Abstract
Endothelin-1 (ET-1), tissue plasminogen activator (tPA), and extracellular signal-regulated kinases-mitogen activated protein kinase (ERK-MAPK) are mediators of impaired cerebral hemodynamics after fluid percussion brain injury (FPI) in piglets. Microparticles (MPs) are released into the circulation from a variety of cells during stress, are pro-thrombotic and pro-inflammatory, and may be lysed with polyethylene glycol telomere B (PEG-TB). We hypothesized that MPs released after traumatic brain injury impair hypotensive cerebrovasodilation and that PEG-TB protects the vascular response via MP lysis, and we investigated the relationship between MPs, tPA, ET-1, and ERK-MAPK in that process. FPI was induced in piglets equipped with a closed cranial window. Animals received PEG-TB or saline (vehicle) 30-minutes post-injury. Serum and cerebrospinal fluid (CSF) were sampled and pial arteries were measured pre- and post-injury. MPs were quantified by flow cytometry. CSF samples were analyzed with enzyme-linked immunosorbent assay. MP levels, vasodilatory responses, and CSF signaling assays were similar in all animals prior to injury and treatment. After injury, MP levels were elevated in the serum of vehicle but not in PEG-TB–treated animals. Pial artery dilation in response to hypotension was impaired after injury but protected in PEG-TB–treated animals. After injury, CSF levels of tPA, ET-1, and ERK-MAPK were all elevated, but not in PEG-TB–treated animals. PEG-TB–treated animals also showed reduction in neuronal injury in CA1 and CA3 hippocampus, compared with control animals. These results show that serum MP levels are elevated after FPI and lead to impaired hypotensive cerebrovasodilation via over-expression of tPA, ET-1, and ERK-MAPK. Treatment with PEG-TB after injury reduces MP levels and protects hypotensive cerebrovasodilation and limits hippocampal neuronal cell injury.
Key words: : endothelin, fluid percussion injury, hypotensive cerebrovasodilation, microparticles, tissue plasminogen activator, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of injury-related death in children.1 While the effects of TBI have been investigated extensively in adult animal models, less is known in the newborn/infant. TBI can cause uncoupling of blood flow and metabolism, resulting in cerebral ischemia or hyperemia. Although hyperemia was historically considered the cause of diffuse brain swelling and secondary injury after TBI in the pediatric setting, more recent evidence suggests that hypoperfusion is the dominant derangement.2,3 Cerebral autoregulation is often impaired after TBI and concomitant hypotension ischemia may ensue, leading to poor outcome.4–7 Since ethical considerations constrain mechanistic studies in children with TBI, we have used an established porcine model of fluid percussion injury (FPI) that mimics TBI to corroborate clinical observations regarding autoregulation after TBI.8 Indeed, we have found that FPI constricts pial arteries, reduces cerebral blood flow (CBF), and disturbs cerebral autoregulation in piglets.8 Piglets offer the unique advantage of a gyrencepahalic brain containing substantial white matter, which is more sensitive to ischemic damage, similar to the human.
Microparticles (MPs) are 0.1–1.0 μm diameter anucleoid cell membrane vesicles that are released after shear stress, trauma, apoptotic activation, or inflammation. MPs have been shown to be highly thrombogenic have been identified as biomarkers in a number of human diseases and implicated in the pathogenesis of a subset therein.9–15 MPs have been recently shown to be elevated in the serum and cerebrospinal fluid (CSF) of patients with TBI, though not yet convincingly linked to injury severity or outcome.16,17 However, in patients with both stroke and subarachnoid hemorrhage, levels of MPs are elevated and correlated with ischemic events.18–20 Because of the enrichment of phosphatidyl serine on their external membranes, as well as their capacity as a source of soluble tissue factor, MPs are capable of being highly thrombogenic. Previous work in mice in a model of decompression injury has shown that MPs may be lysed in vivo with polyethylene glycol telomere B (PEG-TB; a fluorinated hydrocarbon surfactant) and that PEG-TB–induced MP-lysis may reverse the pathologic effects of MP elevation.10
Potassium channels play a central role in resting cerebrovascular tone and in vasodilation in response to hypotension.21–23 In contrast to the adult state, where nitric oxide predominates, prostaglandins are a critical mediator of autoregulatory dilation in response to hypotension in children and neonates.24–26 After TBI, this autoregulatory response to hypotension is blunted, resulting in secondary ischemic insults to the injured brain.27 Several key mediators in the pathway of this dysfunctional cerebrovascular autoregulation after TBI have been identified, including tissue plasminogen activator (tPA), endothelin-1 (ET-1), and activation of the extracellular signal-regulated kinases (ERK) isoform of a family of mitogen-activated protein kinases (ERK-MAPK).23,28–31
We therefore hypothesized that MPs released after TBI impair hypotensive cerebrovasodilation, PEG-TB protects this vascular response via MP lysis, and investigated the relationship between MPs, tPA, ET-1, and ERK-MAPK in that process.
