Abstract
BACKGROUND
Chronic lymphocytic leukemia (CLL) primarily affects older persons who often have coexisting conditions in addition to disease-related immunosuppression and myelosuppression. We conducted an international, open-label, randomized phase 3 trial to compare two oral agents, ibrutinib and chlorambucil, in previously untreated older patients with CLL or small lymphocytic lymphoma.
METHODS
We randomly assigned 269 previously untreated patients who were 65 years of age or older and had CLL or small lymphocytic lymphoma to receive ibrutinib or chlorambucil. The primary end point was progression-free survival as assessed by an independent review committee.
RESULTS
The median age of the patients was 73 years. During a median follow-up period of 18.4 months, ibrutinib resulted in significantly longer progression-free survival than did chlorambucil (median, not reached vs. 18.9 months), with a risk of progression or death that was 84% lower with ibrutinib than that with chlorambucil (hazard ratio, 0.16; P<0.001). Ibrutinib significantly prolonged overall survival; the estimated survival rate at 24 months was 98% with ibrutinib versus 85% with chlorambucil, with a relative risk of death that was 84% lower in the ibrutinib group than in the chlorambucil group (hazard ratio, 0.16; P=0.001). The overall response rate was higher with ibrutinib than with chlorambucil (86% vs. 35%, P<0.001). The rates of sustained increases from baseline values in the hemoglobin and platelet levels were higher with ibrutinib. Adverse events of any grade that occurred in at least 20% of the patients receiving ibrutinib included diarrhea, fatigue, cough, and nausea; adverse events occurring in at least 20% of those receiving chlorambucil included nausea, fatigue, neutropenia, anemia, and vomiting. In the ibrutinib group, four patients had a grade 3 hemorrhage and one had a grade 4 hemorrhage. A total of 87% of the patients in the ibrutinib group are continuing to take ibrutinib.
CONCLUSIONS
Ibrutinib was superior to chlorambucil in previously untreated patients with CLL or small lymphocytic lymphoma, as assessed by progression-free survival, overall survival, response rate, and improvement in hematologic variables. (Funded by Pharmacyclics and others; RESONATE-2 ClinicalTrials.gov number, NCT01722487.)
Chronic lymphocytic leukemia (CLL) is the most common leukemia among adults in Western countries; it affects primarily older persons, with a median age at diagnosis of 72 years.1,2 Chlorambucil has been a standard first-line therapy in CLL, especially for older patients or those with coexisting conditions.1,3 Until recently, no treatment was clearly superior to chlorambucil in this population.3-7 Fludarabine or bendamustine has been associated with higher response rates and longer progression-free survival than those with chlorambucil, but both have also been associated with higher rates of toxic effects, and neither has provided overall survival benefit.3,5,6,8 In previously untreated patients who were younger than 75 years of age, bendamustine was associated with longer progression-free survival as compared with chlorambucil (median, 21.6 months vs. 8.3 months).5
Only recently have data from randomized studies shown improved outcomes with the addition of anti-CD20 monoclonal antibodies to chlorambucil.9,10 In the three-group randomized CLL11 study conducted by the German CLL Study Group, which involved previously untreated patients with coexisting conditions, the median progression-free survival was 29.9 months with the combination of obinutuzumab and chlorambucil, 16.3 months with the combination of rituximab and chlorambucil, and 11.1 months with chlorambucil alone; overall survival was longer with the combination regimens than with chlorambucil.11 In another phase 3 study, which involved previously untreated patients who were not considered to be candidates for fludarabine-containing therapy, the median progression-free survival was 13.1 months with chlorambucil versus 22.4 months with the combination of chlorambucil and ofatumumab.10
Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab is standard in younger patients with CLL,12 but because of treatment-related toxic effects, this regimen is not suitable for older patients or those with coexisting conditions.13 Patients who are 65 years of age or older do not have the same efficacy benefit, and they have more toxic effects than do younger patients treated with this combination chemoimmunotherapy.13-15 Moreover, although the median progression-free survival with first-line fludarabine, cyclophosphamide, and rituximab is approximately 52 months, patients with high-risk genetic abnormalities (chromosome 17p13.1 or 11q22.3 deletion) or unmutated IGHV have inferior outcomes, with approximately 35 to 50% of the patients having progressive disease within 3 years.12
Ibrutinib is a first-in-class oral covalent inhibitor of Bruton's tyrosine kinase (BTK) that has been approved for the treatment of patients with CLL who have received at least one prior therapy and as primary therapy for patients with CLL who have chromosome 17p13.1 deletion.16,17 BTK is essential for signaling by means of the B-cell receptor and chemokine receptors, which CLL cells use for survival, proliferation, and tissue homing.18-22 In pharmacodynamic studies of ibrutinib in vivo in patients with CLL, ibrutinib inhibited leukemia-cell proliferation and accelerated CLL cell death.23-25
In the phase 3 Study of Ibrutinib versus Ofatumumab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (RESONATE) involving patients with previously treated CLL, single-agent ibrutinib showed superior efficacy to ofatumumab, with a risk of progression that was 78% lower and a risk of death that was 57% lower.26 In early-phase data from 31 previously untreated patients with CLL who were 65 years of age or older, the overall response rate with ibrutinib was 84% (with a complete response in 23% of the patients); the estimated rate of progression-free survival at 30 months was 96%, and the overall survival rate was 97%, with 81% of the patients continuing to take daily ibrutinib after 3 years of follow-up.27
These findings suggest a role for single-agent ibrutinib as initial treatment in patients with CLL. We conducted a multicenter, open-label, randomized phase 3 trial (RESONATE-2; study number, PCYC-1115-CA) to evaluate the efficacy and safety of single-agent ibrutinib as compared with chlorambucil in patients 65 years of age or older with previously untreated CLL.
