Abstract
The degree of post-traumatic brain edema and dysfunction of the blood–brain barrier (BBB) influences the neurofunctional outcome after a traumatic brain injury (TBI). Previous studies have demonstrated that the administration of apolipoprotein E-mimetic peptide COG1410 reduces the brain water content after subarachnoid hemorrhage, intra-cerebral hemorrhage, and focal brain ischemia. However, the effects of COG1410 on vasogenic edema following TBI are not known. The current study evaluated the effects of 1 mg/kg daily COG1410 versus saline administered intravenously after a controlled cortical impact (CCI) injury on BBB dysfunction and vasogenic edema at an acute stage in mice. The results demonstrated that treatment with COG1410 suppressed the activity of matrix metalloproteinase-9, reduced the disruption of the BBB and Evans Blue dye extravasation, reduced the TBI lesion volume and vasogenic edema, and decreased the functional deficits compared with mice treated with vehicle, at an acute stage after CCI. These findings suggest that COG1410 is a promising preclinical therapeutic agent for the treatment of traumatic brain injury.
Key words: : apolipoprotein E, blood–brain barrier, COG1410, controlled cortical impact injury, traumatic brain injury, vasogenic edema
Introduction
Secondary injury after traumatic brain injury (TBI), including the loss of ionic homeostasis, excitotoxic damage, increased release of inflammatory mediators, and oxidative factors all contribute to the disruption of the blood–brain barrier (BBB) and formation of vasogenic edema.1,2 The degree of the post-traumatic cerebral edema is one of the main determinants of neurological outcome and survival after a TBI.1 Therefore, reducing vasogenic edema by protecting the BBB has become a focus of recent research to improve neurofunction after a TBI.3
Apolipoprotein E (apoE) is the major apolipoprotein expressed in the human brain and it is the most significantly up-regulated apolipoprotein that is synthesized in the brain after an injury.4 ApoE is a neuroprotective factor in the central nervous system (CNS) with combined beneficial anti-inflammatary,5,6 anti-oxidant,7 and anti-excitotoxic8 activities after a brain injury. ApoE-deficient mice are cognitively impaired and exhibit more severe motor and cognitive deficits after closed head injury, compared with wild-type mice.9,10 One of the reasons for these differences may be that the lack of apoE leads to BBB breakdown, increases BBB susceptibility to injury, and exacerbates brain edema after brain trauma.10–13
However, the intact apoE holoprotein is too large to cross the BBB because of the 34-kDa molecular weight. A series of small apoE-mimetic peptides derived from the receptor-binding region of apoE that cross the BBB and retain the neuroprotective properties of the apoE holoprotein were generated to address this issue.14,15 Several studies, including our previous research, assessed the effects of COG1410 (a peptide derive from apoE amino acid residues 138–149) on reductions in microglial activation, neuronal cell death, traumatic axonal injury, and neurofunctional deficits in animal models of TBI.16–18 However, to our knowledge, the effects of COG1410 on BBB permeability and brain vasogenic edema after TBI have not been reported. The current study examined the protective effects of COG1410 on the BBB and on the reduction of brain vasogenic edema when administered following controlled cortical impact (CCI) injury.
Methods
In this study, all the procedures were evaluated and approved by the Animal Care and Use Committee at the Fist Affiliated Hospital of Chongqing Medical University in Chongqing, China.
Experimental TBI: CCI
Wild type (WT) C57BL/6J male mice, 10 to 12 weeks old and weighing 18–23 g, were used in our experiments. The mice were labeled after removal from the feeding center and randomly divided into different groups (sham injury+saline group, TBI+saline group, and TBI+COG1410 group) using the randomizer of Excel. Subsequent CCI injury was produced on the exposed cortex using a controlled impactor device, TBI-0310 TBI Model system (Precision Systems and Instrumentation, LLC, Fairfax Station, VA), as previously described.19 Briefly, the mice were deeply anesthetized, and a 5-mm right lateral craniotomy centered at 2.7 mm lateral from the midline and 3 mm anterior to the lambda was performed using a motorized drill. The skull was removed without disrupting the dura. The CCI injury was produced using a pneumatic cylinder with a 3-mm diameter flat-tip impounder with an impact velocity of 3 m/sec, a dwell time of 100 msec, and a cortical contusion depth of 1.5 mm. A plastic skull cap was secured over the craniotomy after the injury and the scalp was sutured closed with nylon sutures before the mice recovered from anesthesia. The mice were maintained at 37° throughout the duration of the surgery using a rectal temperature probe and feedback temperature controller. The anesthetized mice were placed in an incubator (37°) until they recovered the ability to ambulate. This procedure produces a moderately severe contusion in the right sensorimotor cortex with pronounced behavioral deficits but virtually no mortality. Sham injury mice underwent the same surgical procedure, including the anesthesia and craniotomy, without the CCI injury.
COG 1410 synthesis and peptide injections
The COG1410 peptide aeetyl-AS-Aib-LRKL-Aib-KRLL-amide, composed of apoE residues 138–149 with aminoiso-butyric acid (Aib) substitutions at positions 140 and 145, was synthesized, purified, and kindly provided by Cognosci (Research Triangle Park, NC). The peptide was dissolved in a sterile 0.9% saline solution immediately prior to use, and the solution was injected into the tail vein at a dose of 1 mg/kg. The peptide was administered immediately after the scalp was sutured closed post-CCI, followed by a once-daily intravenous injection until the day before the mice were sacrificed.20 The vehicle groups underwent injections of only sterile 0.9% saline at each time-point. The investigator who administered the treatment was different from the investigator who performed the surgeries and a single investigator who was blinded to the injury status and treatment regimens of the animals performed all assessments.
