies are needed.
Cerebral blood flow
The effects of TBI on CBF measurements have been reported using multiple methods in various TBI models.48,49 However, the results have been variable with hypoperfusion, hyperperfusion, or a combination of the two found post-TBI in animal models. In our model, CBF was markedly reduced acutely (at 3 h), significantly increased on day 2, and returned toward near-normal values by days 7 and 14. The initial reductions in CBF could be owing to local increases in ICP and/or damaged to blood vessels resulting from mechanical injury during impact. The subsequent hyperperfusion observed at 24 and 48 h postinjury could be related to decreased vasoconstriction or impaired vasoreactivity. Hyperperfusion elevates cerebral blood volume, causing increased ICP altering autoregulation. Dore-Duffy and colleagues suggest that post-TBI hyperperfusion could be caused by altered regulation of blood flow within microvessels by endothelin-1-induced pericytes.50 These findings are also supported by Thomale and colleagues, who found severe hypoperfusion using laser Doppler flowmetry in a similar rat model of moderate CCI in the area of the impact at 0.5–6.0 h and hyperperfusion at 24 and 48 h.51 Other studies found CBF reduction only on day 0 post-TBI, but did not observe subsequent hyperperfusion.52,53 The observed hyperperfusion could lead to detrimental changes by causing increased edema and, potentially, increasing the rate of hemorrhage.54
Interestingly, MB had minimal effects on CBF, when compared to vehicle-treated animals. Previous studies have reported that MB does not affect CBF55 or increased CBF56 only slightly in normal animals. In this study, acute and delayed MB increased CBF values only on day 14, compared to vehicle-treated animals.
Behavioral improvement with methylene blue
We utilized the foot-fault and asymmetry tests to determine functional outcomes post-TBI to the S1FL cortex and the effects of MB on functional recovery. Despite the presence of a significant lesion in vehicle-treated animals at days 7 and 14, forepaw asymmetry scores returned to normal for all groups whereas forelimb foot-fault scores remained significantly abnormal on day 7, returning to normal on day 14. Delayed MB treatment was as effective as acute MB treatment in terms of percent asymmetry measures, despite the fact that lesion volumes were significantly lower in the delayed administration group. Interestingly, the foot-fault test revealed a slower recovery in functional deficits compared to the asymmetry test. This suggests that the foot-fault test may be a more sensitive measure of forelimb functional deficits in mild-to-moderate TBIs. Regression analysis also confirmed increased correlation between lesion volume and percent foot faults scores, when compared to lesion volume and percent asymmetry scores correlation analysis, further supporting that the foot-fault test is a more sensitive measure of motor impairment in TBI rats. In all groups, for both functional tests, animals recovered by day 14. This suggests that there may be functional compensation or reorganization occurring with moderate TBI. Previous studies have revealed a similar phenomenon and have suggested that recovery of brain function may occur owing to diaschisis, behavioral substitution, or that another area of the brain may take over the function.57 For diaschisis to occur, areas outside of the injury that may be depressed in terms of function initially recover over time and begin to function again. Rats may also learn new strategies over time to compensate for functional deficits. However, the tests utilized in this study may also not be sensitive enough to detect more-subtle injuries. Therefore, utilization of enhanced multi-modal imaging methods may provide increased identification of potential outcome measures that are not readily detectable by standard behavioral tests.
Limitations of the current study
There are a few limitations to the current study. 1) Differences in initial lesion volume could confound the experimental data given that each individual animal may start with differing lesion volume. The CCI model utilized in this study minimizes the variability of starting lesion volume and the results demonstrate nonsignificant differences in initial lesion volume between animals, further validating the findings. 2) The functional outcome measures may not be sensitive enough to detect chronic abnormalities in motor function. Therefore, future directions will include behavioral tests with increased sensitivity for motor deficits. We also intend on including assessments for sensory deficits given that this is a common pathology post-TBI. We will assess fine motor impairments using the Vermicili handling test and sensory deficits using the Morris water maze in future studies. 3) Histological data were not included in the current study given that we have previously reported such data and found no significant difference in lesion volume detected by MRI and Nissl or Fluro Jade immunohistology.25 Future studies will include assessment of neurodegeneration and markers of inflammation.
Conclusion
The present study provides additional evidence that delayed MB treatment is neuroprotecitve against mild TBI and further substantiates that targeting the mitochondria provides a mechanism of neuroprotection postinjury. Importantly, delayed administration of MB was also found to attenuate secondary brain injury effects. This has important implications for patients that do not have medical treatment readily available after an acute injury to the brain.