Methods
Patients
Eligible patients were 65 years of age or older and had previously untreated CLL or small lymphocytic lymphoma requiring therapy.28 Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance-status score of 2 or less (on a scale from 0 to 5, with 0 indicating no symptoms and higher numbers indicating increasing disability), an absolute neutrophil count of 1000 cells or more per cubic millimeter, a platelet count of 50,000 or more per cubic millimeter, and adequate liver and kidney function. Patients were ineligible if they had chromosome 17p13.1 deletion. All the patients provided written informed consent.
Study Oversight and Conduct
The study was approved by the institutional review board or independent ethics committee at each institution and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The study was sponsored and designed by Pharmacyclics. All the investigators and their research teams collected the data. The sponsor confirmed the accuracy of the data and compiled the data for analysis. All the authors had full access to the data and were involved in the interpretation of the data.
The first draft of the manuscript was collaboratively written by the first and last authors and two authors who are employees of the sponsor. Editorial support was provided by a professional medical writer, with funding from the sponsor. All the authors contributed to the revisions and final approval of the manuscript and made the decision to submit the manuscript for publication. All the authors vouch for the accuracy and completeness of the reported data and analyses and confirm adherence of the trial to the protocol (available with the full text of this article at NEJM.org). An independent review committee whose members were unaware of the treatment assignments and lymphocyte counts evaluated response and progression.
Randomization and Treatment
Patients were enrolled in the United States, countries in Europe, and other countries (see the Supplementary Appendix, available at NEJM.org). Patients were randomly assigned, in a 1:1 ratio, to receive either oral ibrutinib (at a dose of 420 mg once daily) until disease progression or development of an unacceptable level of toxic effects or up to 12 cycles of chlorambucil (at a dose of 0.5 mg per kilogram of body weight on days 1 and 15 of each 28-day cycle, which was increased to a maximum of 0.8 mg per kilogram, if there was not an unacceptable level of toxic effects) until disease progression, determination of a lack of efficacy (defined as a lack of complete or partial response, as determined by the investigator), or development of an unacceptable level of toxic effects.
Patients with disease progression that was confirmed by the independent review committee were enrolled in a separate extension study (PCYC-1116-CA) for follow-up and second-line treatment according to the investigator's choice. Treatment in the PCYC-1116-CA study could include ibrutinib for chlorambucil-treated patients who had disease that progressed according to the independent review committee and who had an indication for treatment according to the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria28 (Table S1 in the Supplementary Appendix) as determined by the investigator.
Study End Points
The primary end point was progression-free survival, as assessed by the independent review committee according to the iwCLL criteria,28 with modification for treatment-related lymphocytosis such that isolated treatment-related lymphocytosis (in the absence of other clinical, computed tomographic, or laboratory evidence of disease progression) was not considered to indicate progressive disease.29 Key secondary end points included overall survival, overall response (details in Table S2 in the Supplementary Appendix), the rate of sustained improvement in hematologic variables, and safety. Sustained hematologic improvement was defined as an increase in hematologic variables that was sustained continuously for at least 56 days without transfusion or growth factors, as measured by the following: an increase in the platelet count or absolute neutrophil count from baseline of at least 50%, or for hemoglobin, an increase from baseline of ≥2 g per deciliter; or for patients with baseline cytopenia, an increase to a hemoglobin level of more than 11 g per deciliter, a platelet count of more than 100,000 per cubic millimeter, or an absolute neutrophil count of more than 1500 per cubic millimeter.
Safety assessments included evaluation of adverse events and measurement of laboratory variables. The severity of nonhematologic adverse events was graded according to the Common Terminology Criteria for Adverse Events, version 4.03.30 Hematologic adverse events were graded according to the iwCLL criteria.28
Patients were monitored every 2 weeks during cycles 1 and 2, every 4 weeks during cycles 3 through 12, and then every 8 weeks starting at cycle 13. The assessment of response was conducted every 4 cycles until disease progression or until study closure.
Statistical Analysis
The study was powered on the basis of the primary end point, progression-free survival. We calculated that the occurrence of 81 events of death or disease progression would provide the study with approximately 85% power to detect a hazard ratio for progression or death of 0.50 with ibrutinib as compared with chlorambucil, with the use of a one-sided log-rank test at an alpha level of 0.025. No interim analysis was planned. The type I error was controlled with the use of a hierarchical closed-testing procedure for the primary end point and ordered secondary end points including, in order, overall response rate, overall survival, and sustained hematologic improvement.
The primary analysis was a two-sided log-rank test stratified according to two randomization factors: ECOG performance-status score (0 or 1 vs. 2) and disease stage (Rai stage ≤II vs. III or IV). The overall response rate was analyzed by means of the Cochran–Mantel–Haenszel chi-square test, stratified according to the two randomization factors. Overall survival was analyzed with the use of an unstratified log-rank test, owing to small event numbers. The rate of sustained hematologic improvement was compared by a chi-square test for treatment effect.
