Abstract
Adenosine triphosphate-binding cassette (ABC) transport proteins ABCC1 and ABCB1 (also known as multidrug resistance-associated protein 1 and p-glycoprotein, respectively), are key membrane efflux transporters of drugs and endogenous substrates, including in the brain. The impact of traumatic brain injury (TBI) on ABCC1 and ABCB1 expression in humans is unknown. We hypothesized that ABCC1 and ABCB1 expression would be altered in brain tissue from patients acutely after severe TBI. Archived TBI samples (n=10) from our Brain Trauma Research Center and control samples (n=7) from our Alzheimer Disease Research Center were obtained under Institutional Review Board approval. Protein was extracted from fresh frozen cortical brain tissue for Western blot analysis and sections were obtained from fixed cortical tissue for immunohistochemistry. Relative abundance of ABCC1 was increased in samples from TBI versus controls (2.8±2.5 fold; p=0.005). ABCC1 immunohistochemistry was consistent with Western blot data, with increased immunoreactivity in cerebral blood vessel walls, as well as cells with the morphological appearance of neurons and glia in TBI versus controls. Relative abundance of ABCB1 was similar between TBI and controls (p=0.76), and ABCB1 immunoreactivity was primarily associated with cerebral blood vessels in both groups. These human data show that TBI increases ABCC1 expression in the brain, consistent with possible implications for both patients receiving pharmacological inhibitors and/or substrates of ABCC1 after TBI.
Key words: : ABCB1, ABCC1, MDR1, MRP1, multidrug resistance protein, multidrug resistance-associated protein, p-glycoprotein
Introduction
Traumatic brain injury (TBI) affects 1.7 million people each year in the United States.1 Of this number, about 25% are considered moderate to severe injury.2 Patients with severe TBI have a very high risk of death or survival with poor neurological outcome, ranging from 21% to 85% as a person's age increases.3 These statistics have improved only slightly through the years, attributed to development of evidence-based guidelines for supportive management,4 but not to discovery of new, brain-targeted therapeutics. The latter has been the focus of many research laboratories, including our own. While many potential targeted therapies for TBI have shown promise in experimental models, disappointingly no therapies have shown benefit in human trials.5–14
One potential culprit for failure of pharmacological agents targeting TBI is the unknown impact of drug transporters at the blood–brain barrier (BBB) and brain-cerebrospinal fluid (CSF) barrier (BCSFB) in terms of entry into and elimination from injured brain, respectively. In addition to imposing physical barriers, the BBB and BCSFB represent dynamic barriers because they contain a vast number of energy-dependent membrane transporters.15
Of these, adenosine triphosphate (ATP) binding cassette (ABC) transporters are a well-studied family of membrane bound proteins found throughout the body, with the most studied in the brain being P-glycoprotein, or ABCB1 using contemporary nomenclature.16 These ABC transporters contribute in large part to drug resistance, and thus are also commonly referred to as multidrug resistance or resistance-associated proteins (MDR or MRP, respectively). Within the BBB and cells of the central nervous system (CNS), several subtypes of ABC transporters (e.g., ABCB1, ABCC1-7, ABCG2) are present and function in selective active transport of substrates such as glutathione (GSH) conjugates, hormones, cytokines, and xenobiotics.17–20
Surprisingly, changes in ABC transporter expression and/or function after human TBI have not been reported to our knowledge. It could be hypothesized that changes in these ABC transporters after TBI might influence delivery and brain bioavailability of drug substrates and, consequently, outcome. As a first step, we describe the profile of two ABC transporters: ABCC1 (also known as MRP1) and ABCB1 (also known as P-glycoprotein or MDR1) in brain samples from adult patients with severe TBI and neurologically normal controls.
Methods
Subjects
Human brain tissue was acquired for analysis from the University of Pittsburgh Institutional Review Board approved Brain Trauma Research Center and the Alzheimer Disease Research Center (ADRC) tissue banks. Brain biopsy tissue from patients with severe TBI who underwent a decompressive craniectomy for their injury was resected from the pericontusional area of the temporal and/or frontal cortex and flash frozen with liquid nitrogen. Samples were kept frozen and stored at −80°C for Western blot analysis and/or fixed in 4% paraformaldehyde for immunohistochemistry as described in previous studies.21–25 Postmortem control samples of frontal cortex tissue from subjects without neurodegenerative disease were processed in a like manner.
