Abstract
OBJECTIVES
Pulmonary hypertension (PH) is a process of lung vascular remodeling which can lead to right heart dysfunction and significant morbidity. The underlying mechanisms leading to PH are not well understoon and therapies are limited. Using Intermittent hypoxia (IH) as a model of oxidant-induced PH, we identified an important role for endothelial cell mitophagy via mitochondrial uncoupling protein 2 (Ucp2) in the development of IH-induced PH.
APPROACH AND RESULTS
Ucp2 endothelial knockout (VE-KO) and Ucp2 Flox (Flox) mice were subjected to 5 weeks of IH. Ucp2 VE-KO mice exhibited higher RVSP and worse right heart hypertrophy, as measured by increased RV/LV+S ratio, at baseline and after IH. These changes were accompanied by increased mitophagy. Primary mouse lung endothelial cells (MLEC) transfected with Ucp2 siRNA and subjected to cyclical exposures to CoCl2 (chemical hypoxia) showed increased mitophagy, as measured by Pink1 and LC3BII/I ratios, decreased mitochondrial biogenesis and increased apoptosis. Similar results were obtained in primary lung endothelial cells isolated from VE-KO mice. Moreover, silencing Pink1 in the endothelium of Ucp2 KO mice, using endothelial-targeted lentiviral silencing RNA in vivo, prevented IH-induced PH. Human pulmonary artery endothelial cells from people with PH demonstrated changes similar to Ucp2-silenced MLEC.
CONCLUSION
The loss of endothelial Ucp2 leads to excessive Pink1-induced mitophagy, inadequate mitochondrial biosynthesis and increased apoptosis in endothelium. An endothelial Ucp2-Pink1 axis may be effective therapeutic targets in PH.
Keywords: Ucp2, endothelium, mitophagy, intermittent hypoxia, pulmonary hypertension
Introduction
Pulmonary hypertension (PH) is a progressive and incurable disorder associated with the remodeling of lung vessels, with subsequent right heart failure and early death. Therapies as well as our molecular understanding of PH remain limited. Dysregulated angiogenesis (1), growth factor induction and pulmonary smooth muscle cell (PASMC) apoptosis resistance (2) have been invoked as important pathogenetic mechanisms. Recently, the role of the mitochondria in altering PASMC and pulmonary artery endothelial cells to an apoptosis-resistant phenotype has gained attention (3-5). There are a number of different rodent models for PH, both genetic and pharmacological (6, 7), including intermittent hypoxia (IH) (8). We selected IH as a model of oxidant-induced vascular injury, which is thought to be a common mechanism underlying PH-associated disorders, such as parenchymal lung disease, obstructive sleep apnea and heart failure, as well as idiopathic pulmonary arterial hypertension (PAH) (9-11).
Ucp2 belongs to a family of anion transporters that are localized on inner mitochondrial membrane and dissipate proton gradient originated from mitochondrial electron transport chain (12). Thus far 5 Ucp family members have been discovered (13). Ucp1, known as thermogenin, is expressed in brown adipose tissue and dissipates the proton gradient into heat (14). Ucp2 is widely expressed in central nervous system, pancreas, liver and lungs and known to regulate fatty acid metabolism (15), mitochondrial calcium uptake (16) and mitochondrial reactive oxygen species production (16-18). Ucp2 has been studied primarily in central nervous system and pancreas, where its role was established in neurotransmission, synaptic plasticity and neurodegenerative processes (19) as well as in insulin resistance (20). Recently, Ucp2 knockout mice were found to have worse hypoxia-induced PH and the authors attributed the mechanism to cellular dysfunction in PASMC, such as endoplasmic reticulum stress, mitochondrial calcium influx imbalance and mitochondrial hyperpolarization (21, 22). However, little is known about Ucp2 in endothelial cells (EC), which also play a key role in PH pathogenesis. EC apoptosis is thought represent the initial pathological presentation of PH but the early molecular events associated with EC apoptosis in PH are not well defined (23).
Our studies demonstrate a critical role for Ucp2 in preventing excessive EC apoptosis and mitophagy that lead to IH-induced PH and right heart dysfunction. Mitophagy represents selective autophagy of mitochondria – an important mechanism of cellular quality control that eliminates damaged mitochondria, but in excess can contribute to cell death (24). This process is initiated by a change in mitochondrial membrane potential with accumulation of Pink1 on the outer membrane, leading to recruitment of cytoplasmic Parkin and facilitating ubiquitination of damaged mitochondria (25). Changes in mitochondrial potential are known to activate mitophagy/autophagy (26, 27). Given that Ucp2 is a regulator of mitochondrial potential (28, 29) we tested whether the absence of Ucp2 alters mitophagy, leading to the exaggerated PH phenotype of endothelial specific Ucp2 KO mice using an IH model of PH in mice and in EC. We confirmed the clinical relevance of our experimental findings by also investigating lung EC from people with PAH.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Endothelial specific Ucp2 KO mice have increased RVSP and RVH after IH
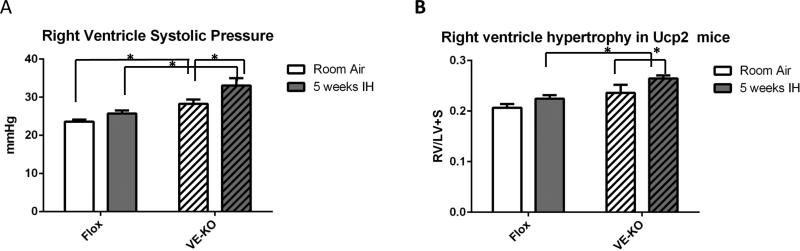
Global Ucp2 KO mice have already reported to be more susceptible to chronic hypoxia-induced PH (21, 22). To specifically determine the role of endothelial Ucp2 in regulating right ventricular systolic pressures (RVSP), a measurement of pulmonary artery pressures, and right ventricular remodeling in response to IH, we generated mice with endothelial specific knockout of the Ucp2 gene by crossing Ucp2 Flox mice (Flox) (30) and mice containing VE-Cadherin driving the transcription of Cre recombinase (VE-KO). Supplemental fig. I shows the absence of Ucp2 expression in EC isolated from total lungs of VE-KO. Animals were subjected to 5 weeks of IH and RVSP was measured. Fig. 1A demonstrates that VE-KO mice have significantly higher RVSP even at baseline compared to Flox mice. Moreover, RVSPs markedly increased after IH in VE-KO mice compared to RVSPs in Flox mice. We also determined that VE-KO mice have significantly increased right ventricular remodeling, as measured by the ratio of right ventricle weight to left ventricle plus septal weight (RV/LV+S), compared to Flox mice (Fig.1B). These data suggest that endothelial Ucp2 is an important determinant of PH at baseline and after IH.
Figure 1.
PH evaluation in Ucp2 Flox and VE-KO mice at room air and after 5 weeks of intermittent hypoxia (IH). A. Right ventricle systolic pressure was measured by direct catheter insertion. B. Right ventricle hypertrophy, as measured by right ventricle weight divided by left ventricle + septal weight (RV/LV+S). Mouse hearts were dissected into right ventricle and left ventricle with septum. Each part was weighed and a RV/LV+S ratio was calculated for each animal. *p<0.05. N=8 -11 in each group.