Results
Patients
Beginning in March 2013, a total of 269 patients underwent randomization (Fig. S1 in the Supplementary Appendix). The characteristics of the patients at baseline were well balanced between the two groups (Table 1). The median age of the patients was 73 years, with 70% of the patients being 70 years of age or older; 45% of the patients had advanced-stage disease (Rai stage III or IV), and 20% had chromosome 11q22.3 deletion.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Ibrutinib (N = 136) | Chlorambucil (N = 133) |
|---|---|---|
| Age | ||
| Median (range) — yr | 73 (65–89) | 72 (65–90) |
| ≥70 yr — no. (%) | 96 (71) | 93 (70) |
| Male sex — no. (%) | 88 (65) | 81 (61) |
| ECOG performance-status score — no. (%)† | ||
| 0 | 60 (44) | 54 (41) |
| 1 | 65 (48) | 67 (50) |
| 2 | 11 (8) | 12 (9) |
| Diagnosis — no. (%) | ||
| Chronic lymphocytic leukemia | 123 (90) | 126 (95) |
| Small lymphocytic lymphoma | 13 (10) | 7 (5) |
| Rai stage III or IV — no. (%) | 60 (44) | 62 (47) |
| Bulky disease ≥5 cm — no. (%)‡ | 54 (40) | 40 (30) |
| Chromosome 11q22.3 deletion — no. (%) | 29 (21) | 25 (19) |
| Unmutated IGHV — no. (%) | 58 (43) | 60 (45) |
| Cytopenia at baseline — no. (%) | ||
| Any cytopenia | 72 (53) | 73 (55) |
| Hemoglobin ≤11 g/dl | 51 (38) | 55 (41) |
| Platelet count ≤100,000/mm3 | 35 (26) | 28 (21) |
| Absolute neutrophil count ≤1500/mm3 | 10 (7) | 7 (5) |
| Lactate dehydrogenase | ||
| Median (range) — U/liter | 199 (52–1188) | 195 (110–1347) |
| >250 U/liter — no. (%) | 39 (29) | 31 (23) |
| β2-Microglobulin | ||
| Median (range) — mg/liter | 5 (2–20) | 5 (1–39) |
| >3.5 mg/liter — no. (%) | 85 (62) | 89 (67) |
| Cumulative Illness Rating Scale score >6 — no. (%)§ | 42 (31) | 44 (33) |
| Creatinine clearance <60 ml/min — no. (%) | 60 (44) | 67 (50) |
| Median time from initial diagnosis (range) — mo | 31 (1–241) | 31 (1–294) |
There were no significant between-group differences at baseline.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and higher numbers indicating increasing disability.
Measurement was based on the longest diameter of the largest lymph node at screening, according to assessment by an independent review committee.
Scores on the Cumulative Illness Rating Scale range from 0 to 52, with higher scores indicating worse health status.
The median follow-up was 18.4 months, with 87% of the patients who had been randomly assigned to ibrutinib still receiving treatment at the time of analysis. In the chlorambucil group, 40% of the patients completed the maximum of 12 cycles of treatment (mean dose per administration, 0.6 mg per kilogram; range, 0.3 to 0.8).
Efficacy
Progression-free Survival
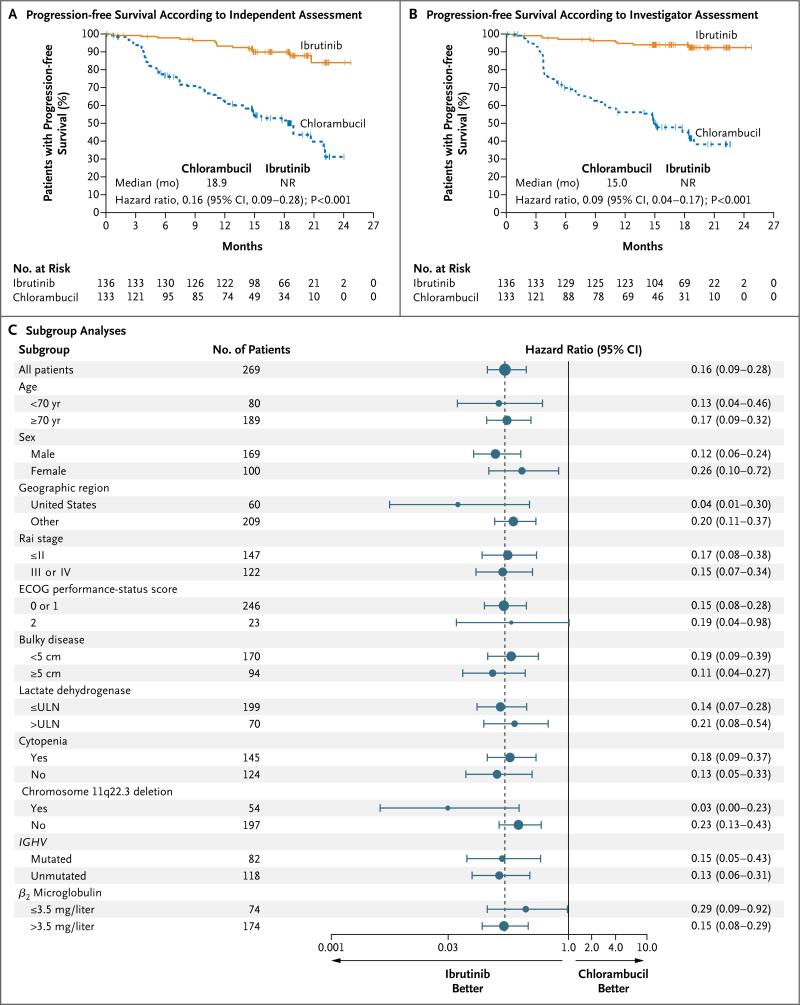
Ibrutinib resulted in significantly longer progression-free survival than that with chlorambucil (median, not reached vs. 18.9 months) as assessed by the independent review committee, with a relative risk of progression or death that was 84% lower than that with chlorambucil (hazard ratio, 0.16; 95% confidence interval [CI], 0.09 to 0.28; P<0.001) (Fig. 1A). The rate of progression-free survival at 18 months was 90% in the ibrutinib group versus 52% in the chlorambucil group.