Methods
Closed cranial window technique
Newborn pigs (1–5 d; 1.0–1.4 kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were anesthetized with isoflurane by mask (1–2%), maintained with a-chloralose (50–80 mg/kg supplemented with 5 mg/kg/h, intravenously). A catheter was inserted into a femoral artery to monitor blood pressure. The trachea was cannulated, and the animals were ventilated with room air. A water circulating heating blanket was used to maintain the animals at 37°-39°C, monitored rectally.
The closed cranial window technique for measuring pial artery diameter and collection of CSF for enzyme-linked immunosorbent assay (ELISA) analysis has been previously described.27 This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37°C and had the following chemistry, which is similar to that of endogenous CSF: pH, 7.33; pCO2, 46 mm Hg; and pO2, 43 mm Hg. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen, and a video microscaler.
The method used to induce brain FPI has been described previously.32 A device designed by the Medical College of Virginia was used. A small opening was made in the fronto-parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura and fluid coupled to the brain injury device (i.e., the shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device). The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9% saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9–2.3 atm, with a constant duration of 19–23 msec) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered concurrently with the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Pial small arteries (resting diameter, 120–160 μm) were examined. Vessel diameters were examined in all animals pre-FPI and 1 h post-FPI in response to hypotension (moderate, severe), papaverine (10−8 M, 10−6 M), and prostaglandin E2 (PGE2; 1 ng/mL, 10 ng/mL). Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 mL blood/Kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25% and 45%, respectively). Such decreases in blood pressure were maintained constant for 3–4 min by titration of additional blood withdrawal or blood reinfusion.
Typically, 2–3 mL of artificial CSF were flushed through the window over a 30 sec period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μL of the total cranial window volume of 500 μL was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side. Eight CSF samples were removed for each animal (four prior to FPI and four starting 50 minutes after FPI). Two 3 mL blood samples were taken from the left femoral arterial line into a Cyto-chex BCT (Streck; Omaha, NE) vacutainer tube. CSF samples were stored at −20°C and blood samples were stored at 4°C.
Three experimental groups were studied, vehicle control, FPI+vehicle, and FPI+PEG-TB. In vehicle control animals, vascular responses were obtained initially, the vehicle administered, and the vascular responses obtained again 1 h later (time control). In TBI animals, vascular responses were similarly obtained initially, vehicle or PEG-TB administered, and the vascular responses obtained again at 1 h post injury. PEG-TB–treated animals (n=7) were given 0.7 μL per gram of piglet weight of a 0.3% PEG-TB solution (weight/volume; Sigma Aldrich; St. Louis, MO) intravenously at 30 min post-FPI. FPI+vehicle animals (n=6) were given a similar volume of saline at 30 min post-FPI. Vehicle control animals (n=6) were surgicated, and vascular responses recorded initially and then again 1 h later after receiving a similar volume of saline at the 30 min time-point, but were not injured.
ELISA and radioimmunoassay
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to quantify CSF, tPA, and ERK-MAPK (Assay Designs, Farmingdale, NY) concentration, while radioimmunoassay kits were used to quantify ET-1 (Phoenix Pharmaceuticals, Burlingame, CA). Phosphorylated ERK-MAPK enzyme values were normalized to total form and then expressed as percent of the control condition.
MP isolation and analysis
All reagents were from Sigma Aldrich unless otherwise stated. Blood samples were mixed via gentle inversion 15–20 times and 1 mL aliquots were then centrifuged at 2300 g for 15 min to prepare platelet-poor plasma and the supernatant carefully removed. Centrifugation was performed at room temperature.
MP analysis was performed using three sets of antibodies for each sample. Each aliquot of sample was combined with 200 μL of Annexin buffer (diluted 1:10 in de-ionized water), 5 μL of each antibody (below), as well as 5 μL of 3 μm reference counting beads (final concentration 105/mL; Spherotech). The diluted annexin buffer was filtered prior to use through a 0.1 μm sterile low-protein-binding filter (Millipore, Danvers, MA). Annexin-V-FITC (BD Bioscience, Sparks, MD) antibody was used to identify annexin+MPs. All MP isolations were performed in triplicate and the average of the three values obtained for each specimen was used for analysis.
Flow cytometry was performed on a 10-color FACSCanto (BD Biosciences). A previously described flow rate–based method was used to quantify MPs in the sample.33 MPs were defined according to size and positive expression of Annexin V.