Rotarod test
A rotarod instrument (ZB-200 Rota-Rod Treadmill; Taimeng Software Co. LTD, Chengdu, China) was used to assess vestibulomotor function of mice. The mice underwent three trials at a stationary rotational speed 16 rpm for 1 min on the day prior to CCI (interval of 15 min between trials). Three trials with an accelerating rotational speed were performed 1 h before CCI as described by Hamm and colleagues.21 The average time to fall from the rotating cylinder in the later three trials was recorded as the baseline latency. The mice underwent three consecutive daily accelerating rotational speed trials on Days 1–3 after CCI or sham injury (interval of 15 min). The average latency of falling from the rotarod device was recorded for each mouse.
Determination of Evans Blue dye extravasation
Evans Blue (EB) dye (2% weight/volume [w/v] in phosphate-buffered saline [PBS]) in a volume of 4 mL/kg (50 μg per gram of body weight) was given via a tail vein injection after CCI and allowed to circulate for 3 h. The mice were sacrificed and transcardially perfused with PBS until colorless PBS came out off the right atrium. The injured hemispheres were removed, and the olfactory bulb and cerebellum were separated, weighed, and homogenized in 2 mL of PBS followed by the addition of 2 mL of 60% trichloroacetic acid (w/v). The homogenates were centrifuged at 18,000 g at 4°C for 10 min, and absorbance of 200 mL of supernatant at 620 nm was measured using a microplate reader (Elx800; Biotek, Winooski, VT). The optical density (OD) of the EB dye in each sample was recorded, and the results are expressed as OD per gram of brain tissue.22
MRI protocol
All magnetic resonance imaging (MRI) scans were performed using a Bruker 7T (70/20) system (BrukerBiospin, Billerica, MA). Pre- and post-CCI mice were anesthetized after the rotarod test using a gas mixture (induction, 5% isoflurane with 1 L/min O2; maintenance, 1% isoflurane with 1 L/min O2), and mounted in a Bruker animal bed. The body temperature was maintained at 37°C, and the respiratory rate was continuously monitored. T2-weighted images were acquired using RARE (repetition time=4000, echo time=45, RARE factor 8, 0.5 mm, field of view 2.5 cm, 256×256). The images were analyzed by using Bruker ParaVision 6.0 software. The lesion volumes were determined as pixels with T2 values higher than the mean plus two standard deviations of the value in the homologous contralesional region.23
Preparation of brain tissue and protein determination
Tissues from each injured hemispheres were separated after CCI or sham injury, weighed and immediately frozen in liquid nitrogen. Tissue samples from each group were lysed in RIPA Lysis Buffer with a protease inhibitor cocktail (05892791001; Roche, Basel, Switzerland) and homogenized at 4°C until lysis was complete. Protein was extracted using a Protein Extraction Kit (P0033B; Beyotime Institute of Biotechnology, Shanghai, China). The protein concentration was determined by enhanced BCA protein assay kit (P0010S; Beyotime Institute of Biotechnology). Half of the protein was boiled 5 min with buffer for western blotting; the other half of the protein sample was not boiled and was used for gelatin zymography.
Gelatin zymography
The matrix metalloproteinase-9 (MMP-9) activity was examined using gelatin zymography as previously described.24 An equal amount of protein from each group was loaded and separated in a 10% polyacrylamide gel copolymerized with gelatin (1 mg/mL). The gels were renatured after separation by electrophoresis, and incubated with developing buffer for 40 h at 37°. Then, the gels were stained with 0.5% Coomassie Blue R-250 for 30 min and destained with 20% ethanol and 10% acetic acid. The MMP-9 activity was quantified using densitometric analyses with the National Institutes of Health Image J software (Image J 1.42q; National Institutes of Health, Bethesda, Maryland). The results expressed as relative OD.
Western blotting
Equal amounts of each protein were processed for electrophoretic separation (SDS-PAGE) and subsequent electrotransfer to polyvinylidene fluoride membranes. The western blots were blocked with 5% bovine serum albumin at room temperature for 1 h. The membrane was incubated at 4°C overnight with a primary antibody (rabbit polyclonal anti-occludin [1:1,000, sc-5562; Santa Cruz Biotechnology, Inc., Dallas, Texas], rabbit polyclonal anti-claudin-5 [1:1,000, sc-28670; Santa Cruz Biotechnology, Inc.], a rabbit polyclonal anti-ZO-1 [1:1,000, sc-10804; Santa Cruz Biotechnology, Inc.], mouse monoclonal anti-β-actin [1:1,000, sc47778; Santa Cruz Biotechnology, Inc.]), followed by incubation for 1 h with a secondary antibody conjugated with horseradish peroxidase. The bands were revealed using ECL Western Blotting kit (32209; Thermo Scientific, Waltham, MA) and photographed by a chemiluminescence imaging system (ChemiDoc XRS+; Bio-Rad, Hercules, CA). The amount of protein in each band was quantified using Quantity One 4.6.2 computer software (Bio-Rad, Hercules, CA).
Statistical analysis
All data were analyzed using the SPSS 19 software package (SPSS, Inc., Chicago, IL). All data are expressed as the mean±standard error of the mean (SEM) of five animals. The rotarod latencies were compared using repeated measures of analysis of variance (ANOVA) with time as the repeated variable, and using Dunnett's post hoc method to correct for repeated comparisons. The EB dye extravasation, MMP-9 activity, and tight junction marker expression were compared with one-way ANOVA using treatment group (COG1410 vs. saline) as the variable. Student's t-test was used to compare lesion volume. Statistical significance was assumed with p<0.05. All values are expressed as the means±SEM.