Ucp2 VE-KO mice demonstrate increased endothelial apoptosis in lungs at baseline and after 1 week IH
We co-stained lung sections from experimental mice for TUNEL staining, a marker for apoptosis, and von Willebrand factor (vWF), an endothelial cell marker. After 5 weeks of IH, we did not detect apoptosis in EC of Flox or VE-KO mice (Supplemental fig. IIA, B, D). However, VE-KO mice showed increased EC apoptosis at baseline (Supplemental fig. IIC), which may account for their baseline changes in RVSP. Given that early endothelial apoptosis has been described as a hallmark of PH (31, 32) and that later stages of PH may be associated with apoptosis resistance (33) we exposed mice to 1 week of IH and performed TUNEL and vWF staining, which showed increased IH-induced endothelial apoptosis in VE-KO mice compared to Flox mice (Supplemental fig.III). These data suggested that endothelial apoptosis may precede the development of overt PH by weeks.
Ucp2 VE-KO mice have increased mitophagy and autophagy markers in lungs
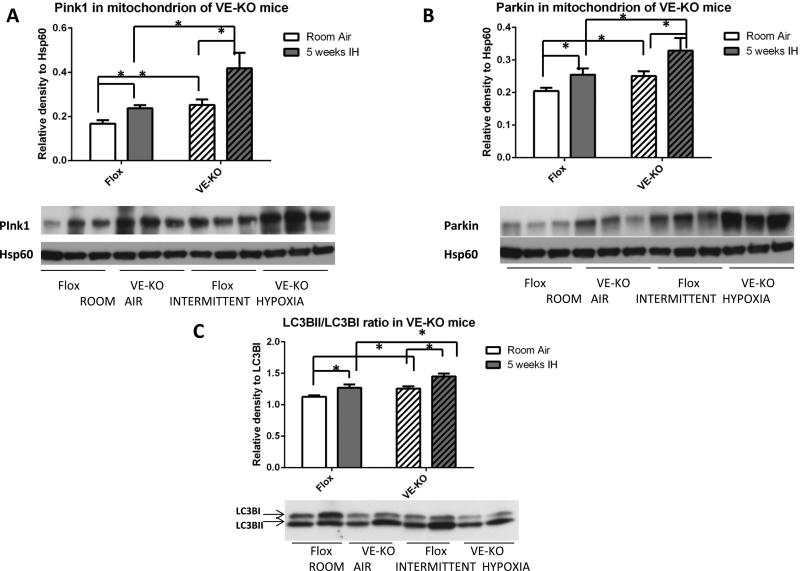
Given that IH is known to generate endothelial oxidant injury and we recently reported that lung endothelial cells undergo autophagy and mitophagy in response to oxidant injury (34, 35), we hypothesized that the absence of endothelial Ucp2 will alter autophagy and mitophagy. Immunoblotting of the mitochondrial fraction of total lung protein lysates prepared by differential centrifugation (see Methods) revealed significantly increased Pink1 and Parkin levels in VE-KO mice at baseline (Room Air) and after IH (Fig. 2A, B). IH significantly increased the mitochondrial content of Pink1 and Parkin in both VE-KO mice and Flox animals. At baseline, VE-KO had increased autophagy, as measured by the LC3BII/LC3BI ratio, compared to Flox animals (Room Air). IH increased autophagy in both Flox and VE-KO animals (Fig. 2C). Similar to the VE-KO mice, global Ucp2KO mice demonstrated more baseline Pink1 and Parkin in the mitochondrial fraction, and an increased ratio of LC3BII/LC3BI in total lung lysates compared to control mice (Supplemental fig.IVA-C). These results suggest that the loss of EC Ucp2 phenocopies the global loss of Ucp2 and is sufficient to cause baseline increases in lung mitophagy, autophagy and PH.
Figure 2.
Immunoblots of lung lysates for proteins associated with mitophagy/autophagy from Ucp2 Flox and VE-KO mice. A. Pink1 protein in the mitochondrial fraction of total lung lysates. B. Parkin protein in the mitochondrial fraction of total lung lysates. C. LC3BII/LC3BI ratio in total lung lysates. *p<0.05.
Mouse lung endothelial cells (MLEC) lacking Ucp2 demonstrate increased mitophagy and autophagy flux
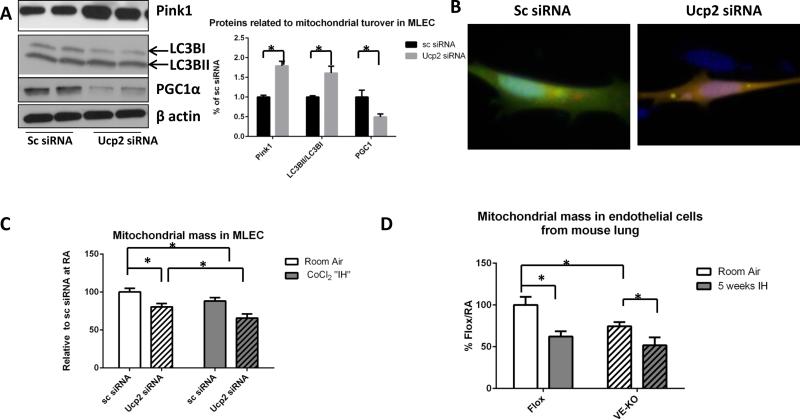
In order to more specifically investigate mitophagy and autophagy in lung endothelial cells, we isolated primary mouse lung endothelial cells (EC) from the lungs Flox and VE-KO mice. For all assays we used EC between passages 5 and 8, during which time cellular senescence does not seem to playing a significant role (Supplemental fig.V). Our protein data showed that cultured EC (which we refer to as cultured MLEC) have increased baseline levels of mitophagy and autophagy-associated proteins (Pink1 and LC3BII/I ratios, respectively), while a major mitochondria biogenesis regulator, peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α), was significantly lower after Ucp2 silencing (Fig. 3A). Moreover, mitochondrial mass in cultured MLEC, as assessed by MitoTracker Green FM staining and detected by flow cytometry, was significantly lower in cells transfected with Ucp2 siRNA (Fig. 3B). Similarly, EC isolated directly from the lungs of VE-KO mice also had less mitochondrial mass compared to EC from Flox animals (Fig. 3C). This suggests that the accelerated removal of damaged mitochondria in MLEC is not compensated by mitochondria biosynthesis. We also transfected cells with ptfLC3 plasmid that contained LC3 tagged with green and red fluorescent proteins (GFP and RFP, respectively). When autophagy flux starts, LC3 localizes to the lysosomes, and only RFP can be detected, since GFP is sensitive to degradation by the acidic environment of the lysosome. Fig. 3D shows that MLEC transfected with Ucp2 siRNA have baseline increased autophagy flux (which we refer to as “hyperactive autophagy”) compared to MLEC transfected with scrambled siRNA.
Figure 3.
Mouse lung endothelial cells (MLEC) after Ucp2 silencing and kept at room air or challenged with CoCl2 “IH”. Scrambled siRNA (Sc) was used as negative control. A. Mitophagy associated protein Pink1, autophagy associated proteins LC3BI and LC3BII and mitochondrial biogenesis-associated protein PGC1α in room air after Sc or Ucp2 siRNA transfection. B. Mitochondrial mass in cultured MLEC as assessed by FACS in room air or after CoCl2 “IH”. C. Mitochondrial mass as assessed by FACS in endothelial cells (EC) isolated from Flox or VE-KO lungs in room air and after 5 weeks of IH. *p<0.05. D. Autophagy levels as assessed by immunofluorescent ptfLC3 plasmid. Original magnification of all photomicrographs: ×40.
