Abstract
Background
Recent data support the renewed interest in hypertriglyceridemia as a possible important therapeutic target for cardiovascular risk reduction. This study was designed to address the question of all-cause mortality during extended follow-up of the BIP trial in patients stratified by baseline triglyceride levels.
Methods
In the BIP trial 3090 patients with proven coronary artery disease were randomized to bezafibrate 400 mg/day or placebo. All-cause mortality data after 20 years of follow-up, were obtained from the National Israeli Population Registry. Patients with hypertriglyceridemia (triglycerides ≥200 mg/dL, n = 458) were equally distributed among the study groups (15 % in both placebo and bezafibrate groups).
Results
During follow-up 1869 patients died (952 in placebo vs. 917 in bezafibrate group). Following multivariate adjustment allocation to bezafibrate was associated with small but significant 10 % mortality risk reduction (HR 0.90; 95 % CI 0.82–0.98, p = 0.026). Variables associated with significantly increased mortality risk were history of a past MI, NYHA class, diabetes, age, higher BMI and glucose level. In patients with hypertriglyceridemia multivariate analysis demonstrated a 25 % all-cause mortality risk reduction associated with allocation to bezafibrate (HR 0.75, CI 95 % 0.60–0.94; p = 0.012). In patients without hypertriglyceridemia bezafibrate had no significant effect on long-term mortality.
Conclusions
During long-term follow-up bezafibrate-allocated patients experienced a modest but significant 10 % reduction in the adjusted risk of mortality. This effect of bezafibrate was more prominent among patients with baseline hypertriglyceridemia (25 % mortality risk reduction).
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0332-6) contains supplementary material, which is available to authorized users.
Keywords: Bezafibrate, Triglycerides, Coronary disease, Prognosis, Mortality, Lipids, Cholesterol, HDL
Background
There is a growing body of recently published genetic and epidemiological evidences which demonstrated a causal role of triglycerides (TG) and TG-rich lipoproteins in the pathogenesis of atherosclerosis and particularly coronary artery disease (CAD) [1–5]. These data support the renewed interest in hypertriglyceridemia as a possible important therapeutic target for cardiovascular risk reduction [6–8]. Historically, one of the main aims of the Bezafibrate Infarction Prevention (BIP) trial was to assess the effect of reducing TG levels (alongside with rising of HDL-C levels) on cardiac risk in patients with established CAD [9]. During the course of the study, TG levels were reduced by 21 % and HDL-C increased by 18 % among patients that had received bezafibrate. Nevertheless, despite these favorable lipid-modifying effects, bezafibrate therapy was associated with only a non-significant trend of a reduction of the incidence of primary end point (fatal or non-fatal myocardial infarction or sudden death). However, a significant 39.5 % reduction in the primary end point in patients with high baseline TG (≥ 200 mg/dl) was observed. Also during post hoc analysis of the BIP trial we have shown that bezafibrate significantly reduced the incidence of myocardial infarction in patients with the metabolic syndrome [10]. The relatively short mean follow-up of 6.2 year which could be achieved during the double-blind phase of the BIP trial precluded a determination of whether bezafibrate affected total mortality. We hypothesized that early favorable effects of bezafibrate on lipids and myocardial infarction during the original trial period could be translated in a subsequent reduction in total mortality during a longer-term observation. Therefore, this study was designed to address the question of mortality during extended 20-year follow-up of the BIP trial.
Methods
The BIP trial
The BIP trial evaluated the effect of bezafibrate versus placebo on major coronary events and mortality in CHD patients. Details of the study design have been previously published [9, 11–13]. Briefly, 3090 male and female patients 45–74 years of age with a history of MI and/or stable angina pectoris and a lipid profile of serum total cholesterol between 180 and 250 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≤180 mg/dL (≤160 mg/dL for patients <50 years), HDL-C ≤45 mg/dl, and triglycerides ≤300 mg/dL were randomized to bezafibrate 400 mg/day or placebo between May 1990 and January 1993 and followed up over a mean period of 6.2 years (median 6.2 years; interquartile range [IQR] 5.3–6.8 years).