Results
COG1410 improves neurological function after CCI
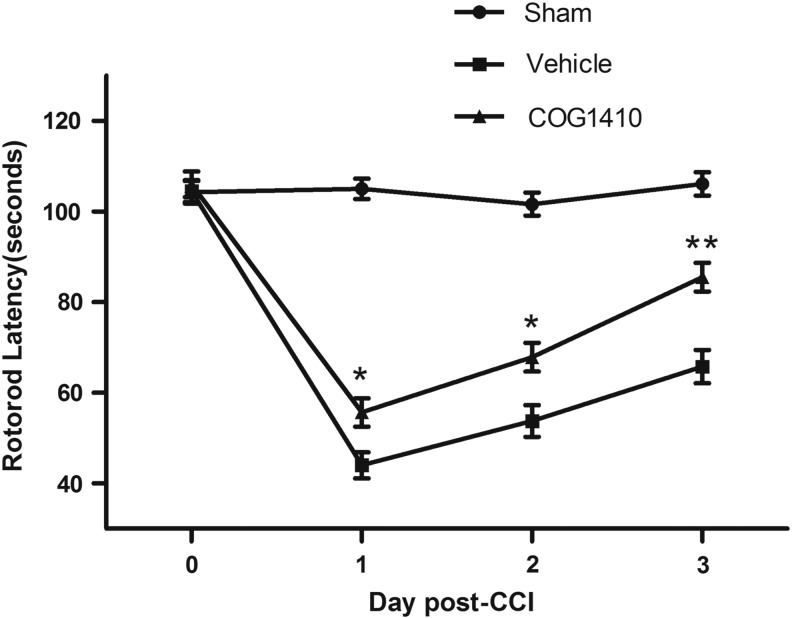
The vestibulomotor function of the mice was assessed using rotarod latencies in different groups (n=5 mice/group). The rotarod latencies were assessed 1 h before injury and on Days 1–3 following brain injury. Mice administered COG1410 continued to exhibit significant improvements in their rotarod performance compared with the vehicle groups on Days 1, 2, and 3 post-CCI (p=0.03, 0.018, and 0.001, respectively; Fig. 1).
FIG. 1.
COG1410 improves functional motor performance on rotarod after controlled cortical impact (CCI). Rotarod test was used to assess the vestibulomotor function of mice. The time of baseline latency at 1 h prior to injury and latencies at Days 1–3 after injury in different groups were continuously demonstrated. Mice administered COG1410 significantly increased rotarod latencies compared with the vehicle groups at 1, 2 and 3 d post-CCI. (mean±standard error of the mean, n=5 per group, *p<0.05, **p<0.01, COG1410 group vs. vehicle group).
COG1410 reduced blood brain barrier permeability after CCI
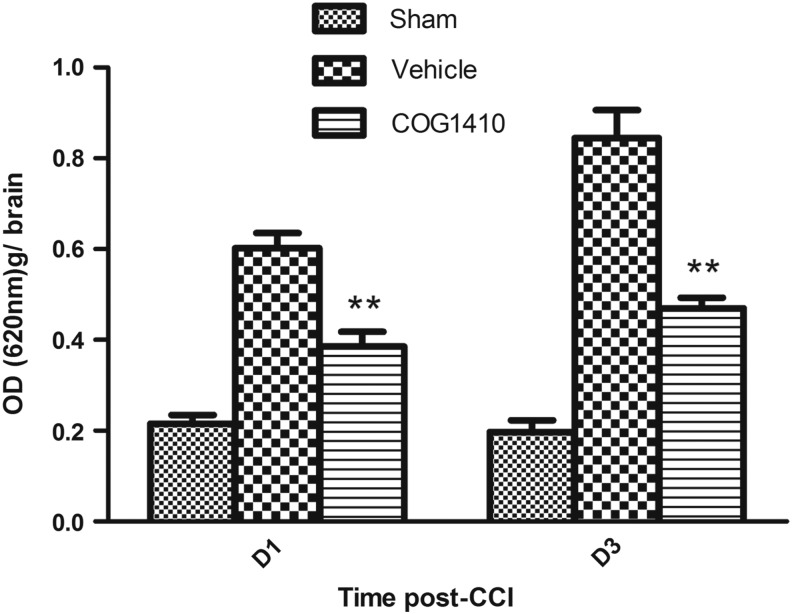
BBB permeability was evaluated using EB dye injection on Day 1 and Day 3 after CCI or sham injury. Systemic changes in skin color were observed immediately following the tail vein injections. The mice were sacrificed and the brains were harvested to evaluate EB dye extravasation 3 h after the injection. The results demonstrated that COG1410 treatment significantly reduced EB dye extravasation on Day 1 (p=0.004) and Day 3 (p=0.006), compared with the vehicle control group (Fig. 2).
FIG. 2.
COG1410 reduced Evans Blue (EB) dye extravasation after controlled cortical impact (CCI). The blood–brain barrier permeability was evaluated by EB dye. The optical density (OD) of EB per gram of brain tissue of sham injury, vehicle- and COG1410-treated mice at Day 1 and Day 3 after brain injury were measured. The results revealed that the EB dye extravasation into brain parenchyma was significantly reduced by COG1410 treatment at Day 1 and Day 3 post-CCI (mean±standard error of the mean, n=5 per group, **p<0.01, COG1410 group vs. vehicle group).