Figure 1. Progression-free Survival with Ibrutinib versus Chlorambucil.
Shown is progression-free survival as assessed by the independent review committee (Panel A) and by the investigators (Panel B). The tick marks indicate patients with censored data. The median progression-free survival in the ibrutinib group was not reached (NR). Panel C shows subgroup analyses of progression-free survival as forest plots of hazard ratios for disease progression or death. The sizes of the circles are proportional to the sizes of the subgroups; error bars indicate 95% confidence intervals. The dashed vertical line represents the overall treatment effect for all patients. The upper limit of the normal range (ULN) for the lactate dehydrogenase level was 250 U per liter. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability.
The results of the analysis of progression-free survival were consistent in the higher-risk subgroups, including patients with Rai stage III or IV disease, worse ECOG performance-status score, presence of chromosome 11q22.3 deletion, and unmutated IGHV status (Fig. 1C). The rate of progression-free survival at 18 months with ibrutinib was approximately 89% both in the subgroup with unmutated IGHV and in the subgroup with mutated IGHV; the corresponding rates of progression-free survival with chlorambucil were 47% and 51%. Investigator-assessed progression-free survival, a key sensitivity analysis, also showed significant prolongation of progression-free survival with ibrutinib (median, not reached vs. 15.0 months), with a relative risk of progression or death that was 91% lower than that with chlorambucil (hazard ratio, 0.09; 95% CI, 0.04 to 0.17; P<0.001) (Fig. 1B). The only case of Richter's transformation (CLL that has evolved into an aggressive, rapidly growing large-cell lymphoma) occurred in the chlorambucil group.
Overall Survival
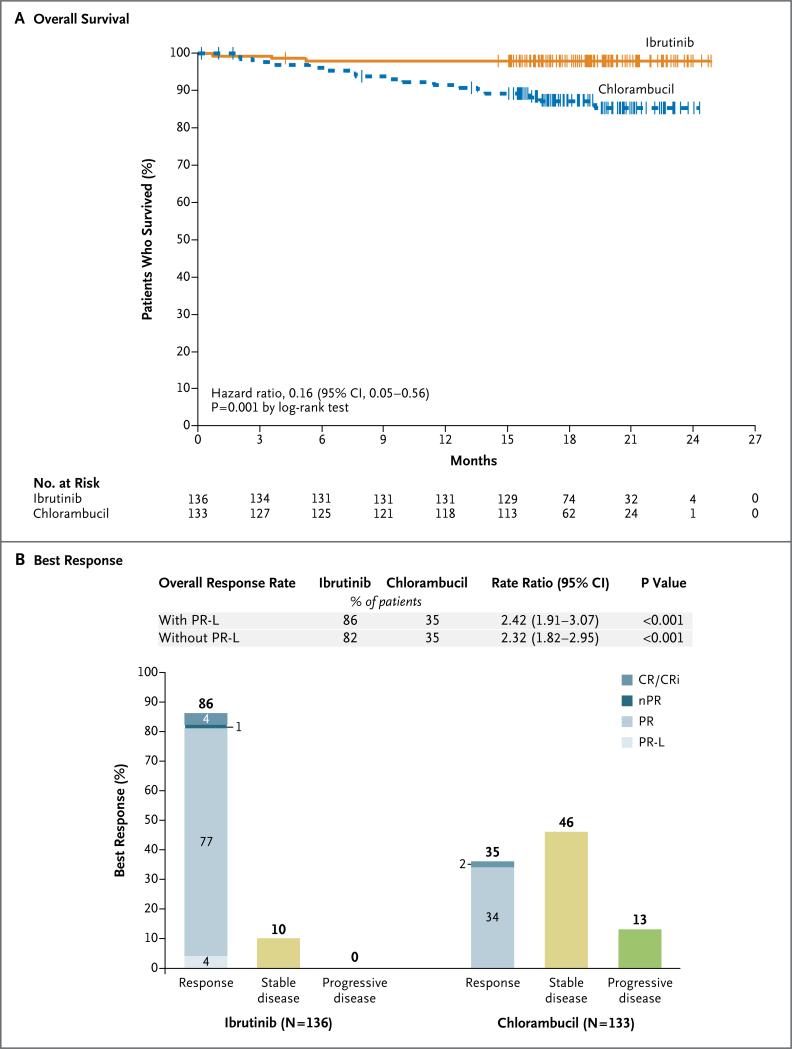
Ibrutinib significantly prolonged overall survival (median, not reached in either group). The overall survival rate at 24 months was 98% with ibrutinib versus 85% with chlorambucil, with a relative risk of death with ibrutinib that was 84% lower than that with chlorambucil (hazard ratio, 0.16; 95% CI, 0.05 to 0.56; P = 0.001) (Fig. 2A).
Figure 2. Overall Survival and Response Rates with Ibrutinib versus Chlorambucil.