Histologic preparation
The brains were prepared for histopathology at 4 h post FPI. Pigs were transcardially perfused with heparinized saline, followed by 4% paraformaldehyde. The brains were then carefully removed from the skull and coronal sections were prepared at 0.6 cm intervals for gross examination and photography. For histopathology, staining was performed on parafiin-embedded slides and serial sections were cut at 30 μm intervals from the front face of each block and mounted on microscope slides. The sections (6 μm) were stained with hematoxylin and eosin (HE). Mean number of degenerating neuorns (±standard error of the mean [SEM]) in CA1 and CA3 hippocampus in vehicle control, FPI+vehicle, and FPI+PEG-TB–treated animals were determined, with data displayed for the side of the brain contralateral to the site of injury (the side where pial artery reactivity was investigated). To determine the extent of neuron degeneration in the hippocampal CA1 and CA3 subfields, six HE-stained sections from each animal were examined and the number of degenerating neurons were counted manually. Three frames (1×1.2 mm per frame) encompassing a total area of 3.6 mm2 in each slide from each hippocampal CA1 and CA3 region were analyzed at×100 magnification.
Statistical analysis
All statistical analyses were performed with Stata v11.2 (StataCorp, College Station, TX). Pial artery diameter values were analyzed using analysis of variance. If the value was significant, the data were then analyzed by Fisher's protected least significant difference test. MP concentrations were not normally distributed and thus a non-parametric test, the Wilcoxon Mann-Whitney test, was used. CSF signaling assay values and counts of degenerating neurons were analyzed with Student's t-tests. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean±SEM of the absolute value or as percentage changes from control value.
Results
Treatment with PEG-TB reduces MP levels
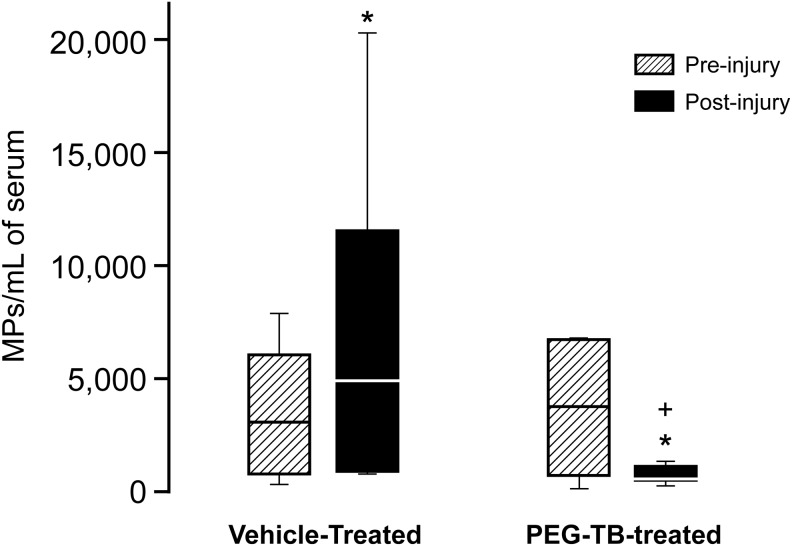
Prior to FPI, total levels of Annexin+ MPs in serum were not significantly different between the FPI+vehicle and FPI+PEG-TB groups (Fig. 1). However, after injury there was a significant difference in total serum MP levels between groups, with MPs in the FPI+vehicle group being markedly elevated and MPs in the FPI+PEG-TB–treated group being suppressed (p=0.02).
FIG. 1.
Influence of fluid percussion injury on microparticles (MPs; mean MP/mL) in blood in absence (vehicle) and presence of polyethylene glycol telomere B (PEG-TB), n=6–7. *p<0.05, compared with corresponding pre-injury value. +p<0.05, compared with corresponding vehicle value.
Treatment with PEG-TB restores cerebral hemodynamics after TBI
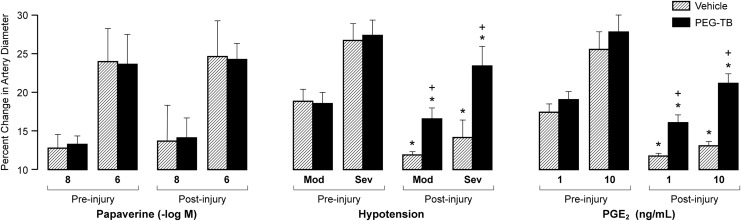
Prior to FPI (and treatment), both the vehicle and PEG-TB–treated groups exhibited pial artery dilation in response to papaverine (positive control), hypotension, and PGE2. There were no significant differences in baseline response to hypotension and PGE2 between groups (p>0.05). Pial artery dilation in response to papaverine was maintained after FPI in the vehicle and PEG-TB–treated groups and not significantly different between vehicle and PEG-TB–treated animals (p>0.05; Fig. 2). After FPI, pial artery dilation during hypotension was blunted in the vehicle group. Treatment with PEG-TB protected pial artery dilation during hypotension (p<0.0001, Fig. 2). Similarly, after FPI, pial artery dilation to PGE2 was markedly reduced in vehicle animals but protected in PEG-TB–treated animals (p<0.0001, Fig. 2).