COG1410 reduced the lesion volume and vasogenic edema after CCI
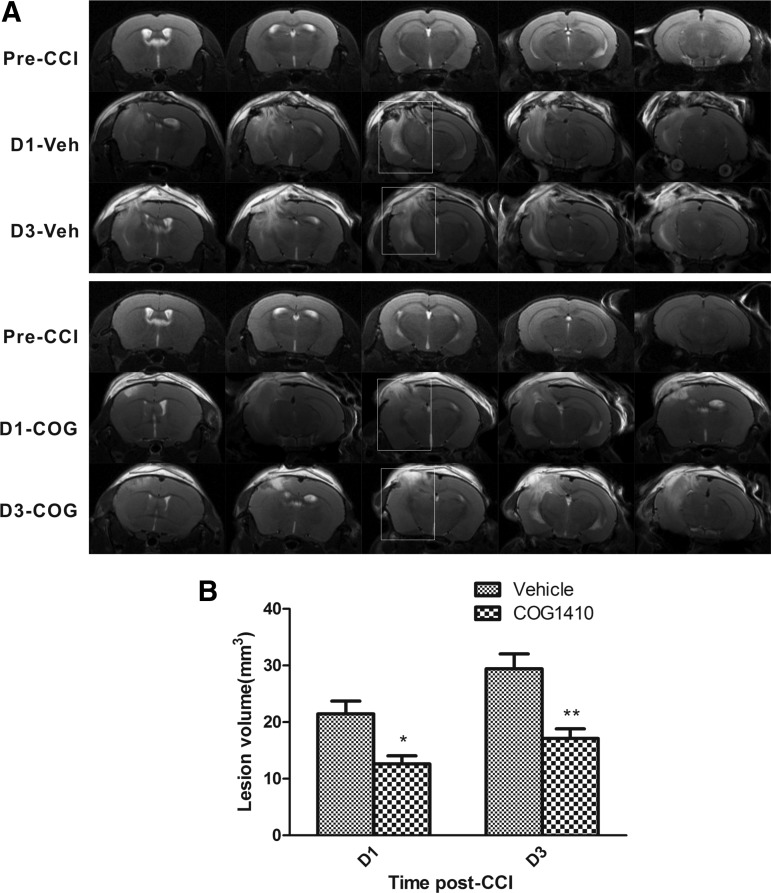
T2WI MRI of a Bruker 7T system was used to evaluate the lesion volume after CCI. The representative T2 maps show entire CCI lesions in vehicle-treated and COG1410-treated animals pre-CCI and on Day 1 and Day 3 post-CCI (Fig. 3A). The hyperintense areas indicate the lesions. The lesion volumes in the COG1410-treated group were significantly smaller than those in the vehicle-treated group on Day 1 (reduced by 41.1%; p=0.011) and Day 3 (reduced by 44.5%; p=0.002) post-CCI (Fig. 3B). These results indicated that COG1410 treatment markedly reduced the TBI lesion volume and vasogenic edema.
FIG. 3.
COG1410 reduced lesion volume after controlled cortical impact (CCI). Representative T 2 maps were shown for the entire CCI lesion volume of a vehicle-treated and a COG1410-treated mouse pre- and post-CCI at Day 1 and Day 3 (A). The lesion volumes in COG1410-treated mice compared with vehicle-treated mice at Day 1 and Day 3 post-CCI are plotted (B). The results demonstrated that the lesion volume was markedly reduced by COG1410 treatment at Day 1 and Day 3 post-CCI. Veh, vehicle-treated mice; COG, COG1410-treated mice; D, day post-CCI. mean±standard error of the mean. n=5 per group. *p<0.05, **p<0.01, COG1410 group vs. vehicle group.
COG1410 reduced MMP-9 activity after CCI
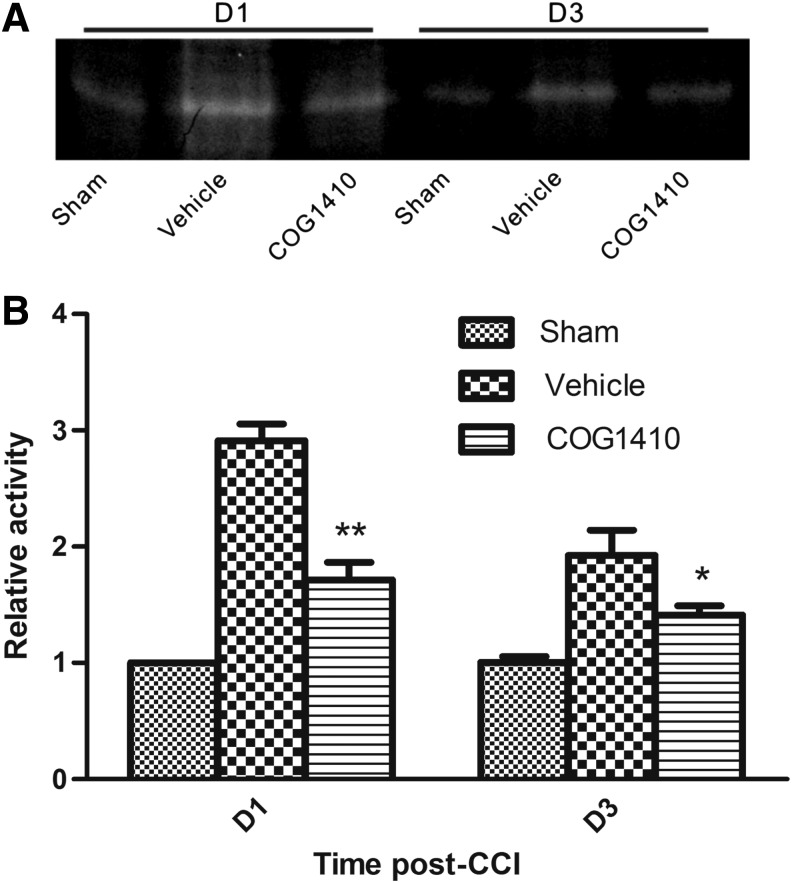
The tight junctions of the BBB are a substrate of MMP-9, and disruption of tight junctions is related to MMP-9 activity. The MMP-9 activity in the injured hemispheres was evaluated using gelatin zymography. Densitometric analysis demonstrated that COG1410 treatment significantly reduced the gelatinase activity of MMP-9 on Day 1 and Day 3 post-CCI (p<0.01 and p<0.05, respectively; Fig 4).