Acknowledgments
This work was supported, in part, by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS45879; to T.Q.D.), a TL1 grant (to J.A.L.) and KL2 (to L.T.W.) through the Clinical Translational Science Awards (CTSA; parent grant 8UL1TR000149, TL1TR001119, and TR001118), and the Mike Hogg Fund (to L.T.W.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Globus M.Y., Alonso O., Dietrich W.D., Busto R., and Ginsberg M.D. (1995). Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 65, 1704–1711 [DOI] [PubMed] [Google Scholar]
- 3.Adelson P.D., Whalen M.J., Kochanek P.M., Robichaud P., and Carlos T.M. (1998). Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir. Suppl. 71, 104–106 [DOI] [PubMed] [Google Scholar]
- 4.Crack P.J., and Taylor J.M. (2005). Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 38, 1433–1444 [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi R.K., and Beal M.F. (2008). Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci. 1147, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L., Aaronson S.A., Abrams J., Alnemri E.S., Andrews D.W., Baehrecke E.H., Bazan N.G., Blagosklonny M.V., Blomgren K., Borner C., Bredesen D.E., Brenner C., Castedo M., Cidlowski J.A., Ciechanover A., Cohen G.M., De Laurenzi V., De Maria R., Deshmukh M., Dynlacht B.D., El-Deiry W.S., Flavell R.A., Fulda S., Garrido C., Golstein P., Gougeon M.L., Green D.R., Gronemeyer H., Hajnoczky G., Hardwick J.M., Hengartner M.O., Ichijo H., Jaattela M., Kepp O., Kimchi A., Klionsky D.J., Knight R.A., Kornbluth S., Kumar S., Levine B., Lipton S.A., Lugli E., Madeo F., Malomi W., Marine J.C., Martin S.J., Medema J.P., Mehlen P., Melino G., Moll U.M., Morselli E., Nagata S., Nicholson D.W., Nicotera P., Nunez G., Oren M., Penninger J., Pervaiz S., Peter M.E., Piacentini M., Prehn J.H., Puthalakath H., Rabinovich G.A., Rizzuto R., Rodrigues C.M., Rubinsztein D.C., Rudel T., Scorrano L., Simon H.U., Steller H., Tschopp J., Tsujimoto Y., Vandenabeele P., Vitale I., Vousden K.H., Youle R.J., Yuan J., Zhivotovsky B., and Kroemer G. (2009). Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16, 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmorstein A.D., Csaky K.G., Baffi J., Lam L., Rahaal F., and Rodriguez-Boulan E. (2000). Saturation of, and competition for entry into, the apical secretory pathway. Proc. Natl. Acad. Sci. U. S. A. 97, 3248–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan P.G., Thompson M.B., and Scheff S.W. (1999). Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 9.Scheff S.W., and Sullivan P.G. (1999). Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 16, 783–792 [DOI] [PubMed] [Google Scholar]
- 10.Sullivan P.G., Rabchevsky A.G., Hicks R.R., Gibson T.R., Fletcher-Turner A., and Scheff S.W. (2000). Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan P.G., Thompson M., and Scheff S.W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161, 631–637 [DOI] [PubMed] [Google Scholar]
- 12.Okonkwo D.O., Buki A., Siman R., and Povlishock J.T. (1999). Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport 10, 353–358 [DOI] [PubMed] [Google Scholar]
- 13.Okonkwo D.O., and Povlishock J.T. (1999). An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 19, 443–451 [DOI] [PubMed] [Google Scholar]
- 14.Scheindlin S. (2008). Something old…something blue. Mol. Interv. 8, 268–273 [DOI] [PubMed] [Google Scholar]
- 15.Rojas J.C., Bruchey A.K., and Gonzalez-Lima F. (2012). Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 96, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas J.C., John J.M., Lee J., and Gonzalez-Lima F. (2009). Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox. Res. 15, 260–273 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Rojas J.C., and Gonzalez-Lima F. (2006). Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox. Res. 9, 47–57 [DOI] [PubMed] [Google Scholar]
- 18.Ishiwata A., Sakayori O., Minoshima S., Mizumura S., Kitamura S., and Katayama Y. (2006). Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta Neurol. Scand. 114, 91–96 [DOI] [PubMed] [Google Scholar]
- 19.Oz M., Lorke D.E., and Petroianu G.A. (2009). Methylene blue and Alzheimer's disease. Biochem. Pharmacol. 78, 927–932 [DOI] [PubMed] [Google Scholar]