FIG. 2.
Influence of papaverine (10-8, 10-6 M), hypotension (moderate, severe), and prostaglandin E2 (PGE2; 1, 10 ng/mL) on pial artery diameter before and after fluid percussion injury in absence (vehicle) and presence of polyethylene glycol telomere B (PEG-TB; n=5–7). *p<0.05, compared with corresponding pre-injury value. +p<0.05, compared with corresponding vehicle value.
Treatment with PEG-TB prevents elevation in tPA, endothelin, and pERK after FPI
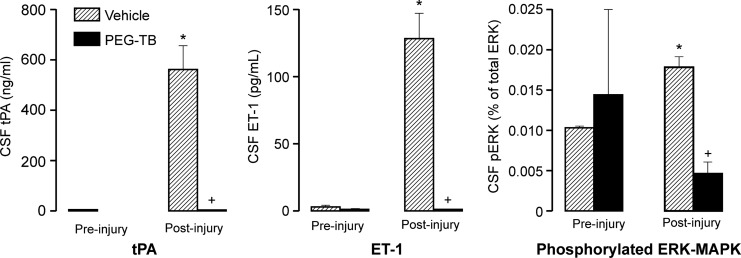
CSF tPA levels were significantly elevated after FPI in vehicle, but not PEG-TB–treated, animals (p<0.0001; Fig. 3). ET-1 levels were not significantly different pre-injury, but after injury, ET-1 was significantly elevated in vehicle, but not PEG-TB–treated, animals (p<0.0001; Fig. 3). Phosphorylated ERK (pERK) as a percent of total ERK was not significantly different between vehicle and PEG-TB–treated animals pre-injury. After injury, pERK increased in vehicle animals, which was blocked in PEG-TB–treated animals (p<0.0001; Fig. 3).
FIG. 3.
Influence of fluid percussion injury on cerebrospinal fluid (CSF) tissue plasminogen activator (tPA; ng/mL), endothelin-1 (ET-1; pg/mL), and phospho extracellular signal-regulated kinases-mitogen activated protein kinase (ERK-MAPK); n=5–7. *p<0.05, compared with corresponding pre-injury value. +p<0.05, compared with corresponding vehicle value.
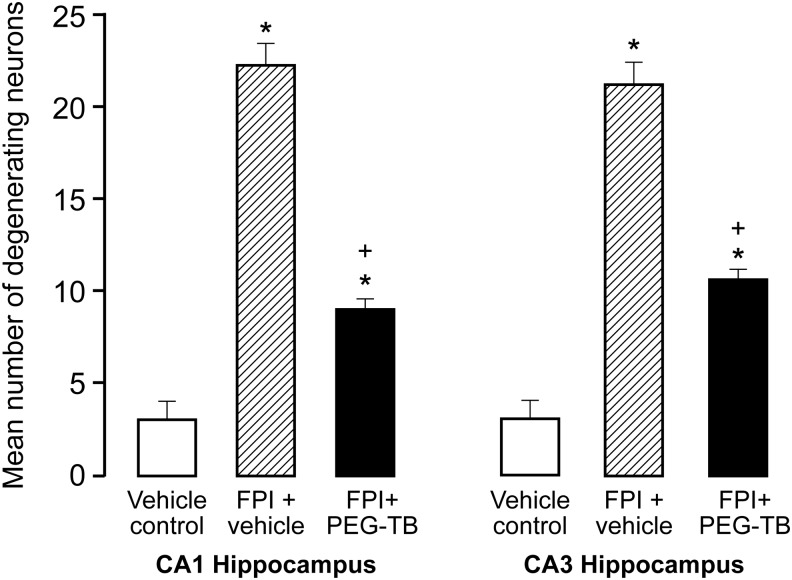
Treatment with PEG-TB prevents neuronal cell death in CA1 and CA3 hippocampus
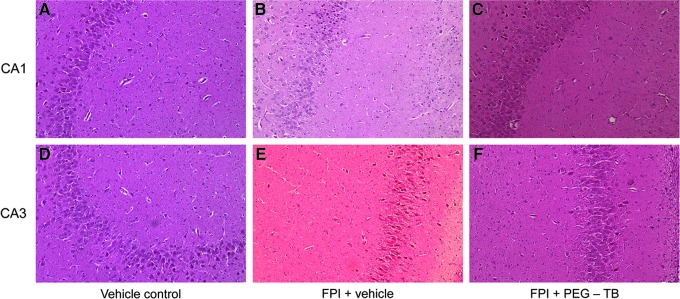
FPI increased the number of degenerating neurons in CA1 and CA3 hippocampus, which was blunted by PEG-TB (p<0.0001 for both regions; Fig. 4 and Fig. 5).