Stable angina pectoris confirmed by coronary angiography, and/or radio- nuclear studies or standard exercise tests. The main exclusion criteria were insulin-dependent diabetes mellitus, severe heart failure, unstable angina pectoris, hepatic or renal failure, known sensitivity to bezafibrate, or current use of lipid-modifying drugs.
After discontinuation of the study medication, patients were observed for coronary events for an additional period, bringing the total follow-up time to a mean of 8.2 years (median 7.9 years; interquartile range 7.2–8.7 years). This study reports all-cause mortality data obtained in 2014 from the National Israeli Population Registry presenting 20 years follow-up [IQR 12–22.6]. The identification number recorded during enrollment was matched to mortality data stored at the national register and verified by matching date of birth. The current analysis is based on over 52,100 patient-years data.
Study population
In the present analysis we included all the subjects included in the original BIP randomized study with the exception of 19 subjects that did not take any study medication. Excluded patients were equally distributed between the Bezafibrate and placebo arm (9 and 10 patients, respectively). IRB approval was obtained both for the original study and for the current long term follow-up data acquisition.
Statistical analysis
Variables were expressed as mean ± SD, and categorical data were summarized as percentages. Baseline characteristics of patients with patients with TG ≥200 mg/dL by the original treatment allocation group were compared using the Chi-square test for categorical parameters and Student t test or Mann–Whitney for continuous variables, as appropriate. Similarly, we compared the pre-specified subgroups of subjects with baseline triglycerides levels ≥200 mg/dL to those with lower levels of triglycerides. The supplemental online data includes characteristics and comparison of the entire study population by original allocation to bezafibrate or placebo.
Survival curves were constructed by the Kaplan–Meier method, and the significance of the variation between them was assessed using a Log-rank test. We compared survival for the entire study population by original treatment allocation (placebo vs. bezafibrate), followed by survival analysis by treatment allocation in the pre-specified sub-groups of subject with TG ≥200 mg/dL and <200 mg/dL.
We used multivariate Cox proportional hazards regression modeling in order to explore the adjusted risk reduction associated with bezafibrate treatment in the entire study population and separately in the subgroup of patients with TG ≥200 mg/dL. Covariates were selected if univariate association was significant (p < 0.05) or clinical studies have demonstrated robust association with prognosis in previous studies. The following covariates were selected using the best subset method: age, gender, BMI, prior MI, history of diabetes mellitus, reported hypertension, NYHA functional class ≥2, and baseline serum values of HDL and glucose. An additional multivariate model was constructed including lipid-lowering medication initiated during the BIP study or the extended follow-up period (total 8 years’ duration) introduced as a time dependent covariate. Proportionality of hazard assumption was verified in all models.
We further undertook interaction term analysis in order to explore mortality associated with fibrate vs. placebo treatment by TG group (below 200 mg/dL vs. ≥200 mg/dL) with the covariates described above.
All statistical tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. The P values for interaction are reported. Analyses were carried out with the use of SPSS software, version 22 (IBM Inc.) and SAS, version 9.3.
Results
The present study included patients from the original BIP cohort: 1548 patients allocated to the bezafibrate group and 1542 patients allocated to the placebo group. Clinical characteristics, laboratory values, and medical therapy were similar in the 2 original study treatment groups (Additional file 1: Table A). Nearly 80 % of the patients had a history of MI, and 10 % had treated diabetes mellitus. Beta-blockers were prescribed to nearly 40 % of study patients, calcium-channel blockers to 50 %, and angiotensin-converting enzyme inhibitors to 12 %.
Patients with triglycerides ≥200 mg/dL (n = 458) were equally distributed among the 2 study groups (15 % in both placebo and bezafibrate groups). There was no significant difference in characteristics of patients with TG ≥200 mg/dL randomized to the placebo (n = 224) vs. fibrate (n = 234) treatment arm (Table 1).
Table 1.
