Abstract
Background
In December 2010, a Plasmodium vivax malaria outbreak occurred among French forces involved in a mission to control illegal gold mining in French Guiana. The findings of epidemiological and entomological investigations conducted after this outbreak are presented here.
Methods
Data related to malaria cases reported to the French armed forces epidemiological surveillance system were collected during the epidemic period from December 2010 to April 2011. A retrospective cohort study was conducted to identify presumed contamination sites. Anopheles mosquitoes were sampled at the identified sites using Mosquito Magnet and CDC light traps. Specimens were identified morphologically and confirmed using molecular methods (sequencing of ITS2 gene and/or barcoding). Anopheles infections with Plasmodium falciparum and P. vivax were tested by both enzyme-linked immunosorbent assay and real-time PCR.
Results
Seventy-two P. vivax malaria cases were reported (three were mixed P. falciparum/P. vivax infections), leading to a global attack rate of 26.5 % (72/272). Lack of compliance with vector control measures and doxycycline chemoprophylaxis was reported by patients. Two illegal gold mining sites located in remote areas in the primary forest were identified as places of contamination. In all, 595 Anopheles females were caught and 528 specimens were formally identified: 305 Anopheles darlingi, 145 Anopheles nuneztovari s.l., 63 Anopheles marajoara and 15 Anopheles triannulatus s.l. Three An. darlingi were infected by P. falciparum (infection rate: 1.1 %) and four An. marajoara by P. vivax (infection rate: 6.4 %).
Discussion
The main drivers of the outbreak were the lack of adherence by military personnel to malaria prevention measures and the high level of malaria transmission at illegal gold mining sites. Anopheles marajoara was clearly implicated in malaria transmission for the first time in French Guiana. The high infection rates observed confirm that illegal gold mining sites must be considered as high level malaria transmission areas in the territory.
Conclusions
Illegal gold mining activities are challenging the control of malaria in French Guiana. Collaboration with neighbouring countries is necessary to take into account mobile populations such as gold miners. Malaria control strategies in the French armed forces must be adapted to P. vivax malaria and sylvatic Anopheles species.
Keywords: Malaria, French Guiana, Illegal gold mining, Military, Plasmodium vivax, Outbreak, Anopheles darlingi, Anopheles marajoara
Background
French Guiana is a French overseas entity and an Outermost Region of the European Union located on the northeast coast of South America (Fig. 1). It is a sparsely populated area—250,000 inhabitants in 2013—and 85 % of the territory is covered by the Amazon rainforest [1]. An estimated 80 % of the population lives on or close to the coastal area, characterized by malaria cases imported from the interior and, more rarely, indigenous transmission [2]. From 2005 to 2014, the number of malaria cases officially reported in French Guiana (all Plasmodium species) decreased from 4479 to 445 [3]. Most of these officially-reported cases currently occur in villages located along the main rivers flowing through the territory, especially those bordering Suriname and Brazil [3]. This is partially offset by an increase in the number of cases related to transmission at illegal gold mining sites. Indeed, such sites located in forested inland French Guiana are probably areas of malaria transmission for Plasmodium vivax, Plasmodium falciparum and Plasmodium malariae [4–7].
Fig. 1.
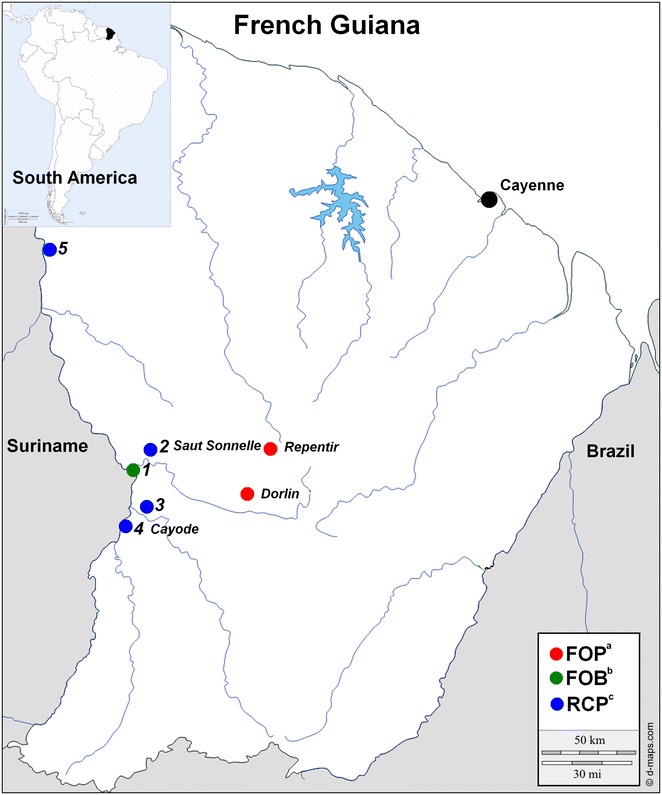
Map of French Guiana and location of French military units in 2010–2011. Legend: a FOP forward operational post, b FOB forward operational base, c RCP river check point. 1—Maripasoula FOB, 2—Saut Sonnelle RCP, 3—Cayodé RCP, 4—Twenké RCP, 5—Providence RCP. Names in italics correspond to mosquito sampling sites
In 2009, Suriname estimated that 1140 cases of malaria diagnosed and treated in its clinics were imported cases, mainly from illegal gold mining sites in French Guiana [8]. In 2014, for the border town of Oiapoque (Oyapock River, Brazil), 21.1 % of malaria cases reported were imported from French Guiana, mostly related to illegal gold mining [9]. In the Amazon, the transmission of malaria due to human migration has been called “frontier malaria” [10]. The impact of illegal gold mining on malaria transmission has been highlighted in most parts of the Amazon Basin [11–14].
In French Guiana, Anopheles (Nyssorhynchus) darlingi, known for its anthropophilia and its adaptation to anthropized environments, is considered as the main vector of malaria [15–18]. In the Amazon Basin, it is described as biting all night with two peaks of activity at dusk and dawn. It can enter shelters or homes to take a blood meal, but rests afterwards in the forest [19–22]. Its breeding sites are linked to river banks and flooded areas when rivers overflow during the rainy season [23, 24]. In addition to An. darlingi, other anopheline species of the subgenera Nyssorhynchus and Anopheles have been incriminated in malaria transmission in the Amazon Basin [24–26]. Moreover, Anopheles of the subgenus Kerteszia are present in French Guiana. These species have been described as biting during the day along forest trails, and they have been suspected of playing a role in malaria transmission [27].