Shown are overall survival with ibrutinib versus chlorambucil (Panel A) and the best response to treatment as assessed by the independent review committee (Panel B). The tick marks indicate patients with censored data. Categories for response assessments included complete response (CR) or complete response with incomplete blood-count recovery (CRi), nodular partial response (nPR; according to the International Workshop on Chronic Lymphocytic Leukemia criteria for response,28 nPR was defined as a complete response with lymphoid nodules in the bone marrow), partial response (PR), partial response with lymphocytosis (PR-L), stable disease, and progressive disease. In the ibrutinib group, five patients (4%) had a complete response and one (1%) had a complete response with incomplete blood-count recovery. In the chlorambucil group, two patients (2%) had a complete response. Data were unknown, missing, or could not be evaluated for six patients in the ibrutinib group and for eight in the chlorambucil group. The rate ratios and P values are based on the Cochran–Mantel–Haenszel chi-square test, stratified according to ECOG performance-status score (0 or 1 vs. 2) and disease stage (Rai stage ≤II vs. III or IV). Percents may not sum as expected owing to rounding.
Over the median follow-up of 18.4 months, 3 patients in the ibrutinib group died, as compared with 17 in the chlorambucil group. The 3 patients in the ibrutinib group who died included 1 who died from a klebsiella infection and 2 who died from unknown causes (Table S3 in the Supplementary Appendix). Among the 17 patients in the chlorambucil group who died, the most common causes were progressive disease and infection. None of the patients in the ibrutinib group who had disease that progressed died during follow-up.
Response
The response rate as assessed by the independent review committee was significantly higher in the ibrutinib group than in the chlorambucil group (86% vs. 35%) (Fig. 2B); 4% of the patients in the ibrutinib group had a partial response with lymphocytosis. Details regarding the frequency and duration of lymphocytosis with ibrutinib are provided in Table S4 in the Supplementary Appendix. Complete responses (including those in patients with incomplete blood-count recovery) occurred in 4% of the patients in the ibrutinib group and in 2% of those in the chlorambucil group (Fig. 2B).
Hematologic Variables
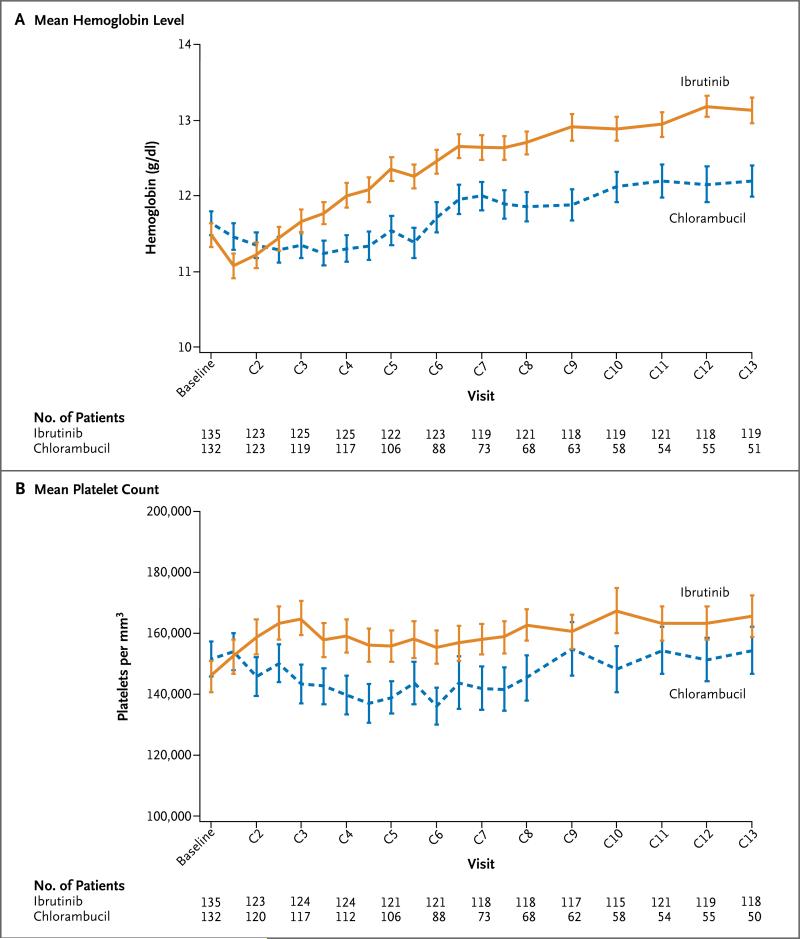
The rates of sustained improvement in hemato-logic variables were significantly higher with ibrutinib than with chlorambucil (Table S5 in Supplementary Appendix). Among patients with anemia at baseline, a significantly higher proportion of patients in the ibrutinib group than in the chlorambucil group had sustained improvement in the hemoglobin level (84% vs. 45%, P<0.001) (Table S6 in the Supplementary Appendix). Similarly, among patients who had thrombocytopenia at baseline, a significantly higher proportion of patients in the ibrutinib group than in the chlorambucil group had sustained improvement in the platelet count (77% vs. 43%, P = 0.005). Changes in hematologic variables over time are shown in Figure 3.
Figure 3. Hematologic Variables over Time in the Safety Population.
Shown are the mean hemoglobin values (Panel A) and mean platelet counts (Panel B) over time in the safety population in each treatment group. The safety population included all patients who received at least one dose of the study drug. Each tick mark represents day 1 of the cycle (C). The baseline measurement was the last measurement on or before day 1 of the first cycle. I bars represent standard errors.
Safety
The most common adverse events, defined as those that occurred in 15% or more of the patients in either treatment group, are shown in Table 2 and in Table S7 in the Supplementary Appendix. The median period of exposure to the study treatment was 17.4 months (range, 0.7 to 24.7) in the ibrutinib group versus 7.1 months (range, 0.5 to 11.7) in the chlorambucil group, hence the corresponding collection period for adverse-event data was longer in the ibrutinib group. In the ibrutinib group, diarrhea was the most frequent adverse event (in 42% of the patients, including grade 3 diarrhea in 4%) (Table S7 in the Supplementary Appendix). Other adverse events that occurred in 20% or more of the patients in the ibrutinib group were fatigue, nausea, and cough. In the chlorambucil group, nausea, fatigue, neutropenia, anemia, and vomiting were observed in 20% or more of the patients; all these events occurred at a higher frequency in the chlorambucil group than in the ibrutinib group (Table S7 in the Supplementary Appendix).