Baseline Clinical and Laboratory characteristics of patients with baseline TG ≥200 mg/dl by original study allocation
| Bezafibrate group (n = 234) | Placebo group (n = 224) | P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 58 ± 7 | 58 ± 7 | 0.62 |
| Male | 210 (90) | 199 (89) | 0.75 |
| Hypertension | 78 (33) | 80 (36) | 0.61 |
| DM | 28 (12) | 21 (9) | 0.37 |
| BMI, kg/m2 | 28 ± 4 | 28 ± 3 | 0.81 |
| NYHA functional class ≥2 | 71 (30) | 53 (25) | 0.41 |
| AP class ≥2 | 72 (30) | 53 (23) | 0.40 |
| Prior MI | 173 (74) | 171 (76) | 0.60 |
| COPD | 4 (2) | 8 (4) | 0.22 |
| Medical therapy | |||
| Anti-platelets | 157 (67) | 155 (69) | 0.63 |
| Beta-blockers | 116 (49) | 94 (42) | 0.10 |
| Nitrates | 114 (51) | 127 (54) | 0.47 |
| Ca2+-blockers | 107 (46) | 116 (52) | 0.11 |
| ACE inhibitors | 30 (13) | 27 (12) | 0.80 |
| Diuretics | 27 (11) | 36 (16) | 0.16 |
| Non-study LLD | 136 (58) | 122 (54) | 0.40 |
| Laboratory values | |||
| Glucose | 104 ± 18 | 103 ± 19 | 0.76 |
| Total cholesterol | 215 ± 18 | 216 ± 18 | 0.46 |
| HDL-C | 31 ± 5 | 32 ± 5 | 0.14 |
| LDL-C | 138 ± 17 | 140 ± 17 | 0.74 |
| Triglycerides | 235 ± 25 | 235 ± 29 | 0.94 |
| Fibrinogen | 361 ± 74 | 355 ± 75 | 0.37 |
Values are presented as n (%) or mean ± SD
AP angina pectoris, ACE angiotensin-converting enzyme, BMI body mass index, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, LLD lipid-lowering drug, MI myocardial infarction, NYHA New York Heart Association
Compared to patients with TG <200 mg/dL patients with baseline TG ≥200 mg/dL presented with similar clinical features, with the exception of a higher BMI and younger age. Laboratory values differed significantly, including lower HDL and lower LDL and higher fasting glucose levels (all p < 0.001; Additional file 2: Table B).
Entire study population mortality
During the follow-up period 1869 patients died (61 %), 952 (62 %) in the placebo group and 917 (60 %) in the bezafibrate group (Fig. 1). Following multivariate adjustment, allocation to the bezafibrate arm was associated with significant 10 % mortality risk reduction (HR 0.90; 95 % CI 0.82–0.98, p = 0.026; Table 2). Variables associated with significantly increased mortality risk were history of a past MI (HR 1.45; CI 0.92–0.98), NYHA class >1 (HR 1.13; CI 1.04–1.22), diabetes (HR 1.34; CI 1.14–1.58), older age, higher BMI and glucose levels (all p < 0.01; Table 2).
Fig. 1.
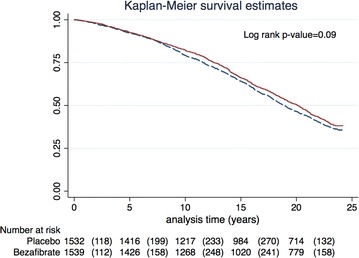
Long-term survival estimates by study drug allocation for the entire study population
Table 2.