Western blotting
Crude membrane fractions were used for Western blot analysis. Briefly, brain tissue was thawed on ice, then placed in buffer (10 mM Tris buffer, pH 7.6, 0.5M MgCl2) containing a protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO) for 15 min, then sonicated. Tonicity restoration buffer (5M NaCl) was added to the sonicated tissue followed by centrifugation at 300×g at 4°C. The resultant supernatant was further centrifuged at 100,000×g at 4°C. The supernatant was discarded; resuspension buffer (0.25M mannitol, 1M sucrose, 10 mM Tris-HCl, 0.1M ethylenediaminetetraacetic acid, and protease inhibitor cocktail) was added to the pellet followed by sonication to resuspend the tissue. Protein concentration was determined using the Pierce BCA Assay Kit (Thermo Scientific, Waltham, MA).
Crude membrane fractions were prepped in a solution of 2×Bio-Rad Laemelli sample buffer (Bio-Rad, Hercules, CA) premixed with B-mercaptoethanol and extra lysis buffer, heated in a 37°C water bath for 30 min, then loaded into NuPAGE Novex 3–8% Tris-Acetate Gels (Invitrogen Life Technologies, Grand Island, NY). Crude membrane fractions from MDCKII cell lines overexpressing human ABCC1 and ABCB1 (kindly provided by Prof. Piet Borst, Netherlands Cancer Institute) were added to respective gels to serve as positive controls, as well as a fluorescently labeled molecular weight marker (Odyssey protein molecular weight marker 10–250 kDa, Li-COR, Lincoln, NE). Electrophoresis was performed at 200V constant in Tris/Acetate/SDS running buffer.
Proteins were transferred to PVDF membranes (EMD Millipore, Billerica, MA) using 30V constant at 4°C overnight. Membranes were washed, blocked with Odyssey blocking buffer (Li-COR) for 1 h and rewashed. Membranes were then incubated in 1:20 dilutions of rat monoclonal antibody against human ABCC1 (P33527, Enzo Life Sciences, Inc., Farmingdale, NY) or mouse monoclonal antibody against ABCB1 (C219, EMD Millipore) at 4°C overnight. For comparison of sample loading, an antibody against human β actin (A2066, Sigma-Aldrich) was also added at a 1:1000 dilution. Washed membranes were then incubated in green fluorescent-conjugated secondary goat antirat antibody (1:5,000) for ABCC1 or goat antimouse antibody (1:15,000) for ABCB1, and red fluorescent-conjugated goat antirabbit antibody (1:20,000) for β actin.
After additional washes, membranes were imaged using an Odyssey scanner and images optimized and quantified using Odyssey Imager Software (Li-COR). Protein bands of interest were identified using positive controls from overexpressing MDCKII cell lines, and relative fluorescent intensity (RFI) for each target protein was quantified against a linear standard curve (r2=0.9917, 0.9262, 0.9573 for ABCC1, ABCB1, and β actin, respectively). To account for loading differences, band intensities are reported as a ratio of actin band intensity within each lane.
Immunohistochemistry
Fixed specimens were paraffin embedded, cut into 10 μm sections, and mounted onto glass slides. Slides were deparaffinized and rehydrated, followed by antigen retrieval using a decloaking solution (Antigen Decloaker, Biocare Medical, Concord, CA). Sections were then blocked in a 3% serum solution for 30 min before incubation in a 1:20 dilution of rat monoclonal anti-ABCC1 antibody (P33527, Enzo Life Sciences, Inc.) or a 1:20 dilution of mouse monoclonal anti-ABCB1 (JSB-1, ABCAM, Cambridge, MA) at 4°C overnight. Negative control slides were prepared in similar fashion but without primary antibody (primary delete).
Slides were washed then incubated in appropriate secondary antibody for 1 h at room temperature, then in Peroxidase Vectastain ABC solutions (Vector Laboratories, Burlingame, CA). Immunohistochemical labeling was visualized using 3, 3'-diaminobenzidine (DAB) per manufacturer's instruction (ImmPACT DAB, Vector Laboratories). Sections were counterstained with hematoxylin and coverslipped using Permount (Thermo Scientific).
Statistical analysis
Data are presented as mean±standard deviation (SD) or median (range) where appropriate. Western blot data are presented as ratios of the RFI of the target protein in relation to β actin, with between group differences determined using the Mann-Whitney rank sum test. Immunohistochemical data were analyzed qualitatively. A p<0.05 was considered significant.
Results
Demographic data are shown in Table 1. For TBI patients, the average age was 43.3±22.0 y. Sixty percent of patients were male. The median initial Glasgow Coma Scale (GCS) score recorded in the emergency department was 6 (range, 3–9). Mean time from TBI to biopsy was 36.8±53.3 h. Of these 10 patients with TBI who underwent decompressive craniectomy, 4 survived and 6 died. For control subjects, the average age was 62.6±16.3 years. Fifty seven percent were male.