- 20.O'Leary J.C., III, Li Q., Marinec P., Blair L.J., Congdon E.E., Johnson A.G., Jinwal U.K., Koren J., III, Jones J.R., Kraft C., Peters M., Abisambra J.F., Duff K.E., Weeber E.J., Gestwicki J.E., and Dickey C.A. (2010). Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol. Neurodegener. 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina D.X., Caccamo A., and Oddo S. (2011). Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 21, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez P., Jiang Z., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue treatment delays progression of perfusion-diffusion mismatch to infarct in permanent ischemic stroke Brain Res. 1588, 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Q., Du F., Huang S., Rodriguez P., Watts L.T., and Duong T.Q. (2013). Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One 8, e79833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifton J., II, and Leikin J.B. (2003). Methylene blue. Am. J. Ther. 10, 289–291 [DOI] [PubMed] [Google Scholar]
- 25.Talley Watts L., Long J.A., Chemello J., Van Koughnet S., Fernandez A., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue is neuroprotective against mild traumatic brain injury. J. Neurotrauma 31, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez T.D., and Schallert T. (1988). Seizures and recovery from experimental brain damage. Exp. Neurol. 102, 318–324 [DOI] [PubMed] [Google Scholar]
- 27.Baskin Y.K., Dietrich W.D., and Green E.J. (2003). Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci. Methods 129, 87–93 [DOI] [PubMed] [Google Scholar]
- 28.Soblosky J.S., Matthews M.A., Davidson J.F., Tabor S.L., and Carey M.E. (1996). Traumatic brain injury of the forelimb and hindlimb sensorimotor areas in the rat: physiological, histological and behavioral correlates. Behav. Brain Res. 79, 79–92 [DOI] [PubMed] [Google Scholar]
- 29.Watts L.T., Long J., Chemello J., Van Koughnet S., Fernandez A., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue is neuroprotective against mild traumatic brain injury. J. Neurotrauma 31, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X., Fisher M., Shen Q., Sotak C.H., and Duong T.Q. (2004). Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann. Neurol. 55, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salaris S.C., Babbs C.F., and Voorhees W.D., III (1991). Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem. Pharmacol. 42, 499–506 [DOI] [PubMed] [Google Scholar]
- 32.Poteet E., Winters A., Yan L.J., Shufelt K., Green K.N., Simpkins J.W., Wen Y., and Yang S.H. (2012). Neuroprotective actions of methylene blue and its derivatives. PLoS One 7, e48279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miclescu A., Sharma H.S., Martijn C., and Wiklund L. (2010). Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit. Care Med. 38, 2199–2206 [DOI] [PubMed] [Google Scholar]
- 34.Bardakci H., Kaplan S., Karadeniz U., Ozer C., Bardakci Y., Ozogul C., Birincioglu C.L., and Cobanoglu A. (2006). Methylene blue decreases ischemia-reperfusion (I/R)-induced spinal cord injury: an in vivo study in an I/R rabbit model. Eur. Surg. Res. 38, 482–488 [DOI] [PubMed] [Google Scholar]
- 35.Ansari M.A., Roberts K.N., and Scheff S.W. (2008). A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma 25, 513–526 [DOI] [PubMed] [Google Scholar]
- 36.Long J.A., Watts L.T., Chemello J., Huang S., Shen Q., and Duong T.Q. (2015). Multiparametric and longitudinal MRI characterization of mild traumatic brain injury in rats. J. Neurotrauma 32, 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenn A.M., Skendelas J.P., Moussa D.N., Muccigrosso M.M., Popovich P.G., Lifshitz J., Eiferman D.S., and Godbout J.P. (2015). Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J. Neurotrauma 32, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marmarou A., Signoretti S., Fatouros P.P., Portella G., Aygok G.A., and Bullock M.R. (2006). Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J. Neurosurg 104, 720–730 [DOI] [PubMed] [Google Scholar]
- 39.Marmarou A., Fatouros P.P., Barzo P., Portella G., Yoshihara M., Tsuji O., Yamamoto T., Laine F., Signoretti S., Ward J.D., Bullock M.R., and Young H.F. (2000). Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J. Neurosurg. 93, 183–193 [DOI] [PubMed] [Google Scholar]
