Abstract
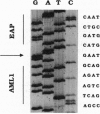
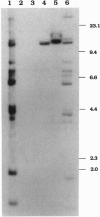
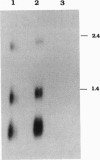
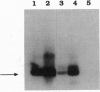
In the 8;21 translocation, the AML1 gene, located at chromosome band 21q22, is translocated to chromosome 8 (q22), where it is fused to the ETO gene and transcribed as a chimeric gene. AML1 is the human homolog of the recently cloned mouse gene pebp2 alpha B, homologous to the DNA binding alpha subunit of the polyoma enhancer factor pebp2. AML1 is also involved in a translocation with chromosome 3 that is seen in patients with therapy-related acute myeloid leukemia and myelodysplastic syndrome and in chronic myelogenous leukemia in blast crisis. We have isolated a fusion cDNA clone from a t(3;21) library derived from a patient with therapy-related myelodysplastic syndrome; this clone contains sequences from AML1 and from EAP, which we have now localized to band 3q26. EAP has previously been characterized as a highly expressed small nuclear protein of 128 residues (EBER 1) associated with Epstein-Barr virus small RNA. The fusion clone contains the DNA binding 5' part of AML1 that is fused to ETO in the t(8;21) and, in addition, at least one other exon. The translocation replaces the last nine codons of AML1 with the last 96 codons of EAP. The fusion does not maintain the correct reading frame of EAP and may not lead to a functional chimeric protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Baron B. W., Nucifora G., McCabe N., Espinosa R., 3rd, Le Beau M. M., McKeithan T. W. Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5262–5266. doi: 10.1073/pnas.90.11.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. P., Rhee S., Craven R. C., Hunter E., Wills J. W. Amino acids encoded downstream of gag are not required by Rous sarcoma virus protease during gag-mediated assembly. J Virol. 1991 Jan;65(1):272–280. doi: 10.1128/jvi.65.1.272-280.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I., Rigault P., Guillou S., Ougen P., Billaut A., Guasconi G., Gervy P., LeGall I., Soularue P., Grinas L. Continuum of overlapping clones spanning the entire human chromosome 21q. Nature. 1992 Oct 1;359(6394):380–387. doi: 10.1038/359380a0. [DOI] [PubMed] [Google Scholar]
- Daga A., Tighe J. E., Calabi F. Leukaemia/Drosophila homology. Nature. 1992 Apr 9;356(6369):484–484. doi: 10.1038/356484b0. [DOI] [PubMed] [Google Scholar]
- Drabkin H., Wright M., Jonsen M., Varkony T., Jones C., Sage M., Gold S., Morse H., Mendez M., Erickson P. Development of a somatic cell hybrid mapping panel and molecular probes for human chromosome 3. Genomics. 1990 Nov;8(3):435–446. doi: 10.1016/0888-7543(90)90029-t. [DOI] [PubMed] [Google Scholar]
- Erickson P., Gao J., Chang K. S., Look T., Whisenant E., Raimondi S., Lasher R., Trujillo J., Rowley J., Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–1831. [PubMed] [Google Scholar]
- Gallant J. A., Lindsley D. Leftward ribosome frameshifting at a hungry codon. J Mol Biol. 1992 Jan 5;223(1):31–40. doi: 10.1016/0022-2836(92)90713-t. [DOI] [PubMed] [Google Scholar]
- Gu Y., Nakamura T., Alder H., Prasad R., Canaani O., Cimino G., Croce C. M., Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992 Nov 13;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Inaba T., Roberts W. M., Shapiro L. H., Jolly K. W., Raimondi S. C., Smith S. D., Look A. T. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science. 1992 Jul 24;257(5069):531–534. doi: 10.1126/science.1386162. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Rashidbaigi A., Lai L. W., Patterson D., Jones C. Sublocalization on chromosome 21 of human interferon-alpha receptor gene and the gene for an interferon-gamma response protein. Somat Cell Mol Genet. 1990 May;16(3):231–240. doi: 10.1007/BF01233359. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Birn D. J., Erickson P., Gao J., LeBeau M. M., Drabkin H. A., Rowley J. D. Detection of DNA rearrangements in the AML1 and ETO loci and of an AML1/ETO fusion mRNA in patients with t(8;21) acute myeloid leukemia. Blood. 1993 Feb 15;81(4):883–888. [PubMed] [Google Scholar]
- Nucifora G., Birn D. J., Espinosa R., 3rd, Erickson P., LeBeau M. M., Roulston D., McKeithan T. W., Drabkin H., Rowley J. D. Involvement of the AML1 gene in the t(3;21) in therapy-related leukemia and in chronic myeloid leukemia in blast crisis. Blood. 1993 May 15;81(10):2728–2734. [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Anastasi J., Winter J. N., Thangavelu M., Vardiman J. W., Rowley J. D., Le Beau M. M. t(3;21)(q26;q22): a recurring chromosomal abnormality in therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 1990 Dec 15;76(12):2594–2598. [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Bitter M. A., Carrino J. J., Le Beau M. M., Diaz M. O., Rowley J. D. Association of a chromosomal 3;21 translocation with the blast phase of chronic myelogenous leukemia. Blood. 1987 Nov;70(5):1338–1342. [PubMed] [Google Scholar]
- Tkachuk D. C., Kohler S., Cleary M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992 Nov 13;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Toczyski D. P., Steitz J. A. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs). EMBO J. 1991 Feb;10(2):459–466. doi: 10.1002/j.1460-2075.1991.tb07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczyski D. P., Steitz J. A. The cellular RNA-binding protein EAP recognizes a conserved stem-loop in the Epstein-Barr virus small RNA EBER 1. Mol Cell Biol. 1993 Jan;13(1):703–710. doi: 10.1128/mcb.13.1.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R., Lindsley D., Falahee B., Gallant J. On the mechanism of ribosomal frameshifting at hungry codons. J Mol Biol. 1988 Sep 20;203(2):403–410. doi: 10.1016/0022-2836(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Müller R. G., Fu X. C., Hynes N. E., Kozma S. Oncogenic activation of the human trk proto-oncogene by recombination with the ribosomal large subunit protein L7a. EMBO J. 1990 Jan;9(1):191–196. doi: 10.1002/j.1460-2075.1990.tb08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]