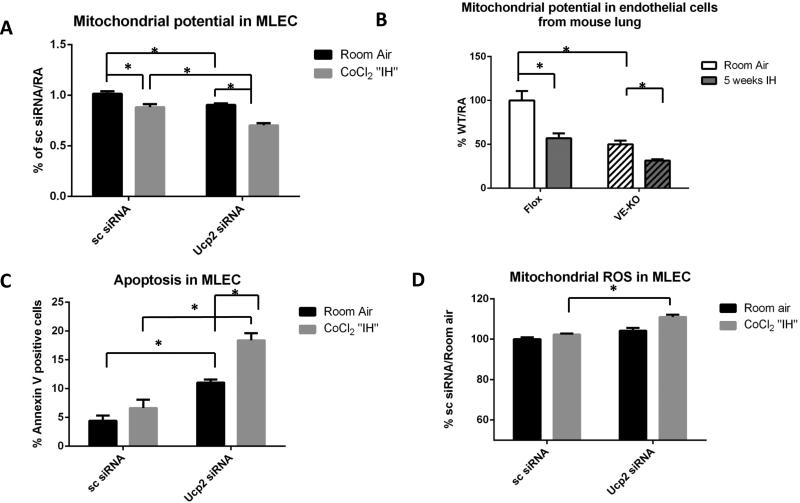
As additional confirmation, cultured MLEC transfected with Ucp2 siRNA had significantly lower mitochondrial membrane potential, as measured by MitoTracker® Red CMXRos, compared to control cells both at room air and after CoCl2 “IH” (Fig. 4A). Mitochondrial membrane potential of EC isolated from the lungs of VE-KO mice also demonstrated significantly lower mitochondrial potential compared to Flox lung EC at room air and after 5 weeks of IH (Fig. 4B). Fig. 4C shows that Ucp2 silencing increased cultured MLEC apoptosis in room air and after IH, suggesting that increased autophagy/mitophagy can lead to MLEC apoptosis, an important mechanism of PH. It has been shown that IH is associated with excessive mitochondrial reactive oxygen species (ROS) production (36) that can induce autophagy (37, 38). To determine if “hyperactive autophagy” in MLEC in the absence of Ucp2 is driven by elevated ROS we assessed mitochondrial ROS content in MLEC with and without Ucp2 siRNA. Fig. 4D showed no significant difference in mitochondrial ROS production between MLEC with and without Ucp2 siRNA at baseline. However, CoCl2 “IH” stimulates a significant increase in mitochondrial ROS levels in Ucp2 siRNA-transfected MLEC. These data suggest that Ucp2 silencing increased baseline mitophagy in MLEC without increasing mitochondrial ROS production.
Figure 4.
Mouse lung endothelial cells (MLEC) after Ucp2 silencing and kept at room air or challenged with CoCl2 “IH”. Scrambled siRNA (Sc) was used as negative control. A. Mitochondrial membrane potential as assessed by FACS in room air or after CoCl2 “IH”. B. Mitochondrial membrane potential as assessed by FACS in EC isolated from Flox and VE-KO lungs in room air and after 5 weeks of IH. C. Apoptosis as assessed by annexin-PI FACS in room air or after CoCl2 “IH”. D. Mitochondrial ROS production as assessed by FACS in room air or after CoCl2 “IH”. *p<0.05.
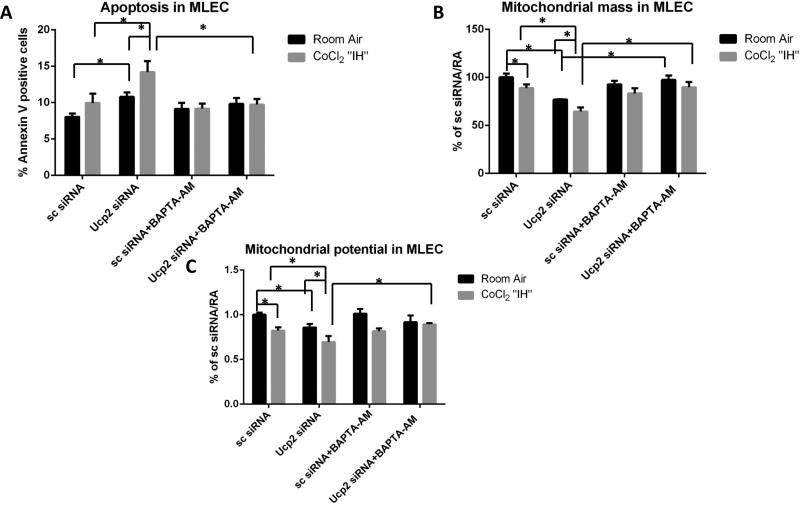
Ucp2 silencing mediates mitochondrial changes and apoptosis via calcium in MLEC
Increased baseline mitophagy appears to be associated with decreased mitochondrial membrane potential in cultured MLEC transfected with Ucp2 siRNA and in EC isolated from VEKO mice, Fig.4). Although a direct effect of Ucp2 loss may be increased mitochondrial membrane potential (39), calcium levels are also important modulators of membrane potential. We determined whether calcium-dependent mechanisms may be mediating Ucp2 silencing effects on mitochondria and apoptosis in MLEC. MLEC were transfected with Ucp2 siRNA and subjected to CoCl2 “IH” in the absence or presence of calcium chelator, BAPTA-AM. Fig. 5A shows that BAPTA-AM eliminated Ucp2 siRNA-induced apoptosis after CoCl2 “IH.” BAPTA-AM also reversed the effects of Ucp2 siRNA on mitochondrial mass and potential in MLEC (Fig. 5B, C). Of note, BAPTA-AM alone did not significantly change apoptosis, mitochondrial mass or mitochondrial potential in MLEC. These data suggested that the effects of Ucp2 silencing on mitochondria and apoptosis may be calcium mediated.
Figure 5.
Mouse lung endothelial cells (MLEC) after Ucp2 silencing and kept at room air or challenged with CoCl2 “IH” with or without incubation with 1uM BAPTA-AM. Scrambled siRNA (Sc) was used as negative control. A. Apoptosis as assessed by annexin-PI FACS in room air or after CoCl2 “IH” with or without incubation with 1uM BAPTA-AM. B. Mitochondrial mass as assessed by FACS in room air or after CoCl2 “IH” with or without incubation with 1uM BAPTA-AM. C. Mitochondrial membrane potential as assessed by FACS with or without incubation with 1uM BAPTA-AM. *p<0.05.
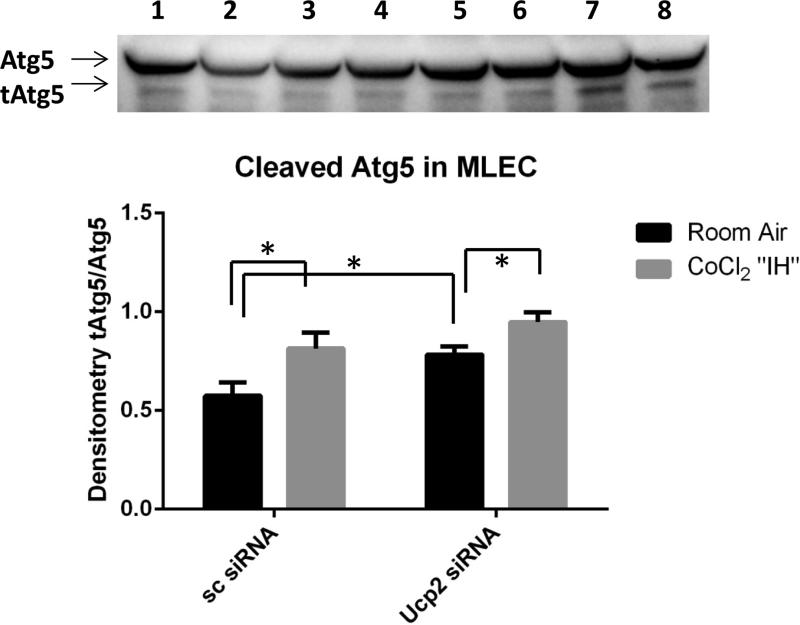
Ucp2 silencing increases Atg5 cleavage in MLEC
Calpains are calcium-dependent proteases which can cleave autophagy protein 5 (Atg5), a major component of autophagosome formation, leading to increased autophagy and apoptosis (40). Fig 6 shows that Ucp2 siRNA-treated cultured MLEC have significantly increased cleaved Atg5 compared to control (sc siRNA) cells. Therefore, Ucp2 silencing may alter calcium signaling, leading to calpain-induced Atg5 cleavage, which results in increased autophagy and apoptosis in MLEC.