Table 2.
Adverse Events and Duration of Treatment.
| Variable | Ibrutinib (N = 135) | Chlorambucil (N = 132) |
|---|---|---|
| Duration of treatment — mo | ||
| Median | 17.4 | 7.1 |
| Range | 0.7–24.7 | 0.5–11.7 |
| Most common adverse event of any grade — no. of patients (%)* | ||
| Diarrhea | 57 (42) | 22 (17) |
| Fatigue | 41 (30) | 50 (38) |
| Cough | 30 (22) | 20 (15) |
| Nausea | 30 (22) | 52 (39) |
| Peripheral edema | 25 (19) | 12 (9) |
| Dry eye | 23 (17) | 6 (5) |
| Arthralgia | 22 (16) | 9 (7) |
| Neutropenia | 21 (16) | 30 (23) |
| Vomiting | 18 (13) | 27 (20) |
| Adverse event of grade ≥3 — no. of patients (%)† | ||
| Neutropenia | 14 (10) | 24 (18) |
| Anemia | 8 (6) | 11 (8) |
| Hypertension | 6 (4) | 0 |
| Pneumonia | 5 (4) | 2 (2) |
| Diarrhea | 5 (4) | 0 |
| Maculopapular rash | 4 (3) | 2 (2) |
| Decreased platelet count | 4 (3) | 1 (1) |
| Abdominal pain | 4 (3) | 1 (1) |
| Hyponatremia | 4 (3) | 0 |
| Thrombocytopenia | 3 (2) | 8 (6) |
| Febrile neutropenia | 3 (2) | 3 (2) |
| Upper respiratory tract infection | 3 (2) | 2 (2) |
| Pleural effusion | 3 (2) | 1 (1) |
| Cellulitis | 3 (2) | 0 |
| Fatigue | 1 (1) | 7 (5) |
| Syncope | 1 (1) | 3 (2) |
| Hemolytic anemia | 0 | 3 (2) |
| Serious adverse event — no. of patients (%)† | ||
| Pneumonia | 5 (4) | 2 (2) |
| Basal-cell carcinoma | 5 (4) | 0 |
| Hyponatremia | 3 (2) | 0 |
| Pyrexia | 1 (1) | 5 (4) |
The events listed are adverse events of any grade that occurred in at least 15% of patients in either treatment group and for which the frequency differed between treatment groups by at least 5%.
The events listed are adverse events of grade 3 or higher or serious adverse events that occurred in at least 2% of the patients in either treatment group. One death due to toxic hepatitis in the chlorambucil group was considered by the investigator to be possibly related to the study treatment; no other deaths were considered by the investigator to be related to the study treatment.
Discontinuation of treatment owing to adverse events occurred less frequently in the ibrutinib group than in the chlorambucil group (in 9% vs. 23% of the patients). Adverse events of grade 3 or higher and serious adverse events are listed in Table 2. Hypertension was observed in 14% of the patients in the ibrutinib group, with grade 3 hypertension occurring in 4% and no events of grade 4 or 5. All six patients with grade 3 hypertension were treated with antihypertensive medication and did not require a dose reduction or discontinuation of ibrutinib. Four of these patients had a history of hypertension; blood-pressure values over time in these patients are shown in Figure S2 in the Supplementary Appendix.
Atrial fibrillation occurred in eight patients (6%) in the ibrutinib group, which was of grade 2 in six patients and grade 3 in two. Atrial fibrillation was managed with discontinuation of the study drug in two patients (1%) and without modification of the ibrutinib dose in the remaining six patients. Seven of these eight patients had a history of hypertension, coronary artery disease, or myocardial ischemia. One patient in the chlorambucil group had atrial fibrillation.
During a median of 17.4 months of exposure to ibrutinib, major hemorrhage (defined as any serious or grade 3 or higher hemorrhage or central nervous system hemorrhage of any grade) occurred in 4% of the patients in the ibrutinib group (six patients, with one having grade 2 hemorrhage, four having grade 3, and one having grade 4) (Table S8 in the Supplementary Appendix). Hemorrhage led to the discontinuation of treatment in three of these patients; three of the six patients were receiving concomitant low-molecular-weight heparin, aspirin, or vitamin E at the time of the event. Major hemorrhage in the central nervous system included one grade 4 intraparenchymal hemorrhage related to transformation of an ischemic stroke in a patient with diabetes and hypertension and one grade 3 post-traumatic subdural hematoma. Major hemorrhage occurred in 2% of the patients in the chlorambucil group over the 7.1-month period of exposure.
Discussion
In this randomized study involving older patients with previously untreated CLL or small lymphocytic lymphoma, ibrutinib was superior to chlorambucil with respect to progression-free and overall survival, response rate, and improvement in hematologic variables. The relative risk of progression was 84% lower and the relative risk of death was also 84% lower with ibrutinib than with chlorambucil. Ibrutinib toxicity was modest in the majority of patients, with 87% of the patients continuing to take the single-agent therapy at a median follow-up of 18.4 months.