Because of illegal activity and violence, no entomological study has ever been conducted to date at illegal gold mining sites in French Guiana. French military personnel have been involved in a specific military operation to control and reduce illegal gold mining activities in French Guiana since 2005. The main purpose of this operation is to disrupt the logistics of illegal gold mining in the primary forest. To that end, 400 military personnel are permanently deployed at river checkpoints (RCP), forward operational bases on major rivers (FOB) and forward operational posts deep in the forest (FOP) (Fig. 1). Most of them come directly from France for 4-month missions in French Guiana. From forward bases, military personnel conduct short (24–48 h, camping in the forest) or long-term (3–4 weeks) interventions at illegal gold mining positions. During these missions, the malaria prevention strategy in the French armed forces is based on three axes: (1) Health education to encourage compliance with protective measures, (2) Personal protection against vectors including repellents (DEET or picaridine), use of permethrin-impregnated combat uniforms, long trousers and long-sleeved shirts from dusk to dawn (patrols and guards), deltamethrin-impregnated cotton bed nets in forward bases and hammocks with mosquito nets when camping deep in the forest (individual vector-bite protection equipment is provided to each soldier) and (3) Chemoprophylaxis based on 100 mg of doxycycline daily during their entire stay and for 4 weeks after leaving the malaria transmission area (terminal prophylaxis) [28]. Despite such strategies, deployments at illegal gold mining sites have resulted in several outbreaks and in an increase of malaria incidence among French forces, with P. vivax accounting for more than 80 % of reported cases [29].
In December 2010, the French military health surveillance system detected a P. vivax outbreak among three military units deployed from France to French Guiana for a 4-month mission. To explain this outbreak, a case series study was first conducted to describe the outbreak, followed by a retrospective cohort study to determine areas at risk for malaria transmission. In a second stage, an entomological study was conducted in the determined areas to identify the vectors involved and determine the level of malaria transmission.
Methods
Epidemiological investigation
Data were collected during the epidemic period from December 2010 to April 2011. A malaria case was mandatorily defined as any pathologic event or symptom associated with confirmed parasitological evidence (Plasmodium spp. in blood smears or quantitative buffy coat or positive malaria rapid diagnosis tests) contracted in French Guiana. Cases were reported to the French armed forces epidemiological surveillance system in French Guiana (indigenous cases) and in continental France for military units that had returned to France (imported cases). During missions, the French armed forces malaria prevention strategy was implemented [28]. The hammocks used were made of a single thin layer of fabric with a non-treated polyester mosquito net. For each malaria case, military physicians had to complete a mandatory specific form containing administrative, geographic and clinical information, compliance with individual protection measures against mosquito bites (repellent applied to skin, use of permethrin-impregnated combat uniform and bed or hammock net), compliance with chemoprophylaxis and biological data [29]. The level of compliance with vector control measures was assessed as either “never”, “seldom”, “often” or “always”, and proper compliance was defined as “always”. Chemoprophylaxis compliance was assessed as a daily 100 mg dose of doxycycline during the 8 days preceding a malaria attack. A retrospective cohort study was conducted to identify places of stay related to a P. vivax attack during the 4-month mission. These different locations were available for the two main military units affected by the epidemic.
Entomological investigation
Mosquito field sampling
Entomological investigation in the field was guided by epidemiological investigation results. Anopheles mosquitoes were sampled from June to July 2011. As classic human landing collections could not be conducted, different traps were used: Mosquito Magnet® (MM) traps (Woodstream Corporation, Lititz, PA) and Center for Disease Control and Prevention (CDC) light traps. One MM trap baited with octenol was deployed along the Maroni River (border with Suriname) at the two river checkpoints of Cayodé and Saut Sonnelle, for 1 month (Fig. 1). Traps were checked after dawn and before dusk every day. Two mosquito collection sessions were conducted at two illegal gold mining sites: Repentir from June 9 to 15, and Dorlin from June 24 to July 15 (Fig. 1). Due to safety and logistical reasons, French military personnel accompanied the research team in the field. Each time, a medical entomologist was present to install traps during the first nights. Three MM traps baited with octenol were in use night and day during the stay and checked after dawn and before dusk. Two CDC light traps were used, but only for the first three nights due to the short lifespan of batteries in the rainforest and logistical limitations.
All mosquitoes were stored individually in numbered vials with desiccant, and were preserved at −20 °C until processing at the medical entomology unit of the Institut Pasteur de la Guyane (IPG), Cayenne (French Guiana), or at the medical entomology unit of the Institute for Biomedical Research of the French Armed Forces (IRBA), Marseille (France). Before storage at −20 °C, mosquitoes were conserved at ambient temperature in the rainforest for 1–15 days according to the date of collection.
Water collections in the study areas were examined for anopheline larvae. Larval surveys were conducted in the immediate vicinity of the settlements. Larvae and pupae were sampled using a standard dipping method in water collections [30]. Bromeliads recovered from fallen trees were also explored for larvae of the subgenus Kerteszia. Larvae and pupae were reared in the field. The neonate adult mosquitoes were stored by date and breeding site in numbered vials with desiccant and preserved at −20 °C, until processing.
Anopheles identification
Adult mosquitoes collected or neonate mosquitoes were sorted by genera, and Anopheles specimens were morphologically identified based on keys in use in the Guiana Shield [31–34].
Mosquitoes caught with traps are often in bad condition, and some specimens are difficult to identify [35]. Furthermore, as no catches had ever been conducted at some of the selected sampling sites, species never described in French Guiana but present in neighbouring countries may have been present deep in the forest. Morphological identifications were then completed by molecular analysis following established protocols. Mosquito DNA was extracted, Internal transcribed spacer 2 (ITS2) gene was processed by PCR-RFLP and sequenced for a selected sample and for specimens infected by P. falciparum or P. vivax [36, 37]. DNA barcodes were also used for the final identification of some specimens [38].