Independent predictors of all-cause mortality at 20 years of the entire study cohort
| HR | 95 % CI | P value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Fibrate treatment | 0.90 | 0.82 | 0.98 | 0.026 |
| Past MI | 1.45 | 1.30 | 1.64 | <0.001 |
| Glucose (per 1 mg/dl) | 1.01 | 1.005 | 1.01 | <0.001 |
| Age (per year increment) | 1.09 | 1.08 | 1.10 | <0.001 |
| BMI (per unit) | 1.03 | 1.01 | 1.04 | <0.001 |
| NYHA functional class ≥ II | 1.13 | 1.04 | 1.22 | 0.005 |
| TG ≥200 mg/dl | 1.04 | 0.92 | 1.18 | 0.52 |
| Diabetes mellitus | 1.34 | 1.14 | 1.58 | <0.001 |
Model further adjusted for hypertension, gender, baseline HDL and use of non-study lipid lowering medication as a time dependent covariate
Mortality in TG sub-groups
Next, we divided our cohort according to their baseline triglyceride levels by original study allocation. Patients with baseline triglycerides ≥200 mg/dL allocated to placebo (n = 224) and bezafibrate groups (n = 234) were compared. Patients that were treated with bezafibrate had a non-significant unadjusted survival benefit (57 vs. 62 %; Log rank p value = 0.14).
Multivariate analysis demonstrated a 25 % adjusted all-cause mortality risk reduction in patients with hypertriglyceridemia treated by bezafibrate (HR 0.75; CI 95 % 0.60–0.94; p = 0.012; Fig. 2). Variables associated with significantly increased mortality risk in this subgroup were: age, past MI, use of non-study lipid lowering medication, and diabetes mellitus (Table 3).
Fig. 2.
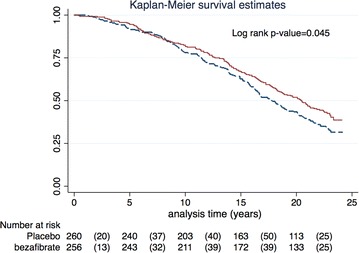
Adjusted survival probability by original study allocation of bezafibrate vs. placebo in patients with TG ≥200 mg/dL&. & Cox proportional hazard regression model adjusted for age, gender, hypertension, past MI, baseline glucose, BMI, NYHA ≥ II, HDL-C and diabetes status
Table 3.
Independent predictors of all-cause mortality at 20 years in the sub group with baseline TG ≥200 mg/dL
| HR | 95 % CI | P value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Fibrate treatment | 0.75 | 0.60 | 0.94 | 0.012 |
| Past MI | 1.53 | 1.15 | 2.00 | 0.004 |
| Glucose | 1.00 | 0.99 | 1.01 | 0.12 |
| Age (per year increment) | 1.08 | 1.07 | 1.11 | <0.001 |
| BMI (per unit) | 1.01 | 0.98 | 1.05 | 0.39 |
| NYHA functional class ≥ II | 1.09 | 0.89 | 1.33 | 0.41 |
| HDL-C (per 1 mg/dl) | 1.00 | 0.97 | 1.02 | 0.76 |
| Diabetes mellitus | 1.88 | 1.36 | 2.61 | <0.001 |
Model further adjusted for hypertension, gender and non-study lipid lowering medication as a time dependent covariate (all p > 0.05)
Interaction term analysis demonstrated that the adjusted risk reduction associated with fibrate treatment compared to placebo is associated with a 21 % risk reduction (HR 0.79; CI 0.63–0.98; p = 0.03) in subjects with elevated TG whereas this effect was not significant in the group with lower TG values (p for interaction = 0.11; Fig. 3).
Fig. 3.
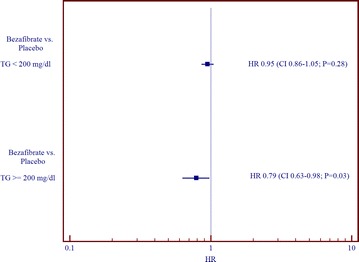
Independent effect of the adjusted risk reduction associated with bezafibrate vs. placebo treatment by pre-specified triglyceride group interaction*. P value for interaction of TG group by treatment allocation = 0.11. *Model further adjusted for age, gender, HDL-C, diabetes, past MI, BMI and glucose levels
Discussion
There are two main findings in this long-term study which based on the intention-to-treat principle with more than 52,000 person-years of follow-up. First, patients treated during original BIP trial period by bezafibrate experienced a modest but significant 10 % reduction in the adjusted risk of mortality compared with patients allocated to the original placebo group. Second, the long-term benefit of bezafibrate was more prominent among patients with hypertriglyceridemia (25 % mortality adjusted risk reduction).