Figure 6.
Immunoblot and densitometry of MLEC lysates demonstrating Atg5 cleavage in MLEC transfected with scrambled (sc) or Ucp2 siRNA in room air and after CoCl2 “IH”: 1,2 – sc siRNA/room air; 3,4 – Ucp2 siRNA/room air; 5,6 – sc siRNA/ CoCl2 “IH”; 7,8 – Ucp2 siRNA/ CoCl2 “IH”.
Pink1 silencing partially reverses “hyperactive autophagy” in MLEC
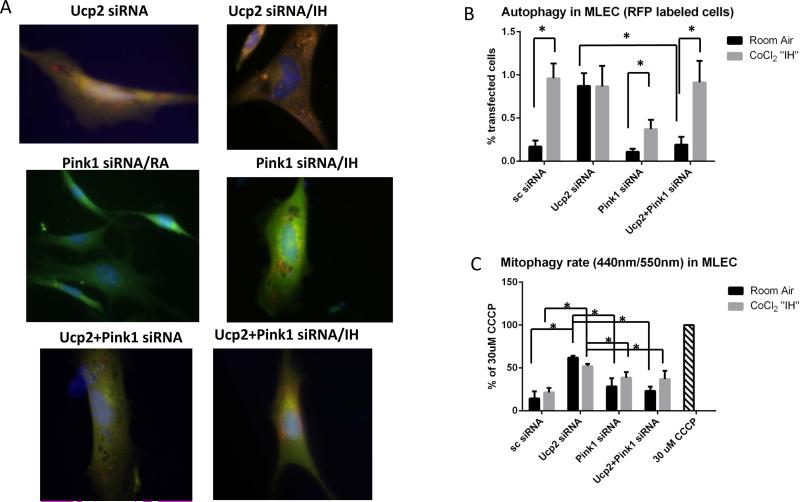
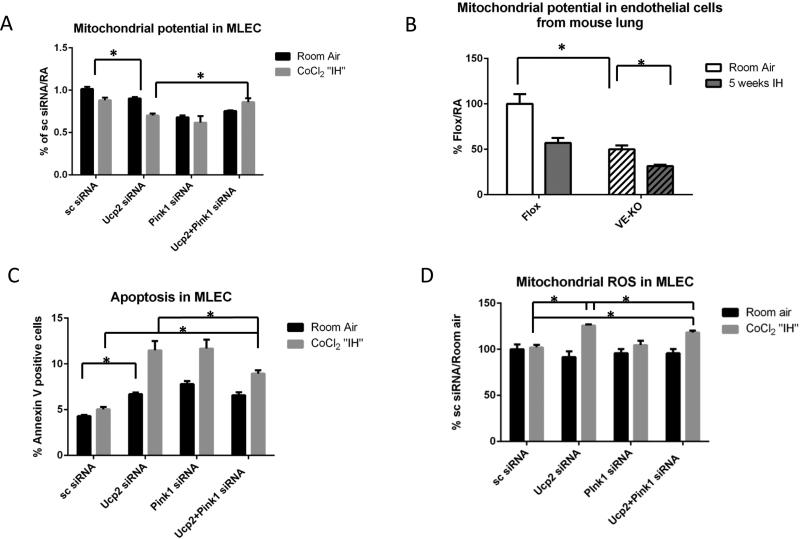
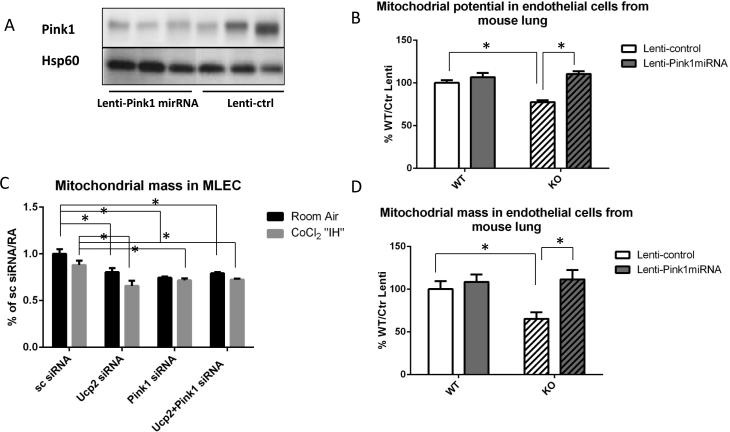
In order to determine whether mitophagy-associated protein, Pink1, mediates the “hyperactive autophagy” associated with Ucp2 silencing, we transfected MLEC with both Ucp2 and Pink1 siRNAs prior to CoCl2 “IH”. Transfection efficiency of simultaneous transfections with Ucp2 and Pink1 siRNAs is shown in Supplemental fig.VIII. Fig. 7A, B shows that Pink1 silencing reduced the “hyperactive autophagy” associated with Ucp2 silencing in MLEC cells. We quantified autophagy in MLEC by counting the percentage of cells labeled with red fluorescent protein in Fig. 7B. We also evaluated the mitophagy rate by co-transfecting cells with mitochondria targeted mKeima-Red plasmid (41). The fluorescent protein Keima has an excitation spectrum that changes with pH. A neutral pH is associated with a short wavelength (~440nm), while an acidic pH, as found in lysosomes, leads to a longer wavelength (~550nm). Keima containing plasmid tagged with a mitochondria-localizing peptide is a mode of detecting mitophagy flux via 440nm/550nm ratios. This ratio determines the amount of mitochondrion outside (440 nm) and inside (550nm) lysosomes, which reflects mitophagy rate. Fig.7C shows that MLEC transfected with Ucp2 siRNA have increased mitophagy flux at baseline that is not changed by CoCl2 “IH”, but significantly reduced by Pink1 silencing. Moreover, Pink1 silencing in MLEC that had been transfected with Ucp2 siRNA restored mitochondrial potential (Fig. 8A) and partially reduced apoptosis as well as ROS production (Fig. 8C and D). We confirmed our MLEC in CoCl2 “IH” results by showing similar changes in mitochondrial potential in MLEC isolated from Flox and VE-KO mice before and after IH (Fig. 8B). To further confirm our results in vivo, we initially constructed an all-cell-targeting Pink1 silencing lentiviral construct (Lenti-Pink1 miRNA) for intranasal delivery, per our recent reports of intranasal lentiviral delivery (34, 35, 42). We first confirmed adequate Pink1 silencing in the mitochondria of lung lysates after WT mice were given intranasal lenti-Pink1 miRNA (Fig. 9A). We then subjected mice to 5 weeks of IH and isolated the mitochondrial fraction from lung lysates. Pink1 silencing restored mitochondrial potential (Fig.9B) and mitochondrial mass (Fig 9D). Of note, in MLEC in which Ucp2 was silenced, Pink1 silencing did not restore mitochondrial mass (Fig 9C). These data suggest that Pink1 silencing has different effects on mitochondrial mass in vitro (MLEC) versus in vivo, which may be due to the contributing role of other cells, as proposed in the discussion section.