Plasmodium falciparum and Plasmodium vivax detection
Heads and thoraces of a sample of Anopheles females were tested by enzyme-linked immunosorbent assay (ELISA) for P. falciparum and P. vivax strains VK210 and VK247 circumsporozoite protein (CSP) [39]. However, using ELISA to research human Plasmodium infection of the Anopheles captured in deep forest has two major drawbacks: the lack of a cold chain and the possibility of non-specific cross-reactions with non-human Plasmodium. The lack of a cold chain leads to a deterioration of parasite proteins detected by ELISA, which could explain the lack of detection of infected Anopheles mosquitoes. Furthermore, zoophilic Anopheles can be infected by “animal” Plasmodium that could generate false positives by cross reaction and thus be considered of importance to human health [40]. Therefore, a RT-PCR detection of Plasmodium infection was systematically carried out for each Anopheles specimen [41]. The CSP index for each Plasmodium species was calculated as the proportion of mosquitoes found to be positive for CSP or for Plasmodium DNA.
Statistical analysis
Univariate analysis was conducted to determine places of stay during the 4-month mission related to the risk of malaria. Relative risk of contracting malaria was calculated for each place of stay. The results are expressed as a percentage of subjects for incidence and attack rates and as a percentage for compliance with protection measures. Excel and SAS 9.3® software were used. Geographic maps used were free open access [42].
Results
Epidemiological investigation
The epidemic lasted 7 months, with a total of 72 P. vivax malaria cases, including three mixed P. falciparum/P. vivax infections. Three military units (N = 272) deployed for 4 months (September 2010–January 2011) were affected by the epidemic: one infantry company (n = 138), one sapper company (n = 116) and one platoon of Gendarmes (n = 18) (Fig. 2). These three units had joint missions during their stay in French Guiana. The first P. vivax malaria case occurred on October 21, 2010, 7 weeks after deployment to French Guiana, and the last case on April 28, 2011, 4 months after returning to France (Fig. 2). The overall attack rate was 26.5 % (72/272). Attack rates for the infantry company, the sapper company and the platoon of Gendarmes were comparable: 24.6 % (34/138), 28.4 % (33/116), 27.8 % (5/18), respectively. The epidemic curve had two peaks, the first at the end of the mission in French Guiana (indigenous cases) and the second after the soldiers’ return to France (imported cases) (Fig. 2). Indigenous cases accounted for 54.2 % (39/72) and imported cases for 45.8 % (33/72). Malaria occurred with a median delay of 36 days [interquartile interval (28–86 days)] after returning to France. In all, 82 % (27/33) of imported cases occurred after terminal doxycycline chemoprophylaxis. One of the patients presented a malarial splenomegaly and a splenic rupture after a seemingly harmless abdominal trauma.
Fig. 2.
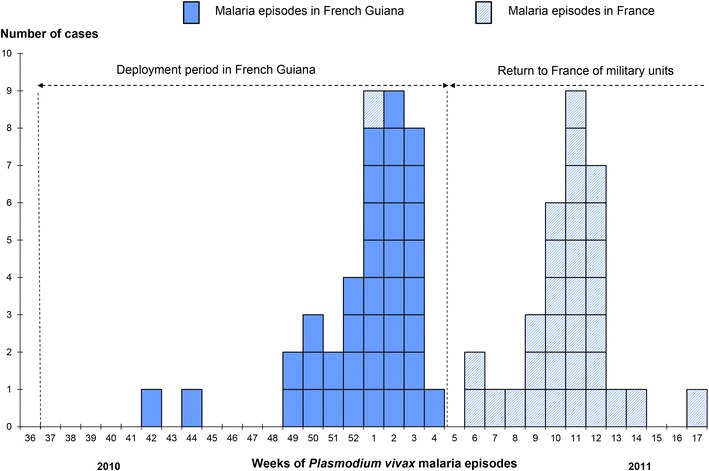
Epidemic curve of Plasmodium vivax episodes among French military personnel 2010–2011 (N = 72)
Compliance with personal protection measures against mosquito bites was completed for 61 cases. Bed or hammock nets were always used by 59 % (36/61), insect repellents by 46 % (28/61) and permethrin-impregnated combat uniforms by 52 % (32/61) of the cases. Several of the patients stated that they had been bitten through the cloth of their hammock during nights spent in deep forest. Evaluation of chemoprophylaxis compliance was not relevant for malaria attacks occurring more than 28 days after returning to France (n = 27). But for other cases, proper compliance (patient’s self-evaluation) with doxycycline in the 8 days preceding a malaria attack was 40 % (18/45).
Places of stay during the 4-month mission in French Guiana were available for infantry and sapper companies (N = 254). Two locations were associated with malaria: the illegal gold mining sites of Dorlin [relative risk = 2.68 CI 95 % (1.61–4.46)] and Repentir [relative risk = 5.35 CI 95 % (3.08–9.30)] (Table 1; Fig. 1).
Table 1.