Fibrates are used in clinical practice for the past half century mainly due to their ability to decrease TG. All fibrates are peroxisome proliferators-activated receptors α agonists with ability to enhance the oxidation of fatty acids in liver and muscle and reduce the rate of hepatic lipogenesis, thereby reducing secretion of very-low-density lipoprotein TG. Other important effects of fibrates include an increase of HDL level, activity of lipoprotein lipase, the size of LDL particles and a decrease in the apolipoprotein CIII concentration [14, 15].
The beneficial effects of all major fibrates (gemfibrozil, fenofibrate, bezafibrate) on cardiovascular events could be clearly demonstrated only in patients with dyslipidemia (mainly hypertriglyceridemia) or metabolic syndrome. Metabolic syndrome is an important prognostic sign [16]. In patients without these metabolic abnormalities these effects were absent [9, 17–21]. In a meta-analysis of dyslipidemic subgroups from 5 randomized control trials (RCT) with fibrates totaling 4726 patients, a 35 % relative risk reduction in cardiovascular events was observed compared with a non-significant 6 % reduction in patients without dyslipidemia [22]. Meta-analysis performed in a general population [23] reflecting a blend of effects in patients with and without dyslipidemia (45,058 participants) effect of fibrate therapy was reduced, producing only a modest but still significant 10 % RR reduction for major cardiovascular events and a 13 % RR reduction for coronary events. In these circumstances, the main determinant of the overall results of the fibrate’s trial is mainly dependent of the number of the included appropriate patients with dyslipidemia and/or metabolic syndrome.
From the modern point of view, patients who fulfilled inclusion criteria of the BIP trial should been treated primary by statins and not by fibrates. In fact, about 50 % of the patients in the BIP trial were presented with high LDL-C and low TG and probably did not need fibrates at all. In 1994 the Scandinavian Simvastatin Survival Study (4S) conclusions became available [24] and statins were administered to an increasing number of patients participating in the BIP trial in direct discordance with its protocol. Because of worse lipids profile of placebo-allocated patients they received statins in significantly greater proportion during the late period of the trial. Consequently, the wide use of non-study statins led to attenuation of the bezafibrate’s effect. After the cessation of the BIP trial the rate of use of statins increased substantially in entire study population and eventually was similar in both treatment groups [25].
After publication of the results from the extended phase of the UKPDS trial [26] the concept of “glycemic legacy” was widely discussed. In the 10-year post-trial follow-up, patients with type 2 diabetes originally allocated to intensive hypoglycemic treatment had a significant reduction in the risk of all-cause mortality after the cessation of randomized interventions. These results were obtained although there were no longer differences in HbA1c values between patients originally assigned to conventional or intensive-treatment groups.
To the best of our knowledge, our study is the first which demonstrated in patients with CAD and particularly in patients with hypertriglyceridemia the presence of long term “metabolic memory” for lipids-modified effects of bezafibrate. Our data together with the results from the extended phase of the UKPDS and STENO 2 trials [26–28] provide evidence that the beneficial results of the effective management of cardio-metabolic risk factors could be seen over many years even after the cessation of treatment. Pooled together, these findings support the possible positive role of a “metabolic legacy” for patients with high cardiovascular risk, rather than only a “glycemic legacy” for diabetic patients.
Currently statins are the cornerstone of the treatment and prevention of cardiovascular diseases related to atherosclerosis including CAD [29–32]. Nonetheless, despite the almost universal use of statins in the setting of secondary prevention of CAD, significant residual cardiovascular risk is still present, especially in patients with hypertriglyceridemia and metabolic syndrome [1, 33]. The current guidelines recommend considering combination fibrate-statin therapy for patients when statin therapy alone is not adequate to achieve lipid goals [29, 32, 34]. Despite the strong theoretical background, there are only few hard outcomes data regarding combined bezafibrate and statin treatment: one small randomized study [35] and number of observational studies [36–38]. In these observations bezafibrate/statin treatment was a safe and was associated with a lower incidence of major cardiovascular events compared with statins alone. Beneficial effects of long-term combination therapy with bezafibrate and ezetimibe in patients with dyslipidemia were also reported [39]. Probably, combined bezafibrate/statin or bezafibrate/ezetimibe therapy will be more effective in achieving a comprehensive lipid control and residual cardiovascular risk reduction and theoretically might prevent statin-associated new-onset diabetes [40].