Table 1.
Patient Demographics and Clinical Characteristics
| Patient | Age (y) | Sex | iGCS | Time (h) | Biopsy region | Assays |
|---|---|---|---|---|---|---|
| TBI 1 | 20 | Female | 5 | 3 | Temporal lobe | WB |
| TBI 2 | 39 | Male | 8 | 6 | Temporal lobe | WB/IHC |
| TBI 3 | 20 | Male | 8 | 10 | Frontal/temporal lobes | WB |
| TBI 4 | 46 | Female | 5 | 16 | Temporal lobe | WB |
| TBI 5 | 58 | Female | 8 | 118 | Temporal lobe | WB |
| TBI 6 | 44 | Male | 7 | 154 | Frontal lobe | WB |
| TBI 7 | 62 | Male | 5 | 23 | Temporal lobe | WB |
| TBI 8 | 18 | Male | 3 | 13 | Temporal lobe | IHC |
| TBI 9 | 41 | Male | 3 | 16 | Temporal lobe | IHC |
| TBI 10 | 87 | Female | 9 | 9 | Temporal lobe | IHC |
| Control 1 | 44 | Male | N/A | N/A | Frontal lobe | WB |
| Control 2 | 51 | Male | N/A | N/A | Frontal lobe | WB |
| Control 3 | 64 | Male | N/A | N/A | Frontal lobe | WB |
| Control 4 | 49 | Female | N/A | N/A | Frontal lobe | WB |
| Control 5 | 82 | Female | N/A | N/A | Frontal lobe | WB |
| Control 6 | 62 | Male | N/A | N/A | Frontal lobe | IHC |
| Control 7 | 86 | Female | N/A | N/A | Frontal lobe | IHC |
iGCS, initial Glasgow Coma Scale score; TBI, traumatic brain injury; WB, Western blot; IHC, immunohistochemistry.
ABCC1
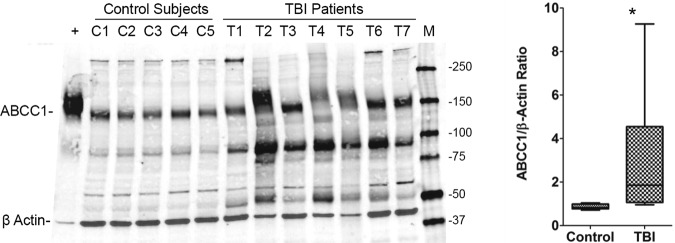
Western blot analysis for ABCC1 is shown in Figure 1. In control and TBI samples, the ABCC1 antibody detected an approximately 150–190 kDa peptide band that was also prominent in the ABCC1 overexpressing cell line positive control. RFI was determined for each subject (images were converted to grayscale) and values adjusted for protein loading. Patients with TBI had increased ABCC1:β-actin ratios versus control subjects (Fig. 1; p<0.01). Note that the peptide bands for both the ABCC1 overexpressing cell line and subjects' samples generally migrated lower than the predicted 190 kDa on Western blot. Please also note what appear to be ABCC1 proteolytic fragments of ∼90 and 50 kDa in the TBI group.
FIG. 1.
ABCC1 protein expression in the human brain. ABCC1 protein abundance was measured using Western blotting and reported as a ratio of β actin band intensity in each lane. Box plot shows median, interquartile range, and 10–90th percentiles. C1–5, control cases; T1–7, TBI patients; +, human ABCC1-overexpressing MDCKII cells (positive control); M, marker. *p<0.05 vs. control cases.
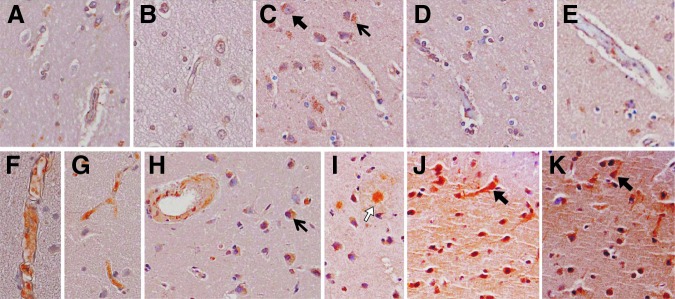
Immunohistochemistry was performed in sections from patients with TBI (T2, T8, T9, T10) and controls (C6, C7) to infer cell-type(s) immunoreactive for ABCC1 (Fig. 2). Cells with the morphological appearance of endothelial cells, astrocytes, and neurons, as well as other smaller, nonvascular cells, were ABCC1-immunoreactive. Intensity of immunolabeling was consistent with Western blot protein data—i.e., appeared increased in patients with TBI versus control subjects.