Places of stay in French Guiana related to Plasmodium vivax attack among French military forces (N = 254, univariate analysis)
| N | Nb P. vivax | Incidence (%) | RR | CI 95 % | p value | |
|---|---|---|---|---|---|---|
| Dorlin | 0.0117 | |||||
| No | 245 | 61 | 24.9 | 1 | ||
| Yes | 9 | 6 | 66.7 | 2.68 | 1.61–4.46 | |
| Repentir | <0.0001 | |||||
| No | 143 | 13 | 9.1 | 1 | ||
| Yes | 111 | 54 | 48.6 | 5.35 | 3.08–9.30 | |
| Twenké | 0.2760 | |||||
| No | 213 | 59 | 27.7 | 1 | ||
| Yes | 41 | 8 | 19.5 | 0.70 | 0.36–1.36 | |
| Cayodé | 0.2631 | |||||
| No | 196 | 55 | 28.1 | 1 | ||
| Yes | 58 | 12 | 20.7 | 0.73 | 0.42–1.28 | |
| Maripasoula | 0.9236 | |||||
| No | 35 | 9 | 25.7 | 1 | ||
| Yes | 219 | 58 | 26.5 | 1.03 | 0.56–1.89 | |
| Saut Sonnelle | 0.4119 | |||||
| No | 136 | 33 | 24.3 | 1 | ||
| Yes | 118 | 34 | 28.8 | 1.19 | 0.79–1.79 | |
| Providence | 0.3565 | |||||
| No | 219 | 60 | 27.4 | 1 | ||
| Yes | 35 | 7 | 20.0 | 0.73 | 0.36–1.47 |
Statistically significant results are in italics
Entomological investigation
Mosquito collection and identification
During the survey in June and July, 595 female Anopheles were caught only at night, including 554 specimens at the two illegal gold mining sites and 41 specimens at both river checkpoints (40 with MM at Cayodé, one with CDC light trap at Saut Sonnelle). In Repentir, 166 Anopheles mosquitoes were caught: 164 using two MM traps over the entire period and two using two CDC traps for three nights. In Dorlin, 388 Anopheles mosquitoes were caught: 370 using two MM traps over the entire period and 18 using two CDC traps for three nights. Morphological identification was difficult due to the damage caused to specimens by traps and the presence of moisture. It allowed the probable species identification of 398 specimens: 249 Anopheles darlingi, 88 Anopheles nuneztovari s.l., 53 Anopheles albitarsis s.l. and seven Anopheles triannulatus s.l. Morphological identification was inconclusive for 199 specimens (199 An. spp.) (Table 2). Molecular identification by ITS2 PCR-RFLP made it possible to formally identify 528 specimens: 305 An. darlingi, 145 An. nuneztovari s.l., 63 An. albitarsis s.l. and 15 An. triannulatus s.l. (Table 2). For 27 specimens (six An. triannulatus s.l., seven An. albitarsis s.l., nine An. triannulatus s.l. and five An. darlingi), the products of ITS2 PCR were sequenced and compared to available sequences. The sequencing confirmed the molecular identification for 26 specimens. The overall consistency of morphological identification was 91 %. Table 3 summarizes the distribution of the species processed with ITS2 PCR-RFLP by location. Furthermore, six members of the An. albitarsis complex caught were molecularly identified using DNA barcoding. At the two sites where they were caught, all specimens were Anopheles marajoara [43].
Table 2.
Distribution by species of the 595 anopheles caught in French Guiana in June–July 2011
| Molecular identification | Morphological identification | |||||
|---|---|---|---|---|---|---|
| An. albitarsis s.l. | An. darlingi | An. nuneztovari s.l. | An. triannulatus s.l. | An. spp. | Total | |
| An. albitarsis s.l. | 48 (90.5 %) | 4 | 11 | 63 | ||
| An. darlingi | 3 | 224 (90.0 %) | 1 | 77 | 305 | |
| An. nuneztovari s.l. | 2 | 83 (94.3 %) | 1 | 59 | 145 | |
| An. triannulatus s.l. | 1 | 3 | 6 (85.7 %) | 5 | 15 | |
| An. spp. | 19 | 1 | 47 | 67 | ||
| Total of KI | 52 | 249 | 88 | 7 | 199 | 595 |
In italic values between parentheses, consistency between morphological identification and molecular analysis for each species
Table 3.
Distribution by site of sampling of the 595 anopheles caught in French Guiana from June to July 2011
| An. marajoara | An. darlingi | An. nuneztovari s.l. | An. triannulatus s.l. | An. spp. | Total | |
|---|---|---|---|---|---|---|
| Dorlin | 62 | 262 | 20 | 2 | 42 | 388 |
| Repentir | 11 | 125 | 13 | 17 | 166 | |
| Cayodé | 32 | 8 | 40 | |||
| Saut Sonnelle | 1 | 1 | ||||
| Total | 63 | 305 | 145 | 15 | 67 | 595 |
Plasmodium falciparum and Plasmodiumvivax detection
Homogenates of the heads and thoraces of a sample of 345 Anopheles females were tested by enzyme-linked immunosorbent assay (ELISA) for P. falciparum andP. vivax strainVK210 and VK247 circumsporozoite protein. According to ELISA, none of those specimens was infected by a human Plasmodium. All the specimens were tested by RT-PCR for P. falciparum and P. vivax. DNA was extracted directly for 243 specimens not tested using ELISA and using the homogenates prepared for ELISA for the 345 others. By RT-PCR, infected anophelines were only found among samples from Dorlin. Three An. darlingi were infected by P. falciparum (infection rate of 1.1 %) and four An. marajoara were infected by P. vivax (infection rate of 6.4 %) (Table 4).
Table 4.
Anopheles species collected in Dorlin and Circumsporozoite Protein Index for P. vivax and P. falciparum
| Anopheline species | MM trapsa | CDC trapsb | P. falciparum | P. vivax | ||
|---|---|---|---|---|---|---|
| n | CSPc (%) | n | CSPc (%) | |||
| An. darlingi | 262 | 3 | 1.1 | 0 | – | |
| An. marajoara | 44 | 18 | 0 | – | 4 | 6.4 |
| An. nuneztovari s.l. | 20 | 0 | – | 0 | – | |
| An. triannulatus s.l. | 2 | 0 | – | 0 | – | |
| An. spp. | 42 | 0 | – | 0 | – | |
| Total | 370 | 18 | 3 | 1.1 | 4 | 6.4 |
aMosquito Magnet® traps baited with octenol
bCenters for Disease Control and Prevention light traps
cCircumsporozoite Protein index
Larvae collection
In Repentir, larval prospecting was done in ponds, pools, puddles and all-terrain vehicle (ATV) ruts. Some bromeliads were recovered from fallen trees. Anopheles larvae were found only in two ATV ruts filled by rainfall. They were reared in the field, but only one adult emerged, morphologically identified as An. nuneztovari s.l., with identification confirmed by ITS2 PCR-RFLP. No Anopheles larvae were found in the different bromeliads. In Dorlin, larval prospecting was done in ponds, pools, puddles and ATV ruts around the illegal settlement. No larvae were found.
Discussion
This study reports on one of the largest malaria outbreaks among French forces deployed to French Guiana [44, 45]. For the first time, an entomological study was conducted in the field, particularly at illegal gold mining sites, to evaluate infection rates and characterize Anopheles species implicated in malaria transmission.
Epidemiological investigation
High P. vivax malaria attack rates, ranging from 24.6 to 27.8 %, were observed in the three military units conducting the same mission: to control illegal gold mining in French Guiana. The epidemic curve presents two peaks.