Figure 7.
Pink1 knockdown in Ucp2-silenced MLEC and kept at room air or challenged with CoCl2 “IH”. A. Autophagy levels as assessed by immunofluorescent ptfLC3 plasmid. Original magnification of all photomicrographs: ×40. B. Quantification of autophagy as a percent of RFP positive cells. C. Quantification of mitophagy as a 444nm/544nm fluorescence ratio. Mitophagy in MLEC treated with 30 μM CCCP for 16 h is used as a positive control. Data presented as a percent of mitophagy undergoing cells relative to positive control. *p<0.05.
Figure 8.
Pink1 knockdown in Ucp2-silenced MLEC and kept at room air or challenged with CoCl2 “IH”. A. Mitochondrial membrane potential after scrambled, Upc2, Pink1 or Ucp2+Pink1 siRNA transfection, assessed by FACS in room air or after CoCl2 “IH”. B. Mitochondrial membrane potential as assessed by FACS in EC isolated from Flox and VE-KO lungs in room air and after 5 weeks of IH. C. Apoptosis in MLEC as assessed by annexin-PI FACS in room air or after CoCl2 “IH”. D. Mitochondrial ROS production in MLEC as assessed by FACS in room air or after CoCl2 “IH”. *p<0.05.
Figure 9.
Lung-targeted Pink1 silencing in vivo. WT and Ucp2 KO mice were given lentiviral Pink1 miRNA intranasally followed by room air or 5 weeks of IH. A. Immunoblot for Pink1 protein in the mitochondrial fraction from lung lysates of mice given lentiviral Pink1 miRNA (Lenti-Pink1 miRNA) or lentiviral control (Lenti-ctrl) ntranasally and maintained in room air. Hsp60 was used as a loading control for mitochondrial protein. B. EC isolated from lungs of WT and KO mice treated with Lenti-control or Lenti-Pink1miRNA and subjected to 5 weeks of IH; C. MLEC after scrambled, Ucp2, Pink1 or Ucp2+Pink1 siRNA transfection, in room air or after CoCl2 “IH”. D. EC isolated from lungs of WT and KO mice treated with Lenti-control or Lenti-Pink1miRNA and subjected to 5 weeks of IH. *p<0.05.
Endothelial Pink1 silencing in Ucp2 KO mice prevents IH-induced PH
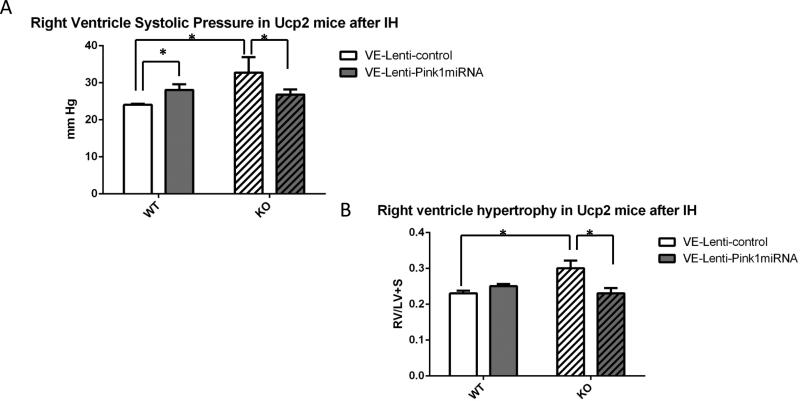
To demonstrate the specific effect of endothelial Pink1 silencing in vivo, we delivered intranasal endothelial-specific Pink1 silencing RNA (VE-Lenti-Pink1 miRNA) to WT and global Ucp2 KO mice prior to 5 weeks of IH. We recently demonstrated endothelial-specificity and effective Pink1 silencing in endothelium using intranasal VE-Lenti-Pink1 miRNA (35). Fig. 10 demonstrates a significant decrease in RVSP and RV/LV+S ratio in Ucp2 KO treated with VE-Lenti-Pink1 miRNA compared to mice treated with control lentivirus. Of note, WT mice treated with VE-Lenti-Pink1 miRNA showed an increase in RVSP compared to control virus treated mice (Fig. 10A). This may be due to the fact the Pink1 silencing in WT endothelium leads to a phenotype similar to Ucp2 silencing, such as increased apoptosis (Fig.8C), decreased mitochondrial potential (Fig. 9B) and mitochondrial mass (Fig. 9C). These data suggest that endothelial Pink1 is an important determinant of the Ucp2 KO PH phenotype in vivo.
Figure 10.
PH evaluation in WT and Ucp2 KO mice given endothelial specific VE-Pink1 miRNA lentivirus intranasally followed by 5 weeks of IH. A. Right ventricle systolic pressure was measured by direct catheter insertion. B. RV/LV+S ratio. Mouse hearts were dissected into right ventricle and left ventricle with septum. Each part was weighed and a RV/LV+S ratio was calculated for each animal. *p<0.05.
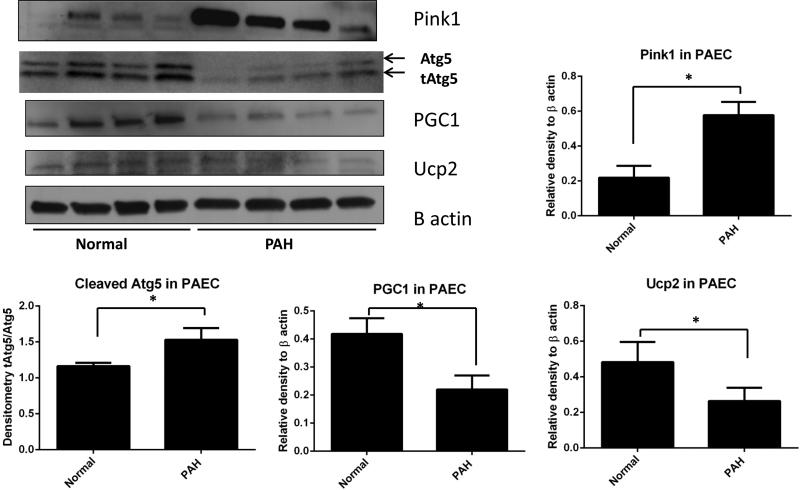
Pulmonary artery endothelial cells (PAEC) from PAH patients demonstrated decreased in Ucp2 and PGC1α, as well as increased Pink1 protein expression
PAECs from were obtained from lung normal and four PAH explanted donor lungs, as we recently described (43). Immunoblotting for Pink1, PGC1α, Atg5 and Ucp2 proteins in total lysates from PAECs showed significantly increased Pink1, Atg5 cleavage (the relative amount of truncated Atg in comparison to Atg), as well as decreased PGC1α and Ucp2 protein expression PAH patients compared to normal donors (Fig.11). These data parallel our findings in IH and in Ucp2 siRNA-treated MLEC.
Figure 11.