All current standards for first-line CLL therapy are based on cytotoxic chemotherapy, including alkylating agents, purine analogues, or combinations thereof, except for patients with chromosome 17p13.1 deletion, for whom ibrutinib is a primary consideration for first-line therapy according to consensus guidelines.16,17,31,32 In addition to their myelosuppressive effects, these cytotoxic chemotherapy approaches may be associated with expansion of subclones with high-risk genetic abnormalities (e.g., TP53 or NOTCH1 mutation)33-35 and an increased risk of secondary cancers, including treatment-related myelodysplasia and acute myeloid leukemia.36,37
When this study was initiated, single-agent chlorambucil was considered to be a standard first-line treatment in older patients with CLL.1,31,38,39 Phase 3 studies have only recently shown improvement in outcomes when chlorambucil is coadministered with anti-CD20 monoclonal antibodies.9,10 Depending on the anti-CD20 agent used in these combinations, the median progression-free survival has been reported as 16.3 months (with rituximab and chlorambucil),11 22.4 months (with ofatumumab and chlorambucil),10 and 29.9 months (with obinutuzumab and chlorambucil).11 The addition of an anti-CD20 agent that requires a slow infusion has been associated with infusion reactions of grade 3 or higher (in 4 to 20% of patients) and with higher rates of neutropenia of grade 3 or higher (in 27 to 35%) than have been observed with chlorambucil alone.9,10
Similar to results observed in patients with relapsed disease, the finding of a positive effect of ibrutinib on progression-free survival in the current study was seen in high-risk subgroups, including patients with Rai stage III or IV disease, those with chromosome 11q22.3 deletion, and those with unmutated IGHV. At 18 months, the rate of progression-free survival with ibrutinib as assessed by the independent review committee was 90%, and the rate as assessed by the investigator was 94%; the median progression-free survival with ibrutinib could not be estimated owing to the small number of progression events. The median progression-free survival of 18.9 months with chlorambucil that was observed in this study appears to be generally longer than that reported in previous trials with chlorambucil in previously untreated patients, in which the median progression-free survival ranged from 8.3 to 20.0 months.3-5,8,10,11 The relatively strong performance of chlorambucil in the current study may have been influenced, in part, by a generally longer exposure to chlorambucil than was used in earlier trials involving previously untreated patients with CLL or by the exclusion of patients with chromosome 17p13.1 deletion (typically 5 to 10% of previously untreated patients with CLL).
Ibrutinib substantially improved overall survival, with an overall survival rate of 98% at 24 months, a finding that is consistent with the 97% rate reported in a phase 2 study of ibrutinib with 3 years of follow-up.27 In these two studies, deaths (3 deaths among 136 patients and 1 death among 31 patients, respectively) were limited to the early part of follow-up with a relative plateau in the survival curve thereafter. The magnitude of the difference in overall survival with ibrutinib as compared with chlorambucil (hazard ratio for death, 0.16) was greater than that observed in studies assessing the addition of anti-CD20 agents to chlorambucil (hazard ratio, 0.47 in one study11 and 0.91 in another study10). Given the availability of crossover for patients who had disease that progressed during chlorambucil treatment, the prolongation of overall survival, which was a major benefit in this study, suggests that patients have benefits with first-line ibrutinib treatment possibly owing to reduced CLL-related or treatment-related mortality before the initiation of second-line therapy. These findings suggest that better results with ibrutinib might be obtained when it is used as first-line treatment rather than for later relapses or in patients with refractory disease.
The response rate was significantly higher with ibrutinib than with chlorambucil (86% vs. 35%). On the basis of results from an early-phase study,27 the rate of complete response is likely to increase with continued ibrutinib therapy. Furthermore, ibrutinib-treated patients had a restoration of bone marrow function, with a significantly higher rate of sustained improvement in hematologic variables. This finding has particular clinical relevance because bone marrow failure is a common cause of complications in patients with CLL, with anemia and thrombocytopenia being frequent indications for initiating treatment in this population.28
The safety of ibrutinib in this older population of patients with CLL who often had clinically significant coexisting conditions (Table 1) was consistent with that in previous reports. Exposure to treatment and adverse-event follow-up was nearly 2.5 times as long with ibrutinib as with chlorambucil. Similar to findings in previous reports about ibrutinib, major hemorrhage was observed in 4% of the patients, with no fatal events, and atrial fibrillation occurred in 6%, with the majority of the events (in six of eight patients) being grade 2 events that were observed over the period of 1.5 years while the patients were taking ibrutinib. Hypertension was reported more frequently with ibrutinib than with chlorambucil, with no events leading to dose modification or having a severity of grade 4 or 5. The rates of fatigue, nausea, vomiting, and myelosuppression were higher with chlorambucil than with ibrutinib. Early discontinuation of treatment due to adverse events was more than twice as frequent with chlorambucil as with ibrutinib.
In conclusion, in this older population of patients with CLL, many of whom had coexisting conditions, oral ibrutinib was administered continuously with a safety profile consistent with that in prior reports, which permitted the vast majority of patients to continue taking the treatment at the completion of the study. As compared with chlorambucil, a standard cytotoxic chemotherapy, ibrutinib was associated with significantly longer progression-free survival and overall survival and with higher rates of response and improvement in hematologic variables among patients with previously untreated CLL or small lymphocytic lymphoma.
Supplementary Material
Acknowledgments
Supported by Pharmacyclics, by grants (CA016672 and 5P01CA081534-14) from the National Institutes of Health, and by the MD Anderson Moon Shot Program in CLL. Dr. Burger is a Scholar of the Leukemia and Lymphoma Society.