- 40.Evgenov O.V., Evgenov N.V., Mollnes T.E., and Bjertnaes L.J. (2002). Methylene blue reduces pulmonary oedema and cyclo-oxygenase products in endotoxaemic sheep. Eur. Respir. J. 20, 957–964 [DOI] [PubMed] [Google Scholar]
- 41.Fenn A.M., Skendelas J.P., Moussa D.N., Muccigrosso M.M., Popovich P.G., Lifshitz J., Eiferman D.S., and Godbout J.P. (2015). Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J. Neurotrauma 32, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmond C.H., Menon D.K., Chatfield D.A., Williams G.B., Pena A., Sahakian B.J., and Pickard J.D. (2006). Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 29, 117–124 [DOI] [PubMed] [Google Scholar]
- 43.Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O., and Grossman R.I. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 103, 298–303 [DOI] [PubMed] [Google Scholar]
- 44.Huisman T.A., Schwamm L.H., Schaefer P.W., Koroshetz W.J., Shetty-Alva N., Ozsunar Y., Wu O., and Sorensen A.G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 25, 370–376 [PMC free article] [PubMed] [Google Scholar]
- 45.Ptak T., Sheridan R.L., Rhea J.T., Gervasini A.A., Yun J.H., Curran M.A., Borszuk P., Petrovick L., and Novelline R.A. (2003). Cerebral fractional anisotropy score in trauma patients: a new indicator of white matter injury after trauma. AJR Am. J. Roentgenol. 181, 1401–1407 [DOI] [PubMed] [Google Scholar]
- 46.Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., and Meyerand M.E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 23, 794–802 [PMC free article] [PubMed] [Google Scholar]
- 47.Ohta M., Higashi Y., Yawata T., Kitahara M., Nobumoto A., Ishida E., Tsuda M., Fujimoto Y., and Shimizu K. (2013). Attenuation of axonal injury and oxidative stress by edaravone protects against cognitive impairments after traumatic brain injury. Brain Res. 1490, 184–192 [DOI] [PubMed] [Google Scholar]
- 48.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 49.Hayward N.M., Tuunanen P.I., Immonen R., Ndode-Ekane X.E., Pitkänen A., and Gröhn O. (2011). Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J. Cereb. Blood Flow Metab. 31, 166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dore-Duffy P., Wang S., Mehedi A., Katyshev V., Cleary K., Tapper A., Reynolds C., Ding Y., Zhan P., Rafols J., and Kreipke C.W. (2011). Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurol. Res. 33, 176–186 [DOI] [PubMed] [Google Scholar]
- 51.Thomale U.W., Schaser K., Kroppenstedt S.N., Unterberg A.W., and Stover J.F. (2002). Cortical hypoperfusion precedes hyperperfusion following controlled cortical impact injury. Acta Neurochir. Suppl. 81, 229–231 [DOI] [PubMed] [Google Scholar]
- 52.Shen Y., Kou Z., Kreipke C.W., Petrov T., Hu J., and Haacke E.M. (2007). In vivo measurement of tissue damage, oxygen saturation changes and blood flow changes after experimental traumatic brain injury in rats using susceptibility weighted imaging. Magn. Reson. Imaging 25, 219–227 [DOI] [PubMed] [Google Scholar]
- 53.Pasco A., Lemaire L., Franconi F., Lefur Y., Noury F., Saint-Andre J.P., Benoit J.P., Cozzone P.J., and Le Jeune J.J. (2007). Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. J. Neurotrauma 24, 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muttaqin Z., Uozumi T., Kuwabara S., Arita K., Kurisu K., Ohba S., Kohno H., Ogasawara H., Ohtani M., and Mikami T. (1993). Hyperaemia prior to acute cerebral swelling in severe head injuries: the role of transcranial Doppler monitoring. Acta Neurochir. (Wien) 123, 76–81 [DOI] [PubMed] [Google Scholar]
- 55.Huang S., Du F., Shih Y.Y., Shen Q., Gonzalez-Lima F., and Duong T.Q. (2013). Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. Neuroimage 72, 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin A.L., Poteet E., Du F., Gourav R.C., Liu R., Wen Y., Bresnen A., Huang S., Fox P.T., Yang S.H., and Duong T.Q. (2012). Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One 7, e46585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feeney D.M. and Baron J.C. (1986). Diaschisis. Stroke 17, 817–830 [DOI] [PubMed] [Google Scholar]