Study limitations
The present study is limited by the post hoc nature of the analysis of the BIP study. It should also be noted that the significant long-term effects of bezafibrate therapy could be observed only after adjustment for important established prognostic predictors including initiation of statins during the double-blind phase of the BIP trial. We do not have information regarding medications or events after the cessation of the BIP trial beyond all-cause mortality nor can we account for changes in medical practice and management guidelines over the long follow-up period. Therefore, further studies are needed to determine the possible long-term mortality benefit of bezafibrate in the current era of universal statin-based secondary prevention in CAD patients.
Conclusions
Patients treated during the original BIP trial period by bezafibrate experienced a modest but significant 10 % reduction in the adjusted risk of mortality during extended follow-up of 20 years. The long-term benefit of bezafibrate was more prominent among patients with baseline hypertriglyceridemia (25 % mortality risk reduction). The present findings suggest that bezafibrate in patients with CAD and hypertriglyceridemia could be associated with a significant long-term mortality reduction that extends far beyond the period of active treatment with the drug.
Authors’ contributions
YA, RK, IG,EVG, AT, AE,NS,EVF participated in the design of the study and performed the statistical analysis and and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- AP
angina pectoris
- BMI
body mass index
- CHD
coronary heart disease
- DM
diabetes mellitus
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- LLD
lipid lowering drugs
- MI
myocardial infarction
- TG
triglycerides
- BIP
Bezafibrate Infarction Prevention
Additional files
10.1186/s12933-016-0332-6 Baseline Clinical and Laboratory Characteristics of the study cohort by original study allocation.
10.1186/s12933-016-0332-6 Baseline Clinical and Laboratory Characteristics of the study cohort by triglyceride level ≥ and < 200 mg/dl.
Footnotes
Yaron Arbel and Robert Klempfner contributed equally to this work
Contributor Information
Yaron Arbel, Phone: 1-416-786-9397, Email: yaronarbel@gmail.com.
Robert Klempfner, Email: yaronarbel@gmail.com.
Aharon Erez, Email: yaronarbel@gmail.com.
Ilan Goldenberg, Email: yaronarbel@gmail.com.
Sagit Benzekry, Email: yaronarbel@gmail.com.
Nir Shlomo, Email: yaronarbel@gmail.com.
Enrique Z. Fisman, Email: yaronarbel@gmail.com
Alexander Tenenbaum, Email: yaronarbel@gmail.com.