Immunoblots for mitophagy-associated protein Pink1, autophagy and apoptosis associated cleaved Atg5 (tAtg5), mitochondrial biosynthesis marker, PGC1, and Ucp2 performed on lysates from PAECs obtained from four PAH patients and four healthy donors. β actin immunoblots used as a loading control. Densitometry measurements are graphically represented. *p<0.05
Discussion
The overall goal of our study was to determine the role of EC Ucp2 and identify previously unrecognized mechanisms whereby Ucp2 deficiency or silencing results in PH. We also demonstrate the relevance of EC Ucp2 and mitophagy to human PH. Unlike previous reports of Ucp2 in PASMC and chronic hypoxia-induced PH, we studied IH as a general model for oxidant-induced PH, which is thought to be a common mechanism underlying both PH and PAH due to a variety of disease states. IH has been reported to lead to pulmonary vascular and systemic hypertension. However, the severity of the vascular changes varies. For example, IH in rats leads to severe systemic hypertension (44), while IH in mice lead to milder changes (45). Campen MJ et al. demonstrated increase RV/LV+septum weight ratio in C57Bl6 mice subjected to 5 weeks of IH to levels similar to what we found in VE-KO mice (45). Fagan KA showed an increase in RVSP in WT mice after IH (29.5±0.6mm Hg in normoxia vs. 36±0.9 mmHg after IH) (46). Our findings were slightly different (23.6±0.7mm Hg in normoxia vs. 25.7±0.8 mmHg after IH) that can be explained by differences in the length of IH exposure (24h/day by Fagan KA vs. 10h/day in our experiments) and the fact that our mice were floxed for Ucp2, which may have some effect on the phenotype. IH has been proposed as an animal model for PH development as a response to cyclical hypoxia-reoxygenation (46). However, others demonstrated that the development of PH in response to IH was predominantly observed in transgenic mice (39, 47, 48). Similarly, our control Flox mice did not show significant increases in RVSP or RV hypertrophy, as measured by RV/LV+septum weight ratio, while VE-KO mice demonstrated both higher RVSP and RV hypertrophy. These results seem to suggest that flox mice are partially protected against PH development which requires future investigation.
We detected apoptosis in endothelium after 1 week of IH but not after 5 weeks, which is the time point when physiologic evidence of PH, such as increased RVSP and RV hypertrophy, are most evident. This data is consistent with reports that early, wide-spread EC apoptosis eventually selects for EC with hyperproliferative and apoptosis-resistant phenotypes (33). We speculate that between 1 and 5 weeks of IH, EC may transition to an apoptosis-resistance phenotype but this would require more extensive time course experiments to confirm. Yamagi-Kegan et al. recently demonstrated endothelial apoptosis during early time points of inducible PH and increased remodeling near the end (32). Interestingly, VE-KO mice had detectable endothelial apoptosis even at baseline, which may account for their baseline increases in RVSP and RV hypertrophy, which will become the basis of future studies.
Our data show that the loss of EC Ucp2 leads to increased Pink1 expression, mitophagy and apoptosis in mouse EC and that PAH in people is associated with similar changes in EC. Based on these data we propose the following mechanism for IH-induced PH: decreased Ucp2 in endothelium results in increased mitophagy-autophagy without adequate replacement of healthy, newly synthesized mitochondria, which leads to increased endothelial loss, at least in part via apoptosis and, ultimately, PH. VE-KO mice allowed us to evaluate the specific role of EC in vivo. Previous reports show endothelial events likely precede PH-associated vasculopathy (49, 50). We demonstrate that VE-KO mice develop PH, even at baseline, and that their lungs have increased mitophagy-associated proteins, Pink1 and Parkin, suggesting higher baseline mitophagy than control mice. Markers of macroautophagy, LC3BII/LC3BI, were also increased in VE-KO lungs. We found parallel findings in MLEC in which Ucp2 was silenced and in global Ucp KO lungs. Increased autophagy has been recently identified by Long et al. in the pathogenesis of monocrotaline-induced PH in rats (51). They showed that blocking autophagy with chloroquine, an inhibitor of autophagic protein degradation, led to decreased proliferation and increased apoptosis of rat PASMC. Mitophagy is a specific type of autophagy in which damaged mitochondria are removed (24).
Mitophagy and mitochondrial biogenesis represent two coordinated processes that determine cellular dysfunction and disease (52). In order to maintain a healthy cellular environment, a balance between removal of damaged mitochondrion and biosynthesis of new mitochondria is necessary. To evaluate mitochondrial “balance” we determined the expression of PGC1α as well as mitochondrial mass. PGC1α is a major transcription factor that controls mitochondrial biogenesis through the co-activation of many nuclear and non-nuclear receptors (53). We evaluated mitochondrial mass not only in cultured MLEC with silenced Ucp2, but also ex vivo in EC isolated from lungs of VE-KO mice. Ryan J et al reported that female rats developed monocrotaline-induced PH through an involvement of PGC1α (54). The same authors showed earlier that mitochondrial fragmentation, increased proliferation, and impaired apoptosis in PASMC were in the basis of PH development (55).
Similar mechanisms may be responsible for IH-induced PH in VE-KO mice and EC. X. Xu and S. Erzurum introduced the concept of increased glycolysis in the EC of PAH patients, potentially due to decreased mitochondrial function and increased ROS production (56). The historical work of Warburg (57), showing a predominance of glycolysis over respiration in tumors, was expanded by R. Tuder into the pathogenesis of PH (3). Condello M et al. (58) demonstrated that tumor cells often have fewer, smaller, more fragile, and highly pleiomorphic mitochondria than their normal counterparts. Decreased quantities of effective mitochondria in EC from Ucp2 KO mice could potentially lead to a glycolytic phenotype, resulting in PH.
To verify our in vivo findings in isolated lung EC, we developed a chemical IH in vitro model. We avoided using gaseous hypoxia, which necessitates serum starvation, because serum deprivation activates autophagy. Gaseous hypoxia also requires at least 2-4 hours before the cell culture media attains hypoxic conditions. In order to mimic our in vivo model of IH, in which cycling between hypoxia and normoxia occurs within minutes, we utilized the rapid anoxic/hypoxic conditions achieved by chemical methods. We instituted chemical “IH” using CoCl2 and checked a number of various conditions (intermittent and sustained hypoxia) based on similar signaling protein profiles between in vitro and in vivo experiments. We analyzed autophagy and mitophagy using complementary assays. In addition to calculating LC3BII/LC3BI ratios, we detected autophagy flux with ptfLC3 plasmid transfection, as others have described (59). To evaluate the mitophagy rate we used a relatively new approach by co-transfecting cells with mitochondrial-targeted mKeima-red plasmid. mKeima protein has a different excitation wavelength depending on the acidity of the environment, such as the acidic environment of the lysosomal compartment (41). Ucp2-silenced MLEC had significantly higher 444nm/544nm ratios than the WT cells, suggesting increased mitophagy rates. Interestingly, CoCl2 “IH” induces autophagy but not mitophagy in wild type MLEC. This data suggests that the increased baseline autophagy markers detected after Ucp2 silencing may primarily be due to increased mitophagy, rather than autophagy.