The first peak corresponds to indigenous malaria cases and could be partly attributed to a lack of adherence to doxycycline chemoprophylaxis. Only 40 % of the patients stated proper chemoprophylaxis compliance in the 8 days before onset of symptoms. Comparable results had already been observed in previous malaria outbreaks among French forces [46, 47]. In a large prospective study conducted on 19 French military units, assessment of levels of compliance with chemoprophylaxis was based on the self-reported daily medication doses missed during the mission, and the average prevalence of appropriate compliance was estimated at 46.2 % [46]. Direct observation therapy is not used in the French forces [28]. During missions in the forest, taking doxycycline is more closely supervised by commanders in direct contact with soldiers. Most of the indigenous cases occurred at the end of the 4-month mission, when soldiers returned from the forest to the coast, which is free of malaria and where taking daily doses of doxycycline becomes an individual behaviour. The perception of lower malaria risk and the lack of group support at the end of the mission could explain poor levels of compliance with chemoprophylaxis [46]. Doxycycline has been shown to be highly effective as a blood schizonticidal agent, killing erythrocytic stages of the malaria parasite [48, 49]. But due to doxycycline’s short half-life and the mechanism of action, its efficiency depends on proper compliance.
The second peak occurred after leaving French Guiana, with 82 % of P. vivax malaria imported cases occurring after terminal doxycycline chemoprophylaxis. These patients did not experience P. vivax malaria or fever during the 4-month mission, and their first clinical episodes were probably hypnozoite-induced relapses. Doxycycline does not affect the liver stage of the parasite, which also means that it does not kill P. vivax hypnozoites and, therefore, does not prevent P. vivax malaria relapses [48, 50, 51]. Considering P. falciparum resistance to malaria drugs, and in order to optimize compliance with a homogeneous prescription, 100 mg of doxycycline once a day is currently the first line chemoprophylaxis in the French armed forces [28]. Malaria chemoprophylaxis strategies in the French armed forces are mainly aimed at preventing P. falciparum malaria during deployments to Africa. The involvement of French forces in endemic P. vivax malaria transmission areas is more recent and has led to an increase in P. vivax incidence [29]. The Australian Defence Force encountered the same problem with P. vivax relapses when they became involved in the United Nations mission in East Timor from 1999 to 2000 [52, 53]. Considering the lack of prophylaxis activity of doxycycline against P. vivax, a daily dose of 100 mg of doxycycline during operations and 22.5 mg of primaquine daily for 2 weeks on returning to Australia were administered [54]. Attack rates observed in their forward battalions ranged from 5 to 13 %, well below those observed in the present study (>25 %). Similarly, in 2006, CDC experts recommended presumptive anti-relapse therapy (terminal prophylaxis) with primaquine for 2 weeks in persons heavily exposed to P. vivax [55]. However, the French National Agency for Medicines and Health (ANSM) has limited the use of primaquine to patients with P. vivax attacks in order to prevent relapses. Therefore, liver-stage prophylaxis with primaquine for delayed-onset malaria cannot currently be used by French forces.
Scrupulously observing individual protection measures against mosquito bites is essential in such contexts. Their combined proper use will theoretically reduce exposure to biting mosquitoes, but not enough to significantly reduce the incidence of malaria among non-immune troops [56, 57]. Military personnel deployed in the interior of French Guiana live in wooden houses with corrugated aluminium roofs, without ceilings or windows. During missions in the forest, they stay directly at illegal gold mining sites, sleeping in hammocks under a waterproof tarp. Poor compliance with personal protection measures against mosquitoes was observed during missions. It suggests a failure by commanders in direct contact with soldiers to apply French armed forces health recommendations. However, hammock protection failure (mosquito bites through the fabric of the hammock during the night) most certainly played an important role in this epidemic.
Entomological investigation
The main entomological investigations were conducted at Repentir and Dorlin. Both sites were identified as presumed contamination locations by the epidemiological investigation. These sites have been subjected to human pressure for many years. After an initial period of legal activity, the mining site of Repentir was rehabilitated before being closed. But the area was then invaded by undocumented gold miners. In December 2010, military intelligence estimated that more than one thousand undocumented people were living and working in this area. The river was diverted for gold mining purposes and ponds and puddles were created. The forest was cleared for gold mining and many excavations were made that were then flooded by the river or filled by rainfall. In some places, the primary forest was destroyed and replaced by savannas, secondary forest or glades. Wells were also dug. The rest of the surrounding primary forest was criss-crossed by footpaths and ATV tracks. In late 2010, French military forces took up positions in this area and undocumented workers left to the nearest illegal gold mining site: Dorlin. Dorlin is an old illegal gold mining site that has been exploited for 20 years. No rehabilitation of the area has ever been carried out. Military intelligence estimated that more than one thousand undocumented people were present here and working at this illegal gold mining site. Many illegal settlements were still present on the site during entomological investigations. Rivers have been diverted; the primary forest has been extensively cleared, replaced by savanna or secondary forest over large areas. Mining excavations have been transformed into lakes and ponds. Backhoes and bulldozers have been used to dig hills in the search for auriferous veins and footpaths and ATV tracks criss-cross the area.
Current entomological investigations confirm that these anthropic modifications are highly favourable to the development of Anopheles mosquitoes. Adult Anopheles from four species that could play a role in malaria transmission were collected with traps, in high densities in some places and periods. Larval prospecting was not significant due to the large size of the areas, the high number of potential breeding sites and the limitations of entomological activities due to security measures.
Morphological and molecular analysis made it possible to identify 89 % (528/595) of the Anopheles mosquitoes caught. Species identified were among the most frequently observed Anopheles species in French Guiana and the Amazon Basin [31]. However, this study confirms for the first time the role of An. marajoara as a P. vivax vector in the French Guiana rainforest. Infected anophelines were found only at the illegal gold mining site of Dorlin. The high infection rates observed confirm that illegal gold mining sites in French Guiana should be considered as high level malaria transmission areas. Thanks to the setting up of a forward operational post and legal mining operations, Repentir site was free of undocumented gold miners for 6 months before the entomological investigation. This could explain the absence of infected anopheles in this area. However, during the 1-week mission spent in Repentir, one P. falciparum malaria case occurred in a legal gold mining operation close to the FOP, suggesting a continuation of low malaria transmission. The use of traps did not enable the calculation of entomological inoculation rates (EIRs) or make it possible to clearly measure the number of infected bites per soldier in Repentir or Dorlin. Nevertheless, the number of Anopheles mosquitoes caught with traps at those sites was very high compared to previous studies in French Guiana. This suggests that anopheles densities were very high at these sites. Furthermore, CDC light traps that seemed to be more attractive to An. marajoara were only used for 3 days, and the abundance of this species was probably underestimated. Considering this, the malaria transmission risk could be considered as very high both at Dorlin and Repentir. These findings are consistent with other studies conducted at illegal gold mining sites including a study in Venezuela where the An. marajoara and An. darlingi association has already been described [58]. The combination of poor adherence of soldiers to protective measures and a stay in an area with a high level of malaria transmission explained the outbreak.