FIG. 2.
ABCC1 immunoreactivity in control cases (A–E) and patients with TBI (F–K). ABCC1 was identified colorimetrically using diaminobenzidine (brown), and sections were counterstained with hematoxylin. A, B. Control 6. C–E. Control 7. F. TBI patient 8. G. TBI patient 9. H–J. TBI patient 10. K. TBI patient 6. Images were taken at 40×; highlighted are ABCC1 immunoreactive cells with the morphologic appearance of neurons (black block arrows) or astrocytes (white block arrows), and other nonvascular immunoreactive cells (arrows).
ABCB1
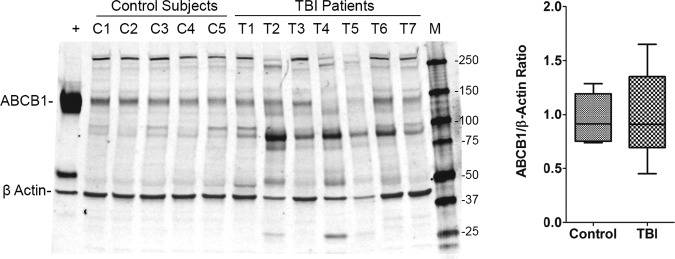
Western blot analysis for ABCB1 is shown in Figure 3. The peptide band for ABCB1 corresponding to the ABCB1 positive control was identified, and RFI was determined for each patient (images were converted to grayscale). The RFI for β actin within each corresponding lane was also calculated to adjust for protein loading. The ABCB1:β actin ratios were similar between patients with TBI and control subjects, although there was greater variability in the TBI versus control subjects (Fig. 3; p>0.05). Note that the peptide bands for both the ABCB1 overexpressing cell line and patient samples migrated below the predicted 170 kDa band on Western blot. Please also note that similar to ABCC1, proteolytic fragments of ∼90 and 50 kDa were also observed in the TBI group.
FIG. 3.
ABCB1 protein expression in the human brain. ABCB1 protein abundance measured using Western blotting and reported as a ratio of β actin band intensity in each lane. Box plot shows median, interquartile range, and 10–90th percentiles. C1–5, control cases; T1–7, patients with traumatic brain injury (TBI); +, human ABCB1-overexpressing MDCKII cells (positive control); M, marker.
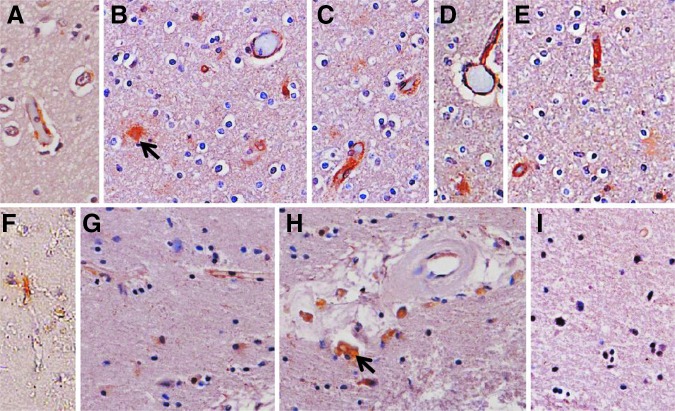
Immunohistochemistry was performed in patients with TBI (T2, T8, T9, T10), and controls (C6, C7) to infer cell-type(s) immunoreactive for ABCB1 (Fig. 4). Cells with the morphological appearance of endothelial cells were primarily seen, although other smaller, perivascular, and nonvascular cells were also ABCB1-immunoreactive. Intensity of immunolabeling appeared to be less robust in patients with TBI versus controls in sections from subjects where immunohistochemistry was performed.
FIG. 4.
ABCB1 immunoreactivity in control cases (A–E) and patients with traumatic brain injury (TBI) (F–I). ABCB1 was identified colorimetrically using diaminobenzidine (brown), and sections were counterstained with hematoxylin. (A) Control 6. (B–E) Control 7. (F) TBI patient 8. (G–I) TBI patient 6. Images were taken at 40×; highlighted are nonvascular immunoreactive cells (arrows).