FIG. 4.
COG1410 reduced MMP-9 activity after controlled cortical impact (CCI). Representative bands for active MMP-9 by zymography (A). The optical density of sham injury group at Day 1 was provided for standard reference values. Relative optical density was compared between in COG1410-treated mice and vehicle-treated mice at Day 1 and Day 3 post-CCI (B). The bar graph demonstrated that COG1410 treatment significantly reduced the activity of MMP-9 at Day 1 and Day 3 post-CCI. (mean±standard error of the mean, n=5 per group, *p<0.05, **p<0.01, COG1410 group vs. vehicle group).
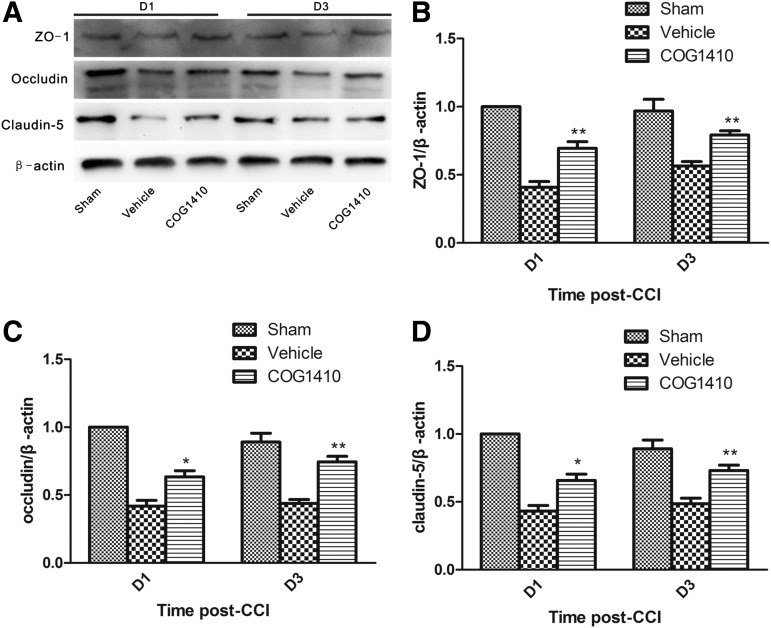
COG1410 reduced BBB disruption after CCI
Tight junctions, which consist of ZO-1, occludin, claudins, and other proteins, are an important component of the BBB. The expression of these tight junction markers was reduced following TBI.25 Tight junctions in the injured hemispheres were evaluated using western blotting and densitometry of protein bands. The densitometry analyses revealed that COG1410 treatment significantly protected the expression of the tight junction component on Day 1 and Day 3 post-CCI (*p<0.05; **p<0.01, respectively; Fig 5).
FIG. 5.
COG1410 reduced tight junction disruption after controlled cortical impact (CCI). Representative western blots of protein components of the tight junction (A). The relative optical density of protein bands for ZO-1 (B), occludin (C), claudin-5 (D) of sham injury group at Day 1 was provided for standard reference values. Relative optical density of tight junction component proteins was compared between in COG1410-treated mice and vehicle-treated mice. The results revealed that COG1410 treatment significantly reduced the loss of tight junction protein components at Day 1 and Day 3 post-CCI (mean±standard error of the mean, n=5 per group, *p<0.05, **p<0.01, COG1410 group vs. vehicle group).
Discussion
We demonstrated that COG1410 administration significantly promoted the functional recovery, reduced the TBI lesion volume, and reduced vasogenic edema following TBI, which supports a therapeutic benefit of this agent. We also found that COG1410 treatment suppressed the MMP-9 activity, disruption of the BBB, and the EB dye extravasation, compared with the vehicle-treated mice in acute stages post-CCI. These results suggest that the therapeutic efficacy of the apoE-mimetic peptide COG1410 is associated with the modulation of BBB integrity.
Numerous findings have demonstrated that apoE deficiency increases the permeability of BBB in vivo and in vitro,11–13,26,27 especially after brain injury.13 There are two known associated mechanisms that account for this effect. First, apoE regulates BBB integrity via the cyclophilin A–nuclear factor-κB–MMP-9 pathway through binding of the apoE receptor LRP111. Second, apoE increases the transendothelial electrical resistance of the BBB by promoting the phosphorylation of occludin at threonine residues by protein kinase C.26 Several studies demonstrated that COG1410 reduced the brain water content after subarachnoid hemorrhage,28 intracerebral hemorrhage,29,30 and focal brain ischemia.31 The present study demonstrated that COG1410 treatment in the acute stages post-CCI the attenuated the functional impairment of the BBB. We also demonstrated that COG1410 reduced the MMP-9 activity and ameliorated the disruption of claudin-5, occludin and ZO-1 on Day 1 and Day 3 post-CCI.