Illegal gold mining has modified the epidemiology of malaria in French Guiana, which was previously limited to the villages located on the main rivers flowing into the territory, in particular the Maroni and Oyapock rivers. Uncontrolled and mobile populations of undocumented gold miners could reintroduce malaria in the malaria-free coastal area of French Guiana, where competent vectors are abundant. The risk of an introduction of P. vivax malaria is probably higher, as P. vivax gametocytes are produced within the first cycles of infection [59]. This situation is challenging for malaria control and elimination in French Guiana, but also in all neighbouring countries of the Guiana Shield. There is a need to join and coordinate the efforts of all countries in order to successfully control malaria in the region.
Conclusions
Despite a decrease in the incidence of malaria in French Guiana, uncontrolled foci of malaria transmission are present in the deep forest. Gold mining is associated with malaria transmission in all the countries of the Guiana Shield. Collaboration with neighbouring countries is necessary to take into account mobile populations such as gold miners. Military personnel are especially exposed during missions at illegal gold mining sites. Malaria control strategies in the French armed forces must be adapted to P. vivax malaria and sylvatic Anopheles mosquitoes. To that end, the French military health service and Institut Pasteur de la Guyane initiated a large research programme in 2012 to evaluate human, mosquito and parasite aspects of P. vivax malaria transmission at illegal gold mining sites in French Guiana.
Authors’ contributions
VPS and FP designed the study and wrote the manuscript; VPS conducted the epidemiological study, analysed data and contributed to field collection; FP performed field collection, analysed and interpreted entomological data and carried out morphological and PCR identification; RG and ID supported morphological identification and ELISA tests and helped draft the manuscript; MM, AM, FS, CC and FD managed the patients and contributed to epidemiological data collection; XD and AD contributed to study design and epidemiological data collection; SB contributed to epidemiological data analysis and helped draft the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
The authors thank Fanny Jarjaval for participating in the entomological study, Pierre Olivier Miloche, Cyril Carfantan, Christophe Rapp and Catherine Verret for data collection. Financial support was made available by the French Military Health Service, for which we are grateful.
Competing interests
The authors declare that they have no competing interest.
Abbreviations
- RCP
river checkpoints (RCP)
- FOB
forward operational bases on main rivers
- FOP
forward operational post in deep forest
- MM traps
Mosquito Magnet® traps
- CDC traps
Centers for Disease Control and Prevention light traps
- PCR
polymerase chain reaction
- ITS2
mosquito internal transcribed spacer 2
- CSP
circumsporozoite protein
Contributor Information
Vincent Pommier de Santi, Email: v.pommierdesanti@gmail.com.
Romain Girod, Email: rgirod@pasteur-cayenne.fr.
Marie Mura, Email: mariemura@yahoo.fr.
Aissata Dia, Email: aiss.dia@gmail.com.
Sébastien Briolant, Email: sbriolant@wanadoo.fr.
Félix Djossou, Email: felix.djossou@wanadoo.fr.
Isabelle Dusfour, Email: idusfour@pasteur-cayenne.fr.
Alexandre Mendibil, Email: alexandre.mendibil@gmail.com.
Fabrice Simon, Email: simon-f@wanadoo.fr.
Xavier Deparis, Email: xavier.deparis@wanadoo.fr.
Frédéric Pagès, Email: frederic.pages@ars.sante.fr.
References
- 1.Institut national de la statistique et des études economiques (INSEE). http://www.insee.fr/fr/regions/guyane/default.asp?page=faitsetchiffres/presentation/presentation.htm. [PubMed]
- 2.Chocho A, Bellony S, Azor P, Chantilly S. Lieux présumés de contamination palustre répertoriés sur le littoral de la Guyane - 2009. Bull Veille Sanitaire Antilles-Guyane. 2011;1:6–10. [Google Scholar]
- 3.Ardillon V, Carvalho L, Prince C, Abboud P, Djossou F. Bilans 2013 et 2014 de la situation du paludisme en Guyane. Bull Veille Sanitaire Antilles-Guyane. 2015;1:16–20. [Google Scholar]
- 4.Berger F, Flamand C, Musset L, Djossou F, Rosine J, Sanquer MA, et al. Investigation of a sudden malaria outbreak in the isolated Amazonian village of Saul, French Guiana, January–April 2009. Am J Trop Med Hyg. 2012;86:591–597. doi: 10.4269/ajtmh.2012.11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carme B. Substantial increase of malaria in inland areas of eastern French Guiana. Trop Med Int Health. 2005;10:154–159. doi: 10.1111/j.1365-3156.2004.01365.x. [DOI] [PubMed] [Google Scholar]
- 6.Carme B, Ardillon V, Girod R, Grenier C, Joubert M, Djossou F, et al. Update on the epidemiology of malaria in French Guiana. Med Trop. 2009;69:19–25. [PubMed] [Google Scholar]
- 7.Musset L, Pelleau S, Girod R, Ardillon V, Carvalho L, Dusfour I, et al. Malaria on the Guiana Shield: a review of the situation in French Guiana. Mem Inst Oswaldo Cruz. 2014;109:522–533. doi: 10.1590/0074-0276140031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiwat H, Hardjopawiro LS, Takken W, Villegas L. Novel strategies lead to pre-elimination of malaria in previously high-risk areas in Suriname, South America. Malar J. 2012;11:10. doi: 10.1186/1475-2875-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Situação Epidemiológica Fronteiras Brasil Guiana Francesa. Portal da Saúde Ministério da Saúde. https://public.tableau.com/profile/mal.ria.brasil#!/vizhome/Fronteiras_2015_05_28/casos_notificados_2013_2014.