Discussion
This is the first study to our knowledge to describe expression of two clinically relevant ABC transporters, ABCC1 and ABCB1, in human neocortex after TBI. These data represent a first step toward uncovering the potential impact of TBI-induced changes in drug transporters in terms of BBB penetration and elimination of therapeutics from the injured brain. Based on our data, a return to bench study in experimental TBI models to determine the role of TBI-induced changes in ABC transporters and subsequent effect on pharmacokinetics of relevant therapeutic and endogenous substrates appears warranted.
In humans, ABCC1 (also known as MRP1) has been reported to be on the luminal side of brain capillary endothelial cells in samples taken after neurosurgical procedures26 and in hippocampal neurons and astrocyte end-feet from patients with mesial temporal lobe epilepsy,27 but not in brain microvessel endothelial cells or perivascular astrocytes isolated from humans without brain disease,28 suggesting upregulation and/or relocation during stress. Undetectable or low level expression of ABCC1 associated with cerebral blood vessels in humans without brain disease,28,29 and the above referenced reports of multiple CNS cell types expressing ABCC1 in humans with brain disease, are consistent with our immunohistochemical data (Fig. 2).
While there are no studies examining ABCC1 in TBI, it has been reported in cultured rat neurons and astrocytes.30,31 In addition, a single nucleotide polymorphism (SNP) for ABCC1 has been shown to be associated with neurological outcome after TBI,32 although whether this represents a gain or loss of function (or neither) SNP is unknown.
There are many known substrates for ABCC1 including reduced GSH, glutathione disulfide, glucuronide, and other glutathione conjugates, anticancer drugs, protease inhibitors, and small peptides.33 Key on this list is GSH, as one could speculate that increased ABCC1 expression may correlate with increased GSH elimination from the brain after TBI. Indeed, the transient increase in CSF GSH reported by Bayir and coworkers34 at 24 h followed by depletion after 3 days in children after severe TBI is consistent with enhanced excretion of GSH from injured brain. Importantly, inhibition of active GSH excretion by ABCC1 prevents neuronal apoptosis in vitro,35 and strategies to replete GSH using drugs modified (esterified or amide conjugates) to circumvent the BBB in experimental models of TBI have shown neuroprotection.36,37
ABCB1 (also known as MDR1 or P-glycoprotein) has been studied more so than ABCC1. ABCB1 is a prominent component of the dynamic BBB, with substrates relevant to the management of TBI that include opioids (e.g., morphine, fentanyl), anti-epileptics (e.g., phenytoin), and antibiotics (e.g., vancomycin).38 Of special relevance to TBI and its potential contribution to an increased risk of developing Alzheimer disease (AD), another ABCB1 substrate is β-amyloid peptide, and studies suggest that ABCB1 is down regulated in patients with AD.39 Further, it has been suggested that drug refractory epilepsy may be related to gain-of-function ABCB1 SNPs.40–44 In experimental models, ABCB1, predominantly expressed in brain microvessels at baseline,45 is reduced 2 months after controlled cortical impact in juvenile rats.46
Our data did not reveal differences between relative protein levels of ABCB1 in TBI versus controls; however, this could be because of the limited sample size and/or the fact that the average time from injury to biopsy was 37 h.
An alternative explanation is that ABCB1 undergoes proteolysis after TBI. Our Western blot data demonstrate prominent ∼90 kDa peptide bands that appear increased in TBI versus control subjects (Fig. 3). Serine protease cleavage of the linker region of isolated ABCB1 produces an ∼80 kDa peptide stimulating its ATPase activity,47 while calpain proteolysis results in ∼98 and 69 kDa fragments that may impact ABCB1 turnover.48 We also observed ABCC1 proteolytic fragments after TBI that were ∼90 and 50 kDa (Fig. 1). Proteolysis of ABCC1 by serine proteases is inhibited by GSH.49
Given the potential role of cysteine and serine proteases after TBI,50–52 it is tempting to speculate that protease activation after TBI may modulate ABCB1 and/or ABCC1 transporter expression and/or function. Further study including identification of these peptide bands using mass spectroscopy and functional assays ex vivo appear justified.
Our study has limitations. It is not possible to attain age- and sex-matched human surgical control tissue. In addition, there is inherent variability in terms of severity and location of injury, time to biopsy, and age in studies using tissue from humans after TBI.21,25,53 As mentioned, sample sizes in both TBI and control groups were limited, and further, the fact that all patients with TBI underwent a decompressive craniectomy dictate that these findings cannot necessarily be extrapolated to patients with TBI with less than severe injury. These valuable human samples, however, provide important insight into relevant mechanisms in the clinical setting.