Although a direct effect of Ucp2 loss is expected to be increased mitochondrial membrane potential (39), there may be counter-balancing, adaptive effects of Ucp2 deficiency that occur in EC. We found that excessive apoptosis, loss of mitochondrial mass and mitochondrial potential in Ucp2 silenced MLEC after CoCl2 “IH” can be prevented by a calcium chelator, BAPTA-AM. Calcium homeostasis is vital for normal cell physiology and its disruption can lead to heart failure, diabetes and neuronal degeneration. In physiological conditions there is constant crosstalk between endoplasmic reticulum, which releases calcium, and mitochondria, which uptakes calcium. Ucp2 has been shown to play a role in mitochondrial uptake of intracellular calcium (60). We postulate that the absence of Ucp2 during IH in EC results in calcium accumulation which leads to mitochondrial depolarization and apoptotic cell death. The precise mechanisms of calcium release and the molecular sequence of events leading to calcium-induced apoptosis merit future investigations. We invoke a potential role for increased cleaved Atg5, which is regulated by calcium-sensitive proteases and has been shown to cause both excessive autophagy and apoptosis (61, 62). Yousefi et al. reported that increased Atg5 cleavage by calcium-sensitive calpains represented the point at which beneficial autophagy becomes excessive or detrimental apoptosis (40). Shi et al. linked calcium influx, Atg5 cleavage by calpains and mitochondrial potential disruption with increased apoptosis (63). Dromparis et al. demonstrated that calcium dysregulation plays a role in PH development in Ucp2-KO mice, but their findings were in PASMC (22). Our data points to a role for Ucp2 in cleavage of Atg5 in MLEC.
Others have reported that decreasing Ucp2 expression with antisense oligonucleotides increased mitochondrial membrane potential and ROS production (64). These apparent discrepancies may be due to the specific cells used for the experiment. For example, Duval et al. used a CRL-2181 cell line, which originates from lymph nodes and are immortalized by SV40. In our study we used primary MLEC. We also studied mitochondrial potential and ROS production in both MLEC and PASMC (not shown). Unlike MLEC, which showed decreased mitochondrial potential with Ucp2 silencing, PASMC transfected with Ucp2 siRNA demonstrated mitochondrial hyperpolarization. Moreover, mitophagy and autophagy-related proteins expression as well as autophagy flux (ptfLC3 plasmid) and mitophagy flux (mKeima plasmid) were different in PASMC (not shown). Therefore, the effect of Ucp2 on mitochondrial membrane potential is likely cell type-specific.
Lastly, we invoked a functional role for Pink1, a mediator of mitophagy, in Ucp2 deficiency-induced PH. In vivo Pink1 silencing, specifically in endothelium, may restore the balance between removal of non functional mitochondrion and biosynthesis of healthy mitochondria. With Pink1 silencing we observed mitochondrial potential restoration in both in vitro and in vivo models. We recently reported that the absence of Pink1 leads to increased apoptosis, defective mitochondrion and increased susceptibility during acute hyperoxia (35) and LPS-induced inflammation (65) in wild type EC. Our current studies show that Pink1 silencing in EC in the absence of Ucp2 seems to have a beneficial effect, which we speculate may be due to the restoration of the balance between mitophagy and mitochondria biogenesis in the setting of Ucp2 deficiency. Therefore, the physiologic role of Pink1-mediated mitophagy is likely context and model dependent. For example, MAP kinase kinase 3 deficient mice are protected against LPS-induced inflammation by their baseline increases in both mitochondrial biosynthesis as well as mitophagy – the net result of which is an increased pool of healthy mitochondria (65). In our model of Ucp2 deficiency, increased mitophagy likely has a detrimental effect because mitochondrial biosynthesis is decreased, the net effect being inadequate mitochondrial replenishment (as evidenced by the decrease in mitochondrial mass we detected in Ucp2 deficient EC). When Pink1 was silenced, mitochondrial mass was restored in vivo only, which also suggests some degree of specificity of Pink1-induced mitochondrial effects and the requirement for alternative cell types in modulating mitochondrial mass. Given that smooth muscle cells proliferation is regulated differently by EC of different sources (66), and the importance of PASMC in PH pathogenesis, specific EC-PASMC interactions involving Ucp2-Pink1 are likely. A more precise understanding of these cell-cell interactions, especially in the context of Ucp2 and mitochondrial biology, is an important area for future investigations.
Mitochondrial markers, such as decreased expression of manganese superoxide dysmutase and pyruvate dehydrogenase, have been associated with PH in people (67, 68). An Ucp2-Pink1 axis has not been previously described in clinical PH. PAECs from people with PAH revealed increased Pink1 and Atg5 cleavage as well as decreased Ucp2 and PGC1α expression, suggestive of depressed mitochondrial biogenesis and increased mitophagy compared to healthy donors. These data are consistent with our mouse and EC IH data further supporting the clinical and therapeutic applicability of the proposed model and pathway.
Supplementary Material
Significance.
Our studies link EC Ucp2 to mitophagy/autophagy and mitochondrial biogenesis. In addition, we identified EC mitophagy as a critical, initiating event in the development of PH. Moreover, we found that Pink1 silencing reversed Ucp2 deficiency-induced PH. Our studies also show that EC from human PH lungs exhibit changes in Ucp2-Pink1-mitochondrial biogenesis that parallel our mouse studies, thus expanding the clinical relevance of these molecules as potential biomarkers and therapeutic targets in PH.
Acknowledgements
We thank Dr. Hyung J Chun for his scientific advice and reading of the manuscript and Drs. Wassim Fares and Terence Trow for their clinical insights and review of the data.
Sources of Funding.
PJL was funded by the National Institutes of Health (R01 HL090660 and HL071595) and FAMRI Clinical Investigator Award 82384. The metabolic studies were supported by the Yale MMPC DK059635.