- 10.Sawyer DR. Malaria on the Amazon frontier: economic and social aspects of transmission and control. Southeast Asian J Trop Med Public Health. 1986;17:342–345. [PubMed] [Google Scholar]
- 11.Valle D, Lima JM. Large-scale drivers of malaria and priority areas for prevention and control in the Brazilian Amazon region using a novel multi-pathogen geospatial model. Malar J. 2014;13:443. doi: 10.1186/1475-2875-13-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer BR. Gold mining and malaria in the Brazilian Amazon. Masters thesis. New Haven:Yale University; 1996.
- 13.Duarte EC, Fontes CJ. Association between reported annual gold mining extraction and incidence of malaria in Mato Grosso-Brazil, 1985–1996. Rev Soc Bras Med Trop. 2002;35:665–668. doi: 10.1590/S0037-86822002000600020. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri AF, Sawyer DO. Heterogeneity of malaria prevalence in alluvial gold mining areas in Northern Mato Grosso State, Brazil. Cad Saude Publica. 2007;23:2878–2886. doi: 10.1590/S0102-311X2007001200009. [DOI] [PubMed] [Google Scholar]
- 15.Hiwat H, Issaly J, Gaborit P, Somai A, Samjhawan A, Sardjoe P, et al. Behavioral heterogeneity of Anopheles darlingi (Diptera: Culicidae) and malaria transmission dynamics along the Maroni River, Suriname, French Guiana. Trans R Soc Trop Med Hyg. 2010;104:207–213. doi: 10.1016/j.trstmh.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Girod R, Roux E, Berger F, Stefani A, Gaborit P, Carinci R, et al. Unravelling the relationships between Anopheles darlingi (Diptera: Culicidae) densities, environmental factors and malaria incidence: understanding the variable patterns of malarial transmission in French Guiana (South America) Ann Trop Med Parasitol. 2011;105:107–122. doi: 10.1179/136485911X12899838683322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girod R, Gaborit P, Carinci R, Issaly J, Fouque F. Anopheles darlingi bionomics and transmission of Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae in Amerindian villages of the Upper-Maroni Amazonian forest, French Guiana. Mem Inst Oswaldo Cruz. 2008;103:702–710. doi: 10.1590/S0074-02762008000700013. [DOI] [PubMed] [Google Scholar]
- 18.Fouque F, Gaborit P, Carinci R, Issaly J, Girod R. Annual variations in the number of malaria cases related to two different patterns of Anopheles darlingi transmission potential in the Maroni area of French Guiana. Malar J. 2010;9:80. doi: 10.1186/1475-2875-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- 20.Pajot FX, Le Pont F, Molez JF, Degallier N. Agressivité d’Anopheles (Nyssorhynchus) darlingi Root, 1926 (Diptera, Culicidae) en Guyane française. Cah ORSTOM SerEnt Med Parasitol. 1977;15:15–22. [Google Scholar]
- 21.Lourenco-de-Oliveira R, Guimaraes AE, Arle M, da Silva TF, Castro MG, Motta MA, et al. Anopheline species, some of their habits and relation to malaria in endemic areas of Rondonia State, Amazon region of Brazil. Mem Inst Oswaldo Cruz. 1989;84:501–514. doi: 10.1590/S0074-02761989000400008. [DOI] [PubMed] [Google Scholar]
- 22.de Barros FS, Honorio NA. Man biting rate seasonal variation of malaria vectors in Roraima, Brazil. Mem Inst Oswaldo Cruz. 2007;102:299–302. doi: 10.1590/S0074-02762007005000024. [DOI] [PubMed] [Google Scholar]
- 23.Molez JF. Myths concerning malarial transmission among Amazonian Indians and their relation with 2 types of transmission encountered in the Amazonian forest. Sante. 1999;9:157–162. [PubMed] [Google Scholar]
- 24.de Oliveira-Ferreira J, Lourenco-de-Oliveira R, Teva A, Deane LM, Daniel-Ribeiro CT. Natural malaria infections in anophelines in Rondonia State, Brazilian Amazon. Am J Trop Med Hyg. 1990;43:6–10. [PubMed] [Google Scholar]
- 25.Dusfour I, Issaly J, Carinci R, Gaborit P, Girod R. Incrimination of Anopheles (Anopheles) intermedius Peryassu, An. (Nyssorhynchus) nuneztovari Gabaldon, An. (Nys.) oswaldoi Peryassu as natural vectors of Plasmodium falciparum in French Guiana. Mem Inst Oswaldo Cruz. 2012;107:429–432. doi: 10.1590/S0074-02762012000300021. [DOI] [PubMed] [Google Scholar]
- 26.Branquinho MS, Lagos CB, Rocha RM, Natal D, Barata JM, Cochrane AH, et al. Anophelines in the state of Acre, Brazil, infected with Plasmodium falciparum, P. vivax, the variant P. vivax VK247 and P. malariae. Trans R Soc Trop Med Hyg. 1993;87:391–394. doi: 10.1016/0035-9203(93)90008-E. [DOI] [PubMed] [Google Scholar]
- 27.Pajot FX, Molez JF, Le Pont F. Anophèles et paludisme sur le haut Oyapock (Guyane française) Cah ORSTOM SerEnt Med Parasitol. 1978;16:105–111. [Google Scholar]
- 28.Migliani R, Pradines B, Michel R, Aoun O, Dia A, Deparis X, et al. Malaria control strategies in French armed forces. Travel Med Infect Dis. 2014;12:307–317. doi: 10.1016/j.tmaid.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Queyriaux B, Texier G, Ollivier L, Galoisy-Guibal L, Michel R, Meynard JB, et al. Plasmodium vivax malaria among military personnel, French Guiana, 1998–2008. Emerg Infect Dis. 2011;17:1280–1282. doi: 10.3201/eid1707.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Service MW. Mosquito ecology. New York: Wiley; 1976. [Google Scholar]
- 31.Shannon RC. Anophelines of the Amazon valley. Proc Entomol Soc Wash. 1933;35:117–143. [Google Scholar]
- 32.Floch H, Abonnenc E. Anophèles de la Guyane Française. Arch Inst Pasteur Guyane. 1951;236:1–92. [Google Scholar]