Conclusion
Our findings provide proof-of-principle and suggest that ABC transporters in experimental models of TBI deserve further exploration—specifically, the impact of TBI on ABC transporter expression and function, and the role of ABC transporters on drug and substrate brain bioavailability. The latter may represent a new therapeutic approach to improving pharmacological effectiveness of promising neuroprotective agents for patients with TBI.
Acknowledgments
This work was supported by NIH grants R01 NS069247 (RSBC, PEE), P50 NS30318 (DOO, AMP, PMK, RSBC), and AG05133 (the University of Pittsburgh ADRC), and NCATS KL2 TR000146 (PEE); and the Children's Hospital of Pittsburgh of UPMC Scientific Program. We would like to thank Professor Piet Borst (Netherlands Cancer Institute) for generously providing the overexpressing cell lines.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention: Atlanta [Google Scholar]
- 2.Gerberding J.L., and Binder S. (2003). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention: Atlanta [Google Scholar]
- 3.Hukkelhoven C.W., Steyerberg E.W., Rampen A.J., Farace E., Habbema J.D., Marshall L.F., Murray G.D., and Maas A.I. (2003). Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J. Neurosurg. 99, 666–673 [DOI] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Haddad S., Tamim H.M., Al-Dawood A., Al-Qahtani S., Ferayan A., Al-Abdulmughni I., Al-Oweis J., and Rugaan A. (2010). Mortality reduction after implementing a clinical practice guidelines-based management protocol for severe traumatic brain injury. J. Crit. Care 25, 190–195 [DOI] [PubMed] [Google Scholar]
- 5.Brown J.I., Baker A.J., Konasiewicz S.J., and Moulton R.J. (1998). Clinical significance of CSF glutamate concentrations following severe traumatic brain injury in humans. J. Neurotrauma 15, 253–263 [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg H.M., Frankowski R.F., Contant C.F., Marshall L.F., and Walker M.D. (1988). High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 69, 15–23 [DOI] [PubMed] [Google Scholar]
- 7.Marshall L.F., and Marshall S.B. (1995). Pitfalls and advances from the international tirilazad trial in moderate and severe head injury. J. Neurotrauma 12, 929–932 [DOI] [PubMed] [Google Scholar]
- 8.Morris G.F., Bullock R., Marshall S.B., Marmarou A., Maas A., and Marshall L.F. (1999). Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J. Neurosurg. 91, 737–743 [DOI] [PubMed] [Google Scholar]
- 9.Muizelaar J.P., Kupiec J.W., and Rapp L.A. (1995). PEG-SOD after head injury. J. Neurosurg 83, 942. [DOI] [PubMed] [Google Scholar]
- 10.Murray G.D., Teasdale G.M., and Schmitz H. (1996). Nimodipine in traumatic subarachnoid haemorrhage: a re-analysis of the HIT I and HIT II trials. Acta Neurochir. (Wien) 138, 1163–1167 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M.L., Tator C.H., Rowed D.W., Reid S.R., Meguro K.,and Andrews D.F. (1984). The University of Toronto head injury treatment study: a prospective, randomized comparison of pentobarbital and mannitol. Can. J. Neurol. Sci. 11, 434–440 [DOI] [PubMed] [Google Scholar]
- 12.Teasdale G., Bailey I., Bell A., Gray J., Gullan R., Heiskanan O., Marks P.V., Marsh H., Mendelow D.A., Murray G. and et al. (1992). A randomized trial of nimodipine in severe head injury: HIT I. British/Finnish Co-operative Head Injury Trial Group. J. Neurotrauma 9 Suppl 2, S545–S550 [PubMed] [Google Scholar]
- 13.Ward J.D., Becker D.P., Miller J.D., Choi S.C., Marmarou A., Wood C., Newlon P.G., and Keenan R. (1985). Failure of prophylactic barbiturate coma in the treatment of severe head injury. J. Neurosurg. 62, 383–388 [DOI] [PubMed] [Google Scholar]
- 14.Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., and Barsan W.G. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potschka H. (2010). Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol. Ther. 125, 118–127 [DOI] [PubMed] [Google Scholar]
- 16.Bendayan R., Lee G., and Bendayan M. (2002). Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc. Res. Tech. 57, 365–380 [DOI] [PubMed] [Google Scholar]
- 17.Begley D.J. (2004). ABC transporters and the blood-brain barrier. Curr. Pharm. Des. 10, 1295–1312 [DOI] [PubMed] [Google Scholar]