FIG. 4.
Top: Typical CA1 hippocampus under conditions of (A) vehicle control, (B) fluid percussion injury (FPI)+vehicle, (C) FPI+polyethylene glycol telomere B (PEG-TB). Bottom: Typical CA3 hippocampus under conditions of (D) vehicle control, (E) FPI+vehicle, and (F) FPI+PEG-TB.
FIG. 5.
Neuronal protection in CA1 and CA3 hippocampus after fluid percussion injury (FPI) with polyethylene glycol telomere B (PEG-TB) administration. Mean number of degenerating neuorns (±standard error of the mean) in CA1 and CA3 hippocampus in vehicle control, FPI+vehicle, and FPI+PEG-TB–treated animals; n=6–7. *p<0.05, compared with corresponding pre-injury value. +p<0.05, compared with corresponding vehicle control value.
Blood chemistry
Blood chemistry values were collected before and after all experiments. There were no statistically significant differences between vehicle control, FPI+vehicle, and FPI+PEG-TB–treated animals. Specifically, the values for vehicle controls were 7.45±0.03, 35±3, and 92±10 at the beginning of the experiment and 7.44±0.04, 37±4, and 90±10 at the end of the experiment for pH, pCO2, and pO2, respectively, while the values for FPI+PEG-TB–treated animals were 7.47±0.03, 34±3, and 90±10 at the beginning of the experiment and 7.45±0.03, 38±4, and 88±10 at the end of the experiment for pH, pCO2, and pO2, respectively.
Discussion
Several new findings of translational relevance have emerged from this study. First, this is the first study to demonstrate a cause and effect relationship between MP elevation after TBI and impaired hypotensive cerebrovasodilation. MP elevations in serum and CSF human TBI patients, compared with healthy controls, were previously demonstrated; however, these studies could only note the association between MP elevation and TBI.16,17 Our study, using a porcine FPI model, demonstrated that post-injury administration of PEG-TB reduced serum MP levels and protected vasodilatory response to hypotension and to direct application of prostaglandin to the pial surface. Because both control and PEG-TB–treated animals demonstrated pial vasodilation in response to papaverine, the effect of MPs is specific to cerebrovascular dilation during hypotension and not reflective of global vasomotor dysfunction.
Second, this study builds on past work to add a step in the pathway that results in dysfunction of endothelial potassium channels leading to altered cerebrovascular autoregulation after TBI. We have identified MP elevations as an important upstream event that leads to impaired cerebrovasodilation during hypotension after FPI via elevations of tPA, ET-1, and pERK-MAPK. Prior work in our lab, and others, has shown that hypotension-induced vasodilation is prostaglandin dependent and K-channel mediated.22,25,26 After FPI, elevated tPA causes dysfunction of K-channels and does so through upregulation of ERK-MAPK.29 Endothelin also is involved in post-traumatic dysfunction of endothelial K-channels.23,34 ERK-MAPK has been identified as the common downstream mediator of both tPA and ET-1.28–30,34,35 We have recently demonstrated the role of tPA in driving ET-1 release after FPI.30 In this study, MP elevation leads to elevations in tPA, ET-1, and ERK-MAPK which are prevented with post-injury MP-lysis by PEG-TB administration, suggesting that MP elevations lead to tPA elevations which in turn drive ET-1 release, ERK-MAPK phosphorylation, and disordered K-channel dilation in response to hypotension and prostaglandins. Hypotension after TBI is associated with poor outcome in observational trials of adult and pediatric patients,4–7 so we believe the role of MPs after TBI is clinically relevant.
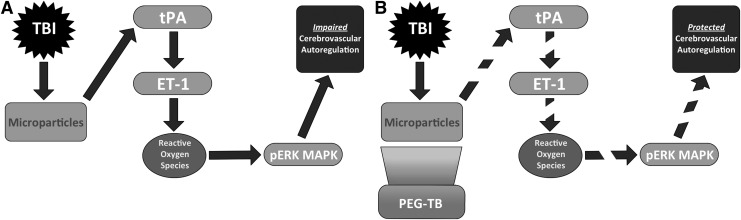
The proposed mechanism for MP-induced disorder of hypotension and prostaglandin-induced vasodilation is illustrated in Figure 6. In the untreated state, TBI leads to release of MPs, leading to elevations of tPA levels. tPA drives endothelin release, which in turn leads to release of reactive oxygen. The latter activates ERK-MAPK, which acts downstream to prevent vasodilation as an autoregulatory response to hypotension. PEG-TB, administered 30 min after injury, emulsifies MPs, preventing tPA and ET-1 release. Through this mechanism, PEG-TB decreases reactive oxygen species, prevents activation of ERK-MAPK and thereby protects prostaglandin-mediated vasodilation in response to hypotension. PEG-TB administration did not change responses to papaverine, suggesting it does not have a global effect on vasodilation and that its effect is limited to the response to hypotension and the pathways analyzed with our experiments.