There are several possible interpretations of these results. First, COG1410 is a peptide that is derived from the receptor binding domain of apoE, which suggests that COG1410 reduces MMP-9 activity via the cyclophilin A-nuclear factor-κB pathway through binding apoE receptor LRP1. Second, because MMP-9 is activated by free radicals, such as nitric oxide,32,33 and COG1410 reduces the oxidative stress post-CCI15; therefore, COG1410 may reduce the MMP-9 activity by inhibiting the oxidative stress after a TBI. Further studies are needed to examine whether COG1410 influences the phosphorylation of BBB tight junction.
Inflammatory stress is another predominant secondary injury after TBI that causes BBB damage.1 ApoE and the apoE-mimetic peptide COG133 demonstrated a strong protective effect of inhibition of inflammatory response in the CNS.6,34 The second generation of apoE-mimetic peptide, COG1040, is more efficacious in reducing CNS inflammation, compared with the first generation of apoE-mimetic peptide COG133,35 and COG1410 increases the therapeutic window post-CCI from 30 min with COG133 to 2 h with COG1410,15 while most clinical studies of TBI have initiated treatment at 6–12 h, and neither 30 min nor 120 min are clinically feasible. Further work is required to address the therapeutic time-window.
Unlike other methods, such as cresyl violet-stained frozen slicing, T2WI MRI is able to dynamically monitor lesion volume in living animals. The present study demonstrated that administration of COG1410 markedly reduced the lesion volumes on Day 1 and Day 3 post-CCI corresponding to the acute stage. Our results were consistent with previous studies reporting that COG1410 reduced the size of the injury cavity in animal models of TBI.17,18
One of the important reasons for the hyper-density of the T2WI is the increase in brain water content; there is a strong linear correlation between the T2WI and brain water content.36 The edema in the acute stage of TBI is a mixed cytotoxic/vasogenic brain edema. However, the vasogenic edema caused by BBB hyperpermeability is the primary reason for the increase of brain water content.3 The whole water content in the focally injured tissue hardly changes during the water transference from extracellular to intracellular sites during the formation of cytotoxic edema.37 Therefore, T2WI is a reliable method, and it has been used to detect lesion volume and vasogenic edema in animal models of cerebral hypoxia-ischemia36,38 and controlled cortical impact.23 Other pathophysiological features beyond cerebral edema, such as hemorrhage, also may influence the T2 signal. However, in our experiments, the interference is very small because the CCI was a moderate injury that caused little hemorrhage. We maintained strict hemostasis post-CCI before closure of the skull with a plastic skull cap, which minimized the impact of hemorrhage. Therefore, our results demonstrated that administration of COG1410 reduced the vasogenic brain edema after CCI.
MMP-9 is activated after brain trauma, and its activity peaks 24 h after TBI.39 The brain water content is maximal 2–3 d after focal contusion in models of TBI,3,23,40 including CCI model. Our previous study demonstrated that this CCI model produces a central contusion and surrounding pericontusional axonal injury.16 The current study demonstrated that the increase in the BBB permeability and edema on Day 3 was more severe than on Day 1 post-CCI. The experimental traumatic brain injuries used in the current study are somewhat similar to human clinical TBI. However, this CCI model produces a central contusion, whereas most of the TBI in humans occurs in a scattered, multifocal distribution pattern. Therefore, the pathophysiology between these injuries is different. Diffuse models of TBI demonstrate an earlier time-point for maximum edema development at 24 h after brain injury.41 Briefly, the acute stage is the very important period for investigating the effect of drugs on MMP-9 activity and brain edema post-CCI. Therefore, we chose the Day 1 and Day 3 post-CCI as our study time-points. Our results demonstrated that COG1410 markedly decreased the peak vasogenic edema after TBI.
The degree of post-traumatic brain edema and BBB dysfunction influences the neurofunctional outcome,1,42 and inhibition of MMP-9 attenuates the behavioral impairments after TBI.43 Therefore, the ability of COG1410 to reduce vasogenic edema and its effects on the vestbulomotor function test demonstrate the potential for this apoE peptide to alter important aspects of neuronal function following TBI. The neuroprotection provided by COG1410 administration after TBI in our study is consistent with previous work reporting that COG1410 reduced the behavioral deficits in different animal models of TBI.15,17,18,20
There are several limitations of the current study. First, the effect of COG1410 on edema was tested only during an acute stage post-CCI. We did not test the effects in the subacute stage or verify whether COG1410 reduced the overall time-course of edema after TBI. The present study specifically assessed the potential effects of COG1410 on MMP-9 activity and vasogenic brain edema. These two parameters change the most during the acute stage after TBI.3,39,40 Therefore, we chose the acute stage as the time-point for our studies. We included a subacute stage time-point (the seventh day post-CCI) in our preliminary experiment, but we found that the EB dye extravasation had returned to normal (data not shown). This result was consistent with previous studies that reported the normalization of EB dye extravasation on the seventh day in different animal models of TBI.13,44,45 Therefore, we used short-term outcomes to evaluate the therapeutic effect of COG1410 in this study. Future investigations of additional time-points are required to address the effects of other secondary injuries following traumatic brain injury.