References
- 1.Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–2275. doi: 10.1016/j.jacc.2015.03.544. [DOI] [PubMed] [Google Scholar]
- 2.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 5.Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 8.Joshi PH, Martin SS, Blumenthal RS. The remnants of residual risk. J Am Coll Cardiol. 2015;65(21):2276–2278. doi: 10.1016/j.jacc.2015.03.543. [DOI] [PubMed] [Google Scholar]
- 9.Prevention The Bezafibrate Infarction (BIP) study group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102(1):21–27. doi: 10.1161/01.CIR.102.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165(10):1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 11.Goldbourt U, Brunner D, Behar S, Reicher-Reiss H. Baseline characteristics of patients participating in the Bezafibrate Infarction Prevention (BIP) Study. Eur Heart J. 1998;19(Suppl H):H42–H47. [PubMed] [Google Scholar]
- 12.Goldbourt U, Behar S, Reicher-Reiss H, Agmon J, Kaplinsky E, Graff E, et al. Rationale and design of a secondary prevention trial of increasing serum high-density lipoprotein cholesterol and reducing triglycerides in patients with clinically manifest atherosclerotic heart disease (the Bezafibrate Infarction Prevention Trial) Am J Cardiol. 1993;71(11):909–915. doi: 10.1016/0002-9149(93)90905-R. [DOI] [PubMed] [Google Scholar]
- 13.Lipids and lipoproteins in symptomatic coronary heart disease Distribution, intercorrelations, and significance for risk classification in 6,700 men and 1,500 women. The Bezafibrate Infarction Prevention (BIP) Study Group, Israel. Circulation. 1992;86(3):839–848. doi: 10.1161/01.CIR.86.3.839. [DOI] [PubMed] [Google Scholar]
- 14.Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125. doi: 10.1186/1475-2840-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7):498–511. doi: 10.1056/NEJM199908123410707. [DOI] [PubMed] [Google Scholar]
- 16.Arbel Y, Havakuk O, Halkin A, Revivo M, Berliner S, Herz I, et al. Relation of metabolic syndrome with long-term mortality in acute and stable coronary disease. Am J Cardiol. 2015;115(3):283–287. doi: 10.1016/j.amjcard.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32(3):493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 20.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85(1):37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 22.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363(7):692–694. doi: 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 23.Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375(9729):1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 24.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 25.Goldenberg I, Benderly M, Goldbourt U. Secondary prevention with bezafibrate therapy for the treatment of dyslipidemia: an extended follow-up of the BIP trial. J Am Coll Cardiol. 2008;51(4):459–465. doi: 10.1016/j.jacc.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi C, Del Prato S. Metabolic memory and individual treatment aims in type 2 diabetes–outcome-lessons learned from large clinical trials. Rev Diabet Stud RDS. 2011;8(3):432–440. doi: 10.1900/RDS.2011.8.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 29.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 31.Jukema JW, Cannon CP, de Craen AJ, Westendorp RG, Trompet S. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60(10):875–881. doi: 10.1016/j.jacc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Fruchart JC, Davignon J, Hermans MP, Al-Rubeaan K, Amarenco P, Assmann G, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. 2014;13:26. doi: 10.1186/1475-2840-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969–2989. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madrid-Miller A, Moreno-Ruiz LA, Borrayo-Sanchez G, Almeida-Gutierrez E, Martinez-Gomez DF, Jauregui-Aguilar R. Ipact of bezafibrate treatment in patients with hyperfibrinogenemia and ST-elevation acute myocardial infarction: a randomized clinical trial. Cir Cir. 2010;78(3):229–237. [PubMed] [Google Scholar]
- 36.Klempfner R, Goldenberg I, Fisman EZ, Amit U, Haitovich A, Matetzky S, et al. Bezafibrate treatment is associated with a reduced rate of re-hospitalization in smokers after acute coronary syndrome. Cardiol J. 2014;21(4):364–369. doi: 10.5603/CJ.a2013.0127. [DOI] [PubMed] [Google Scholar]
- 37.Tenenbaum A, Medvedofsky D, Fisman EZ, Bubyr L, Matetzky S, Tanne D, et al. Cardiovascular events in patients received combined fibrate/statin treatment versus statin monotherapy: Acute Coronary Syndrome Israeli Surveys data. PLoS One. 2012;7(4):e35298. doi: 10.1371/journal.pone.0035298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klempfner R, Goldenberg I, Fisman EZ, Matetzky S, Amit U, Shemesh J, et al. Comparison of statin alone versus bezafibrate and statin combination in patients with diabetes mellitus and acute coronary syndrome. Am J Cardiol. 2014;113(1):12–16. doi: 10.1016/j.amjcard.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Teramoto T, Abe K, Taneyama T. Safety and efficacy of long-term combination therapy with bezafibrate and ezetimibe in patients with dyslipidemia in the prospective, observational J-COMPATIBLE study. Cardiovasc Diabetol. 2013;6(12):163. doi: 10.1186/1475-2840-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenenbaum A, Fisman EZ. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc Diabetol. 2012;11:140. doi: 10.1186/1475-2840-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]