FIG. 6.
Proposed mechanisms for (A) microparticle-induced dysfunction of autoregulatory hypotensive cerebrovasodilation after traumatic brain injury and (B) protection of autoregulatory hypotensive cerebrovasodilation via polyethylene glycol telomere B (PEG-TB). TBI, traumatic brain injury; tPA, tissue plasminogen activator; ET-1, endothelin-1; pERK MAPK, phospho extracellular signal-regulated kinases-mitogen activated protein kinase.
Third, this study demonstrated that PEG-TB treatment reduced hippocampal injury. Previously, we had observed that ET-1 and ERK-MAPK upregulation after FPI in the pig caused histopathology, including neuronal cell necrosis in CA1 and CA3 hippocampal regions.30,36,37 New results in the present study show that PEG-TB blocked ET-1 and ERK-MAPK upregulation after TBI and prevented impairment of hypotensive and PGE2-induced cerebrovasodilation. In the context of the neurovascular unit, hemodynamics are thought to contribute to neuronal cell integrity. PEG-TB may have limited hippocampal neuronal cell injury via preservation of vascular responsiveness to stimuli thought to be important in control of cerebral hemodynamics.8 Mechanistically, these data suggest that PEG-TB is neuroprotective due, in part, to preservation of cerebral hemodynamics and prevention of ERK-MAPK and ET-1 release after brain injury.
Finally, this study has potential near-term translational applications. MPs are being investigated as biomarkers in a variety of human diseases, and this study further supports use of MP as a biomarker for TBI. However, prior studies demonstrating MP elevation after human TBI were small and did not show any link to injury severity or outcome.16,17 A further barrier to translation as a biomarker is need for standardization of the assay, as relatively minor procedural modifications have been shown to lead to significant alterations in MP levels.38 However, MP abatement may have value and relatively low risk as a clinical treatment strategy for TBI. In using PEG-TB, an agent previously shown to be safe in human echocardiography trials at a similar dose to reduce MP levels and restore autoregulation, we have identified an attractive possible translational therapeutic for human use.39,40 PEG-TB is a fluorosurfactant, has a completely different chemical structure, and should not be confused with the PEG-superoxide dismutase, which was previously studied after TBI without benefit.10, 39–41 PEG-TB does not have known antioxidant properties. In fact, aside from its role in stabilizing bubbles for echocardiography or lysing MPs, it is considered relatively pharmacologically inert with no evidence of metabolism within the body and has a half-life of elimination of a few minutes, cleared by the lungs. 10, 39–41
This small, pilot study has several limitations. These were non-survival experiments with pial artery reactivity, histopathology, and biochemical analysis as end-points, rather than more clinically relevant survival endpoints such as behavior. Second, we did not analyze CBF directly but used changes in pial vessel diameter as a proxy for alterations in CBF. Prior work in our lab has shown that changes in CBF closely mirror those observed in pial reactivity27,30,32; however, future studies will likely involve direct CBF measurement. Third, only a single administration of our experimental agent, PEG-TB, was administered at a single time-point. Though the results of this study are encouraging, the MP-lysis and physiologic changes seen after PEG-TB administration may not be lasting, as neither the temporal dynamics of MP elevation after TBI nor the pharmacodynamics of PEG-TB's effect on MPs after TBI have been adequately studied. Finally, we cannot speculate regarding the translational relevance of PEG-TB administration for adult TBI, given the significant age-related differences in cerebrovascular autoregulation and alterations thereof after TBI.5,8,24,31,42 PEG-TB administration should have value in a pediatric population, while other mechanisms might dominate in the adult.
In conclusion, MPs are elevated after TBI, and PEG-TB administration after injury protects hypotensive cerebrovasodilation and limits hippocampal neuronal cell loss.
Author Disclosure Statement
This research was funded by grants from the National Institutes of Health HD57355 (WMA), 5T32NS043126-10 (LEB), and Office of Naval Research Award N00014-08-1-0270 (SRT). No competing financial interests exist.