Dr. Burger reports receiving fees for serving on advisory boards from Janssen and grant support from Pharmacyclics, Gilead Sciences, and Portola Pharmaceuticals; Dr. Barr, receiving consulting fees from Pharmacyclics and AbbVie; Dr. Owen, receiving honoraria and fees for serving on advisory boards from Janssen, Gilead Sciences, Roche, and Lundbeck; Dr. Ghia, receiving fees for serving on advisory boards from AbbVie, Gilead Sciences, H3 Biomedicine, Janssen, Pharmacyclics, and Roche, consulting fees from Adaptive Biotechnologies, lecture fees from Gilead Sciences and Janssen, and grant support from Gilead Sciences, GlaxoSmithKline, and Roche; Dr. Hillmen, receiving lecture fees from Pharmacyclics; Dr. Bartlett, receiving fees for serving on advisory boards from Gilead Sciences and Seattle Genetics; Dr. Gaidano, receiving fees for serving on advisory boards from Janssen, Roche, Amgen, Novartis, GlaxoSmithKline, and Karyopharm Therapeutics, and grant support from Celgene; Dr. Siddiqi, receiving lecture fees from Pharmacyclics and Janssen; Dr. Tam, receiving honoraria from Janssen; Ms. Suri, Dr. Cheng, Dr. Clow, Dr. Styles, and Dr. James, being employees of Pharmacyclics; and Dr. Clow, Dr. Styles, and Dr. James, holding stock in AbbVie. No other potential conflict of interest relevant to this article was reported.
We thank all the patients who participated in this trial and their supportive families; Cathy Zhou, M.S., of Pharmacyclics, for statistical analysis support; and Maoko Naganuma Carter, M.Sc., C.M.P.P., for medical writing support in the preparation of an earlier version of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi50–vi54. doi: 10.1093/annonc/mdr377. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not re sult in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–91. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 4.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 5.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 6.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–7. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 7.Woyach JA, Ruppert AS, Rai K, et al. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential Cancer and Leukemia Group B studies. J Clin Oncol. 2013;31:440–7. doi: 10.1200/JCO.2011.41.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–9. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 9.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 10.Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385:1873–83. doi: 10.1016/S0140-6736(15)60027-7. [DOI] [PubMed] [Google Scholar]
- 11.Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29:1602–4. doi: 10.1038/leu.2015.14. [DOI] [PubMed] [Google Scholar]
- 12.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 13.Eichhorst B, Fink A-M, Busch R, et al. Chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus bendamustine and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): results of a planned interim analysis of the CLL10 trial, an international, randomized study of the German CLL Study Group (GCLLSG). Blood. 2013;122:526. abstract. [Google Scholar]
- 14.Casak SJ, Lemery SJ, Shen YL, et al. U.S. Food and Drug Administration approval: rituximab in combination with fludarabine and cyclophosphamide for the treatment of patients with chronic lymphocytic leukemia. Oncologist. 2011;16:97–104. doi: 10.1634/theoncologist.2010-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imbruvica prescribing information. Pharmacyclics; Sunnyvale, CA: May, 2015. ( https://www.janssenmd.com/pdf/imbruvica/PI-Imbruvica.pdf) [Google Scholar]
- 17.Imbruvica summary of product characteristics. European Medicines Agency; London: Oct, 2014. ( http://ec.europa.eu/health/documents/community-register/2014/20141021129815/anx_129815_en.pdf) [Google Scholar]
- 18.de Gorter DJ, Beuling EA, Kersse-boom R, et al. Bruton's tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 19.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 20.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaargaren M, Beuling EA, Rurup ML, et al. The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J Exp Med. 2003;198:1539–50. doi: 10.1084/jem.20011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger JA, Li K, Keating M, et al. Functional evidence from deuterated water labeling that the Bruton tyrosine kinase inhibitor ibrutinib blocks leukemia cell proliferation and trafficking and promotes leukemia cell death in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma. Blood. 2014;124:326. abstract. [Google Scholar]
- 24.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-κB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–95. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman SE, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28:2188–96. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallek M, Cheson BD, Catovsky D, et al. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. Blood. 2012 Jun 4; ( http://www.bloodjournal.org/content/111/12/5446.e-letters)
- 30.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03. National Institutes of Health; Bethesda, MD: 2010. ( http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf) [Google Scholar]
- 31.NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's Lymphoma version 1.2015. National Comprehensive Cancer Network; Fort Washington, PA: 2015. ( www.nccn.org/professionals/physician_gls/pdf/nhl.pdf) [DOI] [PubMed] [Google Scholar]
- 32.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16:169–76. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landau DA, Carter SL, Stojanov P, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–26. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stilgenbauer S, Sander S, Bullinger L, et al. Clonal evolution in chronic lymphocytic leukemia: acquisition of high-risk genomic aberrations associated with un-mutated VH, resistance to therapy, and short survival. Haematologica. 2007;92:1242–5. doi: 10.3324/haematol.10720. [DOI] [PubMed] [Google Scholar]
- 35.Jethwa A, Hüllein J, Stolz T, et al. Targeted resequencing for analysis of clonal composition of recurrent gene mutations in chronic lymphocytic leukaemia. Br J Haematol. 2013;163:496–500. doi: 10.1111/bjh.12539. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received front-line fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–50. doi: 10.3109/10428194.2014.957203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, Cancer and Leukemia Group B 9011. J Clin Oncol. 2002;20:3878–84. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 38.Ghielmini M, Vitolo U, Kimby E, et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia (CLL). Ann Oncol. 2013;24:561–76. doi: 10.1093/annonc/mds517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gribben JG. How I treat CLL up front. Blood. 2010;115:187–97. doi: 10.1182/blood-2009-08-207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.