- 18.Wijesuriya H.C., Bullock J.Y., Faull R.L., Hladky S.B., and Barrand M.A. (2010). ABC efflux transporters in brain vasculature of Alzheimer's subjects. Brain Res, 1358, 228–238 [DOI] [PubMed] [Google Scholar]
- 19.Sun H., Dai H., Shaik N., and Elmquist W.F. (2003). Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 55, 83–105 [DOI] [PubMed] [Google Scholar]
- 20.Hartz A.M., and Bauer B. (2011). ABC transporters in the CNS—an inventory. Curr. Pharm. Biotechnol 12, 656–673 [DOI] [PubMed] [Google Scholar]
- 21.Clark R.S., Kochanek P.M., Chen M., Watkins S.C., Marion D.W., Chen J., Hamilton R.L., Loeffert J.E., and Graham S.H. (1999). Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 13, 813–821 [DOI] [PubMed] [Google Scholar]
- 22.DeKosky S.T., Abrahamson E.E., Ciallella J.R., Paljug W.R., Wisniewski S.R., Clark R.S., and Ikonomovic M.D. (2007). Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 64, 541–544 [DOI] [PubMed] [Google Scholar]
- 23.Ikonomovic M.D., Uryu K., Abrahamson E.E., Ciallella J.R., Trojanowski J.Q., Lee V.M., Clark R.S., Marion D.W., Wisniewski S.R., and DeKosky S.T. (2004). Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 190, 192–203 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Alber S., Watkins S.C., Kochanek P.M., Marion D.W., Graham S.H., and Clark R.S. (2006). Proteolysis consistent with activation of caspase-7 after severe traumatic brain injury in humans. J. Neurotrauma 23, 1583–1590 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Graham S.H., Kochanek P.M., Marion D.W., Nathaniel P.D., Watkins S.C., and Clark R.S. (2003). Caspase-8 expression and proteolysis in human brain after severe head injury. FASEB J. 17, 1367–1369 [DOI] [PubMed] [Google Scholar]
- 26.Nies A.T., Jedlitschky G., Konig J., Herold-Mende C., Steiner H.H., Schmitt H.P., and Keppler D. (2004). Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience 129, 349–360 [DOI] [PubMed] [Google Scholar]
- 27.Aronica E., Gorter J.A., Ramkema M., Redeker S., Ozbas-Gerceker F., van Vliet E.A., Scheffer G.L., Scheper R.J., van der Valk P., Baayen J.C., and Troost D. (2004). Expression and cellular distribution of multidrug resistance-related proteins in the hippocampus of patients with mesial temporal lobe epilepsy. Epilepsia 45, 441–451 [DOI] [PubMed] [Google Scholar]
- 28.Daood M., Tsai C., Ahdab-Barmada M., and Watchko J.F. (2008). ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 39, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida Y., Ohtsuki S., Katsukura Y., Ikeda C., Suzuki T., Kamiie J., and Terasaki T. (2011). Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 117, 333–345 [DOI] [PubMed] [Google Scholar]
- 30.DeCory H.H., Piech-Dumas K.M., Sheu S.S., Federoff H.J., and Anders M.W. (2001). Efflux of glutathione conjugate of monochlorobimane from striatal and cortical neurons. Drug Metab. Dispos. 29, 1256–1262 [PubMed] [Google Scholar]
- 31.Falcao A.S., Bellarosa C., Fernandes A., Brito M.A., Silva R.F., Tiribelli C., and Brites D. (2007). Role of multidrug resistance-associated protein 1 expression in the in vitro susceptibility of rat nerve cell to unconjugated bilirubin. Neuroscience 144, 878–888 [DOI] [PubMed] [Google Scholar]
- 32.Cousar J.L., Conley Y.P., Willyerd F.A., Sarnaik A.A., Puccio A.M., Empey P.E., Kochanek P.M., Bell M.J., Okonkwo D.O., and Clark R.S. (2013). Influence of ATP-binding cassette polymorphisms on neurological outcome after traumatic brain injury. Neurocrit. Care 19, 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakos E., and Homolya L. (2007). Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflugers Arch. 453, 621–641 [DOI] [PubMed] [Google Scholar]
- 34.Bayir H., Kagan V.E., Tyurina Y.Y., Tyurin V., Ruppel R.A., Adelson P.D., Graham S.H., Janesko K., Clark R.S., and Kochanek P.M. (2002). Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res 51, 571–578 [DOI] [PubMed] [Google Scholar]
- 35.Hammond C.L., Marchan R., Krance S.M., and Ballatori N. (2007). Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J. Biol. Chem. 282, 14337–14347 [DOI] [PubMed] [Google Scholar]