- 33.Fauran P, Pajot FX. Complement to the catalog of the Culidae Recorded from French Guiana (South America) Mosq News. 1974;6:99–110. [Google Scholar]
- 34.Fauran P. Catalogue annoté des Culicidés signalés en Guyane Française. Arch Inst Pasteur Guyane. 1961;22:1–15. [Google Scholar]
- 35.Dusfour I, Carinci R, Gaborit P, Issaly J, Girod R. Evaluation of four methods for collecting malaria vectors in French Guiana. J Econ Entomol. 2010;103:973–976. doi: 10.1603/EC09328. [DOI] [PubMed] [Google Scholar]
- 36.Cienfuegos AV, Rosero DA, Naranjo N, Luckhart S, Conn JE, Correa MM. Evaluation of a PCR-RFLP-ITS2 assay for discrimination of Anopheles species in northern and western Colombia. Acta Trop. 2011;118:128–135. doi: 10.1016/j.actatropica.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zapata MA, Cienfuegos AV, Quiros OI, Quinones ML, Luckhart S, Correa MM. Discrimination of seven Anopheles species from San Pedro de Uraba, Antioquia, Colombia, by polymerase chain reaction-restriction fragment length polymorphism analysis of its sequences. Am J Trop Med Hyg. 2007;77:67–72. [PubMed] [Google Scholar]
- 38.Ruiz-Lopez F, Wilkerson RC, Conn JE, McKeon SN, Levin DM, Quinones ML, et al. DNA barcoding reveals both known and novel taxa in the Albitarsis Group (Anopheles: Nyssorhynchus) of Neotropical malaria vectors. Parasit Vectors. 2012;5:44. doi: 10.1186/1756-3305-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkot TR, Zavala F, Gwadz RW, Collins FH, Nussenzweig RS, Roberts DR. Identification of malaria-infected mosquitoes by a two-site enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984;33:227–231. doi: 10.4269/ajtmh.1984.33.227. [DOI] [PubMed] [Google Scholar]
- 40.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vo TK, Bigot P, Gazin P, Sinou V, De Pina JJ, Huynh DC, et al. Evaluation of a real-time PCR assay for malaria diagnosis in patients from Vietnam and in returned travellers. Trans R Soc Trop Med Hyg. 2007;101:422–428. doi: 10.1016/j.trstmh.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 42.d-maps.com. http://d-maps.com/pays.php?num_pay=243&lang=fr.
- 43.Dusfour I, Jarjaval F, Gaborit P, Mura M, Girod R, Pages F. Confirmation of the occurrence of Anopheles (Nyssorhynchus) marajoara in French Guiana. J Am Mosq Control Assoc. 2012;28:309–311. doi: 10.2987/12-6248R.1. [DOI] [PubMed] [Google Scholar]
- 44.Verret C, Cabianca B, Haus-Cheymol R, Lafille JJ, Loran-Haranqui G, Spiegel A. Malaria outbreak in troops returning from French Guiana. Emerg Infect Dis. 2006;12:1794–1795. doi: 10.3201/eid1211.060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michel R, Ollivier L, Meynard JB, Guette C, Migliani R, Boutin JP. Outbreak of malaria among policemen in French Guiana. Mil Med. 2007;172:977–981. doi: 10.7205/MILMED.172.9.977. [DOI] [PubMed] [Google Scholar]
- 46.Resseguier N, Machault V, Ollivier L, Orlandi-Pradines E, Texier G, Pradines B, et al. Determinants of compliance with malaria chemoprophylaxis among French soldiers during missions in inter-tropical Africa. Malar J. 2010;9:41. doi: 10.1186/1475-2875-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ollivier L, Michel R, Carlotti MP, Mahe P, Romand O, Todesco A, et al. Chemoprophylaxis compliance in a French battalion after returning from malaria-endemic area. J Travel Med. 2008;15:355–357. doi: 10.1111/j.1708-8305.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 48.Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517–531. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briolant S, Almeras L, Fusai T, Rogier C, Pradines B. Cyclines and malaria. Med Trop (Mars) 2007;67:86–96. [PubMed] [Google Scholar]
- 50.Schwartz E, Parise M, Kozarsky P, Cetron M. Delayed onset of malaria—implications for chemoprophylaxis in travelers. N Engl J Med. 2003;349:1510–1516. doi: 10.1056/NEJMoa021592. [DOI] [PubMed] [Google Scholar]
- 51.Clyde DF, Miller RM, DuPont HL, Hornick RB. Antimalarial effects of tetracyclines in man. J TropMed Hyg. 1971;74:238–242. [PubMed] [Google Scholar]
- 52.Peragallo MS, Croft AM, Kitchener SJ. Malaria during a multinational military deployment: the comparative experience of the Italian, British and Australian Armed Forces in East Timor. Trans R Soc Trop Med Hyg. 2002;96:481–482. doi: 10.1016/S0035-9203(02)90409-8. [DOI] [PubMed] [Google Scholar]
- 53.Kitchener S, Nasveld P, Russell B, Elmes N. An outbreak of malaria in a forward battalion on active service in East Timor. Mil Med. 2003;168:457–459. [PubMed] [Google Scholar]
- 54.Kitchener S. Epidemiology of malaria from East Timor among Australian Defence Force personnel. Trans R Soc Trop Med Hyg. 2002;96:376–377. doi: 10.1016/S0035-9203(02)90365-2. [DOI] [PubMed] [Google Scholar]
- 55.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- 56.Pages F. Vector control for armed forces: a historical requirement requiring continual adaptation. Med Trop. 2009;69:165–172. [PubMed] [Google Scholar]
- 57.Deparis X, Frere B, Lamizana M, N’Guessan R, Leroux F, Lefevre P, et al. Efficacy of permethrin-treated uniforms in combination with DEET topical repellent for protection of French military troops in Cote d’Ivoire. J Med Entomol. 2004;41:914–921. doi: 10.1603/0022-2585-41.5.914. [DOI] [PubMed] [Google Scholar]
- 58.Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 59.Claustre J, Venturin C, Nadire M, Fauran P. Malarial vectors in French Guiana: study in an epidemic focus near Cayenne (1989–1998) Bull Soc Pathol Exot. 2001;94:353–357. [PubMed] [Google Scholar]