References
- 1.Langlois J.A., Rutland-Brown W., and Thomas K.E. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 20, 229–238 [DOI] [PubMed] [Google Scholar]
- 2.Adelson P.D., Clyde B., Kochanek P.M., Wisniewski S.R., Marion D.W., and Yonas H. (1997). Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr. Neurosurg. 26, 200–207 [DOI] [PubMed] [Google Scholar]
- 3.Adelson P.D., Srinivas R., Chang Y., Bell M., and Kochanek P.M. (2011). Cerebrovascular response in children following severe traumatic brain injury. Childs. Nerv. Syst. 27, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 4.Coates B.M., Vavilala M.S., Mack C.D., Muangman S., Suz P., Sharar S.R., Bulger E., and Lam A.M. (2005). Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit. Care Med. 33, 2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman S.S., Udomphorn Y., Armstead W.M., Fisk D.M., and Vavilala M.S. (2008). Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology 108, 588–595 [DOI] [PubMed] [Google Scholar]
- 6.Chaiwat O., Sharma D., Udomphorn Y., Armstead W.M., and Vavilala M.S. (2009). Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma 26, 657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vavilala M.S., Bowen A., Lam A.M., Uffman J.C., Powell J., Winn H.R., and Rivara F.P. (2003). Blood pressure and outcome after severe pediatric traumatic brain injury. J. Trauma 55, 1039–1044 [DOI] [PubMed] [Google Scholar]
- 8.Armstead W.M. (2000). Age-dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation 7, 225–2035 [PubMed] [Google Scholar]
- 9.Blann A., Shantsila E., and Shantsila A. (2009). Microparticles and arterial disease. Semin. Thromb. Hemost. 35, 488–496 [DOI] [PubMed] [Google Scholar]
- 10.Thom S.R., Yang M., Bhopale V.M., Huang S., and Milovanova T.N. (2011). Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J. Appl. Physiol. 110, 340–351 [DOI] [PubMed] [Google Scholar]
- 11.Mackman N. (2009). On the trail of microparticles. Circ. Res. 104, 925–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meziani F., Delabranche X., Asfar P., and Toti F. (2010). Bench-to-bedside review: circulating microparticles—a new player in sepsis? Crit. Care 14, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidot L., Jy W., Bidot C., Jimenez J.J., Fontana V., Horstman L.L., and Ahn Y.S. (2008). Microparticle-mediated thrombin generation assay: increased activity in patients with recurrent thrombosis. J. Thromb. Haemost. 6, 913–919 [DOI] [PubMed] [Google Scholar]
- 14.Amabile N., Guérin A.P., Leroyer A., Mallat Z., Nguyen C., Boddaert J., London G.M., Tedgui A., and Boulanger C.M. (2005). Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 16, 3381–3388 [DOI] [PubMed] [Google Scholar]
- 15.Agouni A., Lagrue-Lak-Hal A.H., Ducluzeau P.H., Mostefai H.A., Draunet-Busson C., Leftheriotis G., Heymes C., Martinez M.C., and Andriantsitohaina R. (2008). Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 173, 1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morel N., Morel O., Petit L., Hugel B., Cochard J.F., Freyssinet J.M., Sztark F., and Dabadie P. (2008). Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J. Trauma 64, 698–704 [DOI] [PubMed] [Google Scholar]
- 17.Nekludov M., Mobarrez F., Gryth D., Bellander B.M., and Wallen H. (2014). Formation of microparticles in the injured brain of patiens with severe isolated traumatic brain injury. J. Neurotrauma 31, 1927–1933 [DOI] [PubMed] [Google Scholar]
- 18.Simak J., Gelderman M.P., Yu H., Wright V., and Baird A.E. (2006). Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J. Thromb. Haemost. 4, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 19.Sanborn M.R., Thom S.R., Bohman L.-E., Stein S.C., Levine J.M., Milovanova T., Maloney-Wilensky E., Frangos S., and Kumar M.A. (2012). Temporal dynamics of microparticle elevation following subarachnoid hemorrhage. J. Neurosurg. 117, 579–586 [DOI] [PubMed] [Google Scholar]
- 20.Lackner P., Dietmann A., Beer R., Fischer M., Broessner G., Helbok R., Marxgut J., Pfausler B., and Schmutzhard E. (2010). Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke. 41, 2353–2357 [DOI] [PubMed] [Google Scholar]
- 21.Faraci F.M., and Heistad D.D. (1998). Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 78, 53–97 [DOI] [PubMed] [Google Scholar]
- 22.Armstead W.M. (1999). Hypotension dilates pial arteries by KATP and kca channel activation. Brain Res. 816, 158–164 [DOI] [PubMed] [Google Scholar]
- 23.Armstead W.M. (2001). Endothelin-Induced cyclooxygenase-dependent superoxide generation contributes to K+ channel functional impairment after brain injury. J. Neurotrauma 18, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 24.Armstead W.M. (1999). Age-dependent impairment of K(ATP) channel function following brain injury. J. Neurotrauma 16, 391–402 [DOI] [PubMed] [Google Scholar]