- 36.Lai Y., Hickey R.W., Chen Y., Bayir H., Sullivan M.L., Chu C.T., Kochanek P.M., Dixon C.E., Jenkins L.W., Graham S.H., Watkins S.C., and Clark R.S. (2008). Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J. Cereb. Blood Flow Metab. 28, 540–550 [DOI] [PubMed] [Google Scholar]
- 37.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., and Sullivan P.G. (2014). N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 257, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva R., Vilas-Boas V., Carmo H., Dinis-Oliveira R.J., Carvalho F., de Lourdes Bastos M., and Remiao F. (2015). Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol. Ther. 149, 1–123 [DOI] [PubMed] [Google Scholar]
- 39.Cascorbi I., Fluh C., Remmler C., Haenisch S., Faltraco F., Grumbt M., Peters M., Brenn A., Thal D.R., Warzok R.W., and Vogelgesang S. (2013). Association of ATP-binding cassette transporter variants with the risk of Alzheimer's disease. Pharmacogenomics 14, 485–494 [DOI] [PubMed] [Google Scholar]
- 40.Loscher W., and Potschka H. (2002). Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J. Pharmacol. Exp. Ther. 301, 7–14 [DOI] [PubMed] [Google Scholar]
- 41.Sisodiya S.M., Lin W.R., Harding B.N., Squier M.V., and Thom M. (2002). Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 125, 22–31 [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui A., Kerb R., Weale M.E., Brinkmann U., Smith A., Goldstein D.B., Wood N.W., and Sisodiya S.M. (2003). Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N. Engl. J. Med. 348, 1442–1448 [DOI] [PubMed] [Google Scholar]
- 43.Basic S., Hajnsek S., Bozina N., Filipcic I., Sporis D., Mislov D., and Posavec A. (2008). The influence of C3435T polymorphism of ABCB1 gene on penetration of phenobarbital across the blood-brain barrier in patients with generalized epilepsy. Seizure 17, 524–530 [DOI] [PubMed] [Google Scholar]
- 44.Bournissen F.G., Moretti M.E., Juurlink D.N., Koren G., Walker M., and Finkelstein Y. (2009). Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia 50, 898–903 [DOI] [PubMed] [Google Scholar]
- 45.Gazzin S., Strazielle N., Schmitt C., Fevre-Montange M., Ostrow J.D., Tiribelli C., and Ghersi-Egea J.F. (2008). Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J. Comp. Neurol. 510, 497–507 [DOI] [PubMed] [Google Scholar]
- 46.Pop V., Sorensen D.W., Kamper J.E., Ajao D.O., Murphy M.P., Head E., Hartman R.E., and Badaut J. (2013). Early brain injury alters the blood-brain barrier phenotype in parallel with beta-amyloid and cognitive changes in adulthood. J. Cereb. Blood Flow Metab. 33, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuti S.L., and Rao U.S. (2002). Proteolytic cleavage of the linker region of the human P-glycoprotein modulates its ATPase function. J. Biol. Chem. 277, 29417–29423 [DOI] [PubMed] [Google Scholar]
- 48.Ohkawa K., Asakura T., Takada K., Sawai T., Hashizume Y., Okawa Y., and Yanaihara N. (1999). Calpain inhibitor causes accumulation of ubiquitinated P-glycoprotein at the cell surface: possible role of calpain in P-glycoprotein turnover. Int. J. Oncol. 15, 677–686 [DOI] [PubMed] [Google Scholar]
- 49.Ren X.Q., Furukawa T., Nakajima Y., Takahashi H., Aoki S., Sumizawa T., Haraguchi M., Kobayashi M., Chijiiwa K., and Akiyama S. (2005). GSH inhibits trypsinization of the C-terminal half of human MRP1. J. Biol. Chem. 280, 6231–6237 [DOI] [PubMed] [Google Scholar]
- 50.Clark R.S., Kochanek P.M., Watkins S.C., Chen M., Dixon C.E., Seidberg N.A., Melick J., Loeffert J.E., Nathaniel P.D., Jin K.L., and Graham S.H. (2000). Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem. 74, 740–753 [DOI] [PubMed] [Google Scholar]
- 51.Kampfl A., Posmantur R.M., Zhao X., Schmutzhard E., Clifton G.L., and Hayes R.L. (1997). Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: implications for pathology and therapy: a review and update. J. Neurotrauma 14, 121–134 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Luo W., and Reiser G. (2008). Trypsin and trypsin-like proteases in the brain: proteolysis and cellular functions. Cell. Mol. Life Sci. 65, 237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark R.S., Bayir H., Chu C.T., Alber S.M., Kochanek P.M., and Watkins S.C. (2008). Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 4, 88–90 [DOI] [PubMed] [Google Scholar]