Abbreviations
- Ucp2
uncoupling protein 2
- PH
pulmonary hypertension
- Pink1
PTEN induced putative kinase 1
- PGC1α
peroxisome proliferator activated receptor gamma coactivator 1-alpha
- MLEC
mouse lung endothelial cells
- PASMC
pulmonary artery smooth muscle cells
- EC
endothelial cells
- TAPSE
tricuspid annular plane systolic excursion
- PAT
pulmonary acceleration time
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- PAEC
pulmonary artery endothelial cells
- ROS
reactive oxygen species
- RVSP
right ventricle systolic pressure
- VE-KO
endothelial specific knockout driven by VE-cadherin promoter
Footnotes
Disclosures. None
References
- 1.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 2.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the vegf receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. The FASEB Journal. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 3.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: A new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am J Physiol Lung Cell Mol Physiol. 302:L977–991. doi: 10.1152/ajplung.00362.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron RM, Choi AJ, Owen CA, Choi AM. Genetically manipulated mouse models of lung disease: Potential and pitfalls. Am J Physiol Lung Cell Mol Physiol. 302:L485–497. doi: 10.1152/ajplung.00085.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosc LV, Resta T, Walker B, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med. 14:3–17. doi: 10.1111/j.1582-4934.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steudel W, Watanabe M, Dikranian K, Jacobson M, Jones RC. Expression of nitric oxide synthase isoforms (nos ii and nos iii) in adult rat lung in hyperoxic pulmonary hypertension. Cell Tissue Res. 1999;295:317–329. doi: 10.1007/s004410051238. [DOI] [PubMed] [Google Scholar]
- 10.Herget J, Wilhelm J, Novotna J, Eckhardt A, Vytasek R, Mrazkova L, Ostadal M. A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol Res. 2000;49:493–501. [PubMed] [Google Scholar]
- 11.Fessel JP, Flynn CR, Robinson LJ, Penner NL, Gladson S, Kang CJ, Wasserman DH, Hemnes AR, West JD. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. Am J Respir Cell Mol Biol. 49:778–787. doi: 10.1165/rcmb.2012-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenberg M. Uncoupling protein--a useful energy dissipator. J Bioenerg Biomembr. 1999;31:419–430. doi: 10.1023/a:1005440221914. [DOI] [PubMed] [Google Scholar]
- 13.Jezek P, Zackova M, Rehakova Z, Ruzicka M, Borecky J, Skobisova E, Brucknerova J, Garlid KD, Gimeno RE, Tartaglia LA. Existence of uncoupling protein-2 antigen in isolated mitochondria from various tissues. FEBS Lett. 1999;455:79–82. doi: 10.1016/s0014-5793(99)00853-4. [DOI] [PubMed] [Google Scholar]
- 14.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 15.VAN DER LEE KAJM, WILLEMSEN PHM, VAN DER VUSSE GJ, VAN BILSEN M. Effects of fatty acids on uncoupling protein-2 expression in the rat heart. The FASEB Journal. 2000;14:495–502. doi: 10.1096/fasebj.14.3.495. [DOI] [PubMed] [Google Scholar]
- 16.Teshima Y, Akao M, Jones SP, Marbán E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circulation Research. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 17.Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 18.Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F, Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- 19.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the cns: In support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 20.Chan CB, Harper ME. Uncoupling proteins: Role in insulin resistance and insulin insufficiency. Curr Diabetes Rev. 2006;2:271–283. doi: 10.2174/157339906777950660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, Sydykov A, Haag D, Esfandiary A, Kojonazarov B, Veit F, et al. Mitochondrial hyperpolarization in pulmonary vascular remodeling - ucp2 deficiency as disease model. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2012-0361OC. [DOI] [PubMed] [Google Scholar]
- 22.Dromparis P, Paulin R, Sutendra G, Qi AC, Bonnet S, Michelakis ED. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ Res. 113:126–136. doi: 10.1161/CIRCRESAHA.112.300699. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y, Choi AM. Cross talk between autophagy and apoptosis in pulmonary hypertension. Pulm Circ. 2:407–414. doi: 10.4103/2045-8932.105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates pink1 processing, import and parkin recruitment. EMBO Rep. 13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 27.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 28.Mills EM, Xu D, Fergusson MM, Combs CA, Xu Y, Finkel T. Regulation of cellular oncosis by uncoupling protein 2. J Biol Chem. 2002;277:27385–27392. doi: 10.1074/jbc.M111860200. [DOI] [PubMed] [Google Scholar]
- 29.Marti A, Larrarte E, Novo FJ, Garcia M, Martinez JA. Ucp2 muscle gene transfer modifies mitochondrial membrane potential. Int J Obes Relat Metab Disord. 2001;25:68–74. doi: 10.1038/sj.ijo.0801484. [DOI] [PubMed] [Google Scholar]
- 30.Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Bruning JC, Lowell BB. Glucose stimulation of hypothalamic mch neurons involves k(atp) channels, is modulated by ucp2, and regulates peripheral glucose homeostasis. Cell Metab. 12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: From experimental models to clinical trials. Pharmacol Ther. 126:1–8. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (fizz1/relmalpha) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 306:L1090–1103. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Jiang G, Sauler M, Lee PJ. Lung endothelial ho-1 targeting in vivo using lentiviral mirna regulates apoptosis and autophagy during oxidant injury. FASEB J. doi: 10.1096/fj.13-231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, Shan P, Mannam P, Lee PJ. Endothelial pink1 mediates the protective effects of nlrp3 deficiency during lethal oxidant injury. J Immunol. 192:5296–5304. doi: 10.4049/jimmunol.1400653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via hif-mediated ros. Respir Physiol Neurobiol. 174:230–234. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, Ding S, Lee JC, Shi X. Cadmium induces autophagy through ros-dependent activation of the lkb1-ampk signaling in skin epidermal cells. Toxicol Appl Pharmacol. 255:287–296. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karna P, Zughaier S, Pannu V, Simmons R, Narayan S, Aneja R. Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent. J Biol Chem. 285:18737–18748. doi: 10.1074/jbc.M109.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 41.Kogure T, Karasawa S, Araki T, Saito K, Kinjo M, Miyawaki A. A fluorescent variant of a protein from the stony coral montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat Biotechnol. 2006;24:577–581. doi: 10.1038/nbt1207. [DOI] [PubMed] [Google Scholar]
- 42.Siner JM, Jiang G, Cohen ZI, Shan P, Zhang X, Lee CG, Elias JA, Lee PJ. Vegf-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 2007;21:1422–1432. doi: 10.1096/fj.06-6661com. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, et al. An endothelial apelin-fgf link mediated by mir-424 and mir-503 is disrupted in pulmonary arterial hypertension. Nat Med. 19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: Importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- 45.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in c57bl/6j mice. J Appl Physiol (1985) 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 46.Fagan KA. Selected contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol (1985) 2001;90:2502–2507. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- 47.Fagan KA, Morrissey B, Fouty BW, Sato K, Harral JW, Morris KG, Jr., Hoedt-Miller M, Vidmar S, McMurtry IF, Rodman DM. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res. 2001;2:306–313. doi: 10.1186/rr74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial hif-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 49.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 50.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: Pulmonary arterial hypertension. Nat Rev Cardiol. 8:443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type ii receptor degradation. Circulation Research. 112:1159–1170. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J, Wang KZ, Chu CT. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 9:1663–1676. doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 54.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, et al. Pgc1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med. 187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol. 1:357–372. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 58.Condello M, Caraglia M, Castellano M, Arancia G, Meschini S. Structural and functional alterations of cellular components as revealed by electron microscopy. Microsc Res Tech. 76:1057–1069. doi: 10.1002/jemt.22266. [DOI] [PubMed] [Google Scholar]
- 59.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged lc3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 60.Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, Imamura H, Malli R, Graier WF. Leucine zipper ef hand-containing transmembrane protein 1 (letm1) and uncoupling proteins 2 and 3 (ucp2/3) contribute to two distinct mitochondrial ca2+ uptake pathways. Journal of Biological Chemistry. 286:28444–28455. doi: 10.1074/jbc.M111.244517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 62.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi M, Zhang T, Sun L, Luo Y, Liu DH, Xie ST, Song XY, Wang GF, Chen XL, Zhou BC, et al. Calpain, atg5 and bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis. 18:435–451. doi: 10.1007/s10495-012-0786-2. [DOI] [PubMed] [Google Scholar]
- 64.Duval C, Negre-Salvayre A, Dogilo A, Salvayre R, Penicaud L, Casteilla L. Increased reactive oxygen species production with antisense oligonucleotides directed against uncoupling protein 2 in murine endothelial cells. Biochem Cell Biol. 2002;80:757–764. doi: 10.1139/o02-158. [DOI] [PubMed] [Google Scholar]
- 65.Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, Merkel J, Kang MJ, Dela Cruz CS, Ahasic AM, et al. Mkk3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 306:L604–619. doi: 10.1152/ajplung.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chanakira A, Dutta R, Charboneau R, Barke R, Santilli SM, Roy S. Hypoxia differentially regulates arterial and venous smooth muscle cell proliferation via pdgfr-î2 and vegfr-2 expression. American Journal of Physiology - Heart and Circulatory Physiology. 302:H1173–H1184. doi: 10.1152/ajpheart.00411.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, Michelakis ED. Pyruvate dehydrogenase inhibition by the inflammatory cytokine tnfalpha contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med (Berl) 89:771–783. doi: 10.1007/s00109-011-0762-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.