Second, cytotoxic brain edema is one type of predominant edema after TBI.3 We examined the effect of COG1410 on vasogenic edema post-CCI but we did not directly examine its effect on cytotoxic brain edema. Further studies are needed to determine whether apoE and apoE-mimetic peptides such as COG1410 influence cytotoxic brain edema after TBI.
Third, dynamic contrast-enhanced MRI (DCE-MRI) and its volume transfer coefficient (Ktrans) can be used to quantitatively analyze BBB permeability after TBI.46 However, this method requires quantitative intravascular injections of MRI-contrast agent at a constant rate using a microinfusion pump. It is technically challenging to administer contrast agent via tail vein of C57BL/6J mice and maintain a stable dose of contrast agent. Our further studies will verify the effect of COG1410 on Ktrans after TBI in larger species.
In conclusion, these findings support a protective effect of COG1410 by reducing the disruption of the BBB and the development of vasogenic brain edema in an animal model of TBI. Our current findings and the results of others suggests that COG1410 is a promising preclinical therapeutic agent for the treatment of traumatic brain injury.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81371319, 81371378 & 81000528), the Program for New Century Excellent Talents in University (NCET-12-1057), and the Foundation for outstanding youth academic technology leaders of Sichuan province (2014JQ0022). We thank Cognosci Inc. for providing COG1410.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Unterberg A.W., Stover J., Kress B., and Kiening K.L. (2004). Edema and brain trauma. Neuroscience 129, 1021–1029 [DOI] [PubMed] [Google Scholar]
- 2.Bains M. and Hall E.D. (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 1822, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donkin J.J. and Vink R. (2010). Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr. Opin. Neurol. 23, 293–299 [DOI] [PubMed] [Google Scholar]
- 4.Poirier J. (1994). Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 17, 525–530 [DOI] [PubMed] [Google Scholar]
- 5.Lynch J.R., Morgan D., Mance J., Matthew W.D., and Laskowitz D.T. (2001). Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J. Neuroimmunol. 114, 107–113 [DOI] [PubMed] [Google Scholar]
- 6.Mace B.E., Wang H., Lynch J.R., Moss J., Sullivan P., Colton H., Morgan K., Renauld J.C., and Laskowitz D.T. (2007). Apolipoprotein E modifies the CNS response to injury via a histamine-mediated pathway. Neurol. Res. 29, 243–250 [DOI] [PubMed] [Google Scholar]
- 7.Lomnitski L., Chapman S., Hochman A., Kohen R., Shohami E., Chen Y., Trembovler V., and Michaelson D.M. (1999). Antioxidant mechanisms in apolipoprotein E deficient mice prior to and following closed head injury. Biochim. Biophys. Acta 1453, 359–368 [DOI] [PubMed] [Google Scholar]
- 8.Zhou S., Wu H., Zeng C., Xiong X., Tang S., Tang Z. and Sun X. (2013). Apolipoprotein E protects astrocytes from hypoxia and glutamate-induced apoptosis. FEBS Lett. 587, 254–258 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Lomnitski L., Michaelson D.M. and Shohami E. (1997). Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience 80, 1255–1262 [DOI] [PubMed] [Google Scholar]
- 10.Lynch J.R., Pineda J.A., Morgan D., Zhang L., Warner D.S., Benveniste H., and Laskowitz D.T. (2002). Apolipoprotein E affects the central nervous system response injury and the development of cerebral edema. Ann. Neurol. 51, 113–117 [DOI] [PubMed] [Google Scholar]
- 11.Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., and Zlokovic B.V. (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullerton S.M., Shirman G.A., Strittmatter W.J., and Matthew W.D. (2001). Impairment of the blood-nerve and blood-brain barriers in apolipoprotein e knockout mice. Exp. Neurol. 169, 13–22 [DOI] [PubMed] [Google Scholar]
- 13.Methia N., Andre P., Hafezi-Moghadam A., Economopoulos M., Thomas K.L., and Wagner D.D. (2001). ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 7, 810–815 [PMC free article] [PubMed] [Google Scholar]
- 14.Laskowitz D.T., Thekdi A.D., Thekdi S.D., Han S.K., Myers J.K., Pizzo S.V., and Bennett E.R. (2001). Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp. Neurol. 167, 74–85 [DOI] [PubMed] [Google Scholar]
- 15.Laskowitz D.T., McKenna S.E., Song P., Wang H., Durham L., Yeung N., Christensen D., and Vitek M.P. (2007). COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J. Neurotrauma 24, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y. and Brody D.L. (2012). Administration of COG1410 reduces axonal amyloid precursor protein immunoreactivity and microglial activation after controlled cortical impact in mice. J. Neurotrauma 29, 2332–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman N.A., Beare J.E., Tan A.A., Vitek M.P., McKenna S.E., and Hoane M.R. (2010). COG1410, an apolipoprotein E-based peptide, improves cognitive performance and reduces cortical loss following moderate fluid percussion injury in the rat. Behav. Brain Res. 214, 395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoane M.R., Kaufman N., Vitek M.P., and McKenna S.E. (2009). COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J. Neurotrauma 26, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris C.M. and Scheff S.W. (2009). Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J. Neurotrauma 26, 2269–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoane M.R., Pierce J.L., Holland M.A., Birky N.D., Dang T., Vitek M.P., and McKenna S.E. (2007). The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J. Neurotrauma 24, 1108–1118 [DOI] [PubMed] [Google Scholar]