- 25.Leffler C.W., Busija D.W., Beasley D.G., and Fletcher A.M. (1986). Maintenance of cerebral circulation during hemorrhagic hypotension in newborn pigs: role of prostanoids. Circ. Res. 59, 562–567 [DOI] [PubMed] [Google Scholar]
- 26.Leffler C.W. and Busija D.W. (1987). Prostanoids and pial arteriolar diameter in hypotensive newborn pigs. Am. J. Physiol. 252, H687–H691 [DOI] [PubMed] [Google Scholar]
- 27.Armstead W.M. and Kurth C.D. (1994). Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma 11, 487–497 [DOI] [PubMed] [Google Scholar]
- 28.Armstead W.M., Riley J., and Vavilala M.S. (2012). TBI sex dependently upregulates ET-1 to impair autoregulation, which is aggravated by phenylephrine in males but is abrogated in females. J. Neurotrauma 29, 1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstead W.M., Riley J., Cines D.B., and Higazi A.A. (2011). tPA contributes to impairment of ATP and Ca sensitive K channel mediated cerebrovasodilation after hypoxia/ischemia through upregulation of ERK MAPK. Brain Res. 1376, 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstead W.M., Bohman L.-E., Riley J., Yarovoi S., Higazi A.A., and Cines D.B. (2013). tPA-S(481)A prevents impairment of cerebrovascular autoregulation by endogenous tPA after traumatic brain injury by upregulating p38 MAPK and inhibiting ET-1. J. Neurotrauma 30:1898–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstead W.M., Cines D.B., and Higazie A.A. (2005). Plasminogen activators contribute to age-dependent impairment of NMDA cerebrovasodilation after brain injury. Brain Res. Dev. Brain Res. 156, 139–146 [DOI] [PubMed] [Google Scholar]
- 32.Armstead W.M., Kiessling J.W., Cines D.B., and Higazi A.A. (2011). Glucagon protects against impaired NMDA-mediated cerebrovasodilation and cerebral autoregulation during hypotension after brain injury by activating cAMP protein kinase A and inhibiting upregulation of tPA. J. Neurotrauma 28, 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nantakomol D., Chimma P., Day N.P., Dondorp A.M., Combes V., Krudsood S., Looareesuwan S., White N.J., Pattanapanyasat K., and Chotivanich K. (2008). Quantitation of cell-derived microparticles in plasma using flow rate based calibration. Southeast Asian J. Trop. Med. Public Health 39, 146–153 [PubMed] [Google Scholar]
- 34.Zubkov A.Y., Rollins K.S., Parent A.D., Zhang J., and Bryan R.M. (2000). Mechanism of endothelin-1-induced contraction in rabbit basilar artery. Stroke 31, 526–533 [DOI] [PubMed] [Google Scholar]
- 35.Armstead W.M., Riley J., and Vavilala M.S. (2013). Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr. Crit. Care Med. 14, e103–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstead WM, Raghupathi R. (2011). Endothelin and the neurovascular unit in pediatricntraumatic brain injury. Neurol. Res. 33, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstead W.M., Riley J., Yarovoi S., Cines D.B., Smith D.H., and Higazi A.A. (2012). tPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J. Neurotrauma 29, 1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayers L., Kohler M., Harrison P., Sargent I., Dragovic R., Schaap M., Nieuwland R., Brooks S.A., and Ferry B. (2011). Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb. Res. 127, 370–377 [DOI] [PubMed] [Google Scholar]
- 39.Grayburn P.A., Weiss J.L., Hack T.C., Klodas E., Raichlen J.S., Vannan M.A., Klein A.L., Kitzman D.W., Chrysant S.G., Cohen J.L., Abrahamson D., Foster E., Perez J.E., Aurigemma G.P., Panza J.A., Picard M.H., Byrd B.F., Segar D.S., Jacobson S.A., Sahn D.J., and DeMaria A.N. (1998). Phase III multicenter trial comparing the efficacy of 2% dodecafluoropentane emulsion (EchoGen) and sonicated 5% human albumin (Albunex) as ultrasound contrast agents in patients with suboptimal echocardiograms. J. Am. Coll. Cardiol. 32, 230–236 [DOI] [PubMed] [Google Scholar]
- 40.Beppu S., Matsuda H., Shishido T., Matsumura M., and Miyatake K. (1997). Prolonged myocardial contrast echocardiography via peripheral venous administration of QW3600 Injection (EchoGen): its efficacy and side effects. J. Am. Soc. Echocardiogr. 10, 11–24 [DOI] [PubMed] [Google Scholar]
- 41.Young B., Runge J.W., Waxman K.S., Harrington T., Wilberger J., Muizelaar J.P., Boddy A., and Kupiec J.W. (1996). Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. JAMA 276, 538–543 [PubMed] [Google Scholar]
- 42.Armstead W.M. (2002). Age dependent NMDA contribution to impaired hypotensive cerebral hemodynamics following brain injury. Brain Res. Dev. Brain Res. 139, 19–28 [DOI] [PubMed] [Google Scholar]