- 21.Hamm R.J., Pike B.R., O'Dell D.M., Lyeth B.G., and Jenkins L.W. (1994). The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196 [DOI] [PubMed] [Google Scholar]
- 22.Kaya M. and Ahishali B. (2011). Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol. Biol. 763, 369–382 [DOI] [PubMed] [Google Scholar]
- 23.Talley Watts L., Long J.A., Chemello J., Van Koughnet S., Fernandez A., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue is neuroprotective against mild traumatic brain injury. J. Neurotrauma 31, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z.D., Wu H.T., Sun X.C., Zhang X.D., and Zhang J.H. (2011). Protection of minocycline on early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir. Supplement 110, 71–74 [DOI] [PubMed] [Google Scholar]
- 25.Thal S.C., Luh C., Schaible E.V., Timaru-Kast R., Hedrich J., Luhmann H.J., Engelhard K., and Zehendner C.M. (2012). Volatile anesthetics influence blood-brain barrier integrity by modulation of tight junction protein expression in traumatic brain injury. PloS One 7, e50752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishitsuji K., Hosono T., Nakamura T., Bu G., and Michikawa M. (2011). Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 286, 17536–17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafezi-Moghadam A., Thomas K.L., and Wagner D.D. (2007). ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am. J. Physiol. Cell Physiol. 292, C1256–C1262 [DOI] [PubMed] [Google Scholar]
- 28.Gao J., Wang H., Sheng H., Lynch J.R., Warner D.S., Durham L., Vitek M.P., and Laskowitz D.T. (2006). A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit. Care 4, 25–31 [DOI] [PubMed] [Google Scholar]
- 29.Laskowitz D.T., Lei B., Dawson H.N., Wang H., Bellows S.T., Christensen D.J., Vitek M.P., and James M.L. (2012). The apoE-mimetic peptide, COG1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit. Care 16, 316–326 [DOI] [PubMed] [Google Scholar]
- 30.James M.L., Sullivan P.M., Lascola C.D., Vitek M.P., and Laskowitz D.T. (2009). Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke 40, 632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Anderson L.G., Lascola C.D., James M.L., Venkatraman T.N., Bennett E.R., Acheson S.K., Vitek M.P., and Laskowitz D.T. (2012). ApolipoproteinE mimetic peptides improve outcome after focal ischemia. Exp. Neurol. 241, 67–74 [DOI] [PubMed] [Google Scholar]
- 32.Gu Z., Kaul M., Yan B., Kridel S.J., Cui J., Strongin A., Smith J.W., Liddington R.C., and Lipton S.A. (2002). S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297, 1186–1190 [DOI] [PubMed] [Google Scholar]
- 33.Kim G.W., Gasche Y., Grzeschik S., Copin J.C., Maier C.M., and Chan P.H. (2003). Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J. Neurosci. 23, 8733–8742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch J.R., Tang W., Wang H., Vitek M.P., Bennett E.R., Sullivan P.M., Warner D.S., and Laskowitz D.T. (2003). APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 278, 48529–48533 [DOI] [PubMed] [Google Scholar]
- 35.Laskowitz D.T., Fillit H., Yeung N., Toku K., and Vitek M.P. (2006). Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol. Scand. Suppl. 185, 15–20 [DOI] [PubMed] [Google Scholar]
- 36.Qiao M., Malisza K.L., Del Bigio M.R., and Tuor U.I. (2001). Correlation of cerebral hypoxic-ischemic T2 changes with tissue alterations in water content and protein extravasation. Stroke 32, 958–963 [DOI] [PubMed] [Google Scholar]
- 37.Simard J.M., Kent T.A., Chen M., Tarasov K.V., and Gerzanich V. (2007). Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loubinoux I., Volk A., Borredon J., Guirimand S., Tiffon B., Seylaz J., and Meric P. (1997). Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 28, 419–426 [DOI] [PubMed] [Google Scholar]
- 39.Truettner J.S., Alonso O.F., and Dalton Dietrich W. (2005). Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 25, 1505–1516 [DOI] [PubMed] [Google Scholar]
- 40.Marmarou A. (2003). Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir. Supplement 86, 7–10 [DOI] [PubMed] [Google Scholar]
- 41.Cao F., Chen M., Li G., Ye K., Huang X., and Zheng X. (2012). Altered expression of metabotropic glutamate receptor 1 alpha after acute diffuse brain injury: effect of the competitive antagonist 1-aminoindan-1, 5-dicarboxylic acid. Neural Regen. Res. 7, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alves J.L. (2014). Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 92, 141–147 [DOI] [PubMed] [Google Scholar]
- 43.Hadass O., Tomlinson B.N., Gooyit M., Chen S., Purdy J.J., Walker J.M., Zhang C., Giritharan A.B., Purnell W., Robinson C.R., 2nd Shin D., Schroeder V.A., Suckow M.A., Simonyi A., Sun G.Y., Mobashery S., Cui J., Chang M., and Gu Z. (2013). Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PloS One 8, e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapira Y., Setton D., Artru A.A., and Shohami E. (1993). Blood-brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth. Analg. 77, 141–148 [DOI] [PubMed] [Google Scholar]
- 45.Baskaya M.K., Rao A.M., Dogan A., Donaldson D., and Dempsey R.J. (1997). The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 226, 33–36 [DOI] [PubMed] [Google Scholar]
- 46.Wei X.E., Wang D., Li M.H., Zhang Y.Z., Li Y.H., and Li W.B. (2011). A useful tool for the initial assessment of blood-brain barrier permeability after traumatic brain injury in rabbits: dynamic contrast-enhanced magnetic resonance imaging. J. Trauma 71, 1645–1650 [DOI] [PubMed] [Google Scholar]