Abstract
Purpose
The purpose of this study was to assess whether providing medication adherence information with or without motivational interviewing improves diabetes and lipid control.
Methods
Study participants were adult members of a health system in southeast Michigan, were using both oral diabetes and lipid-lowering medications, and had glycated hemoglobin (HbA1C) or low-density lipoprotein cholesterol (LDL-C) levels not at goal. Participants were randomly assigned to receive usual care (UC) – n=567; have medication adherence information provided to their physician (AI) – n=569; or have AI and receive motivational interviewing though trained staff (AI + MI) – n=556. Primary outcomes were HbA1C and LDL-C levels at 18 months post-randomization.
Results
Primary outcomes were not significantly different between patients in the AI or AI + MI study arms when compared with UC. Similarly, neither oral diabetes nor lipid-lowering medication adherence was significantly different between groups. Patient participation in the AI + MI arm was low and limit the interpretation of the study results, but post-hoc analysis of the AI + MI study arm showed that the number of MI sessions received was positively associated with only oral diabetes medication adherence.
Conclusion
Neither AI nor MI significantly improved diabetes and lipid control when compared with UC. Moreover, patient participation appeared to be a particular barrier for MI. (ClinicalTrials.gov identifier: NCT00754741)
Keywords: Medication Adherence, Motivational Interviewing, Behavior Therapy, Randomized Controlled Trial, Diabetes Mellitus, Type 2, Hypercholesterolemia
Non-adherence is common among patients who take prescription medications chronically.1, 2 It has been previously shown that approximately 40% of patients with hyperlipidemia and diabetes use less than 80% of their prescribed dose of medications,3 and these adherence estimates comport the findings of others.4–7 Non-adherence to glucose lowering drugs among patients with diabetes increases the risk of hospitalization and mortality8, 9 and healthcare costs.10
Interventions to improve medication adherence for chronic conditions are often disappointingly ineffective, especially when one considers the degree of change observed for the amount of resources invested.11, 12 Some studies suggest that providing patients feedback on their level of medication use13, 14 and having them share in treatment decisions15 may increase adherence, while other studies have shown that providing adherence information to patients or physicians has limited impact on adherence.16–18
Motivational interviewing (MI) is a non-confrontational, patient-centered technique to elicit positive behavioral change.19 Reflective listening is used to guide patients to identify discrepancies between their current behavior and their broader personal values.20 The technique also attempts to promote patient motivation and self-confidence (i.e., self-efficacy) to make these changes.21 Therefore, MI encourages patient-driven behavioral change and may be useful in improving medication adherence, as has been suggested in a number of small studies.22–24 The effectiveness of MI on improving diabetes outcomes is not known. Although small studies seem to indicate potential benefits,25 recent trials in clinical settings and with longer durations of follow-up have failed to demonstrate positive results.26–28 The overall effectiveness of MI in improving diabetes medication adherence is also not clear.29, 30
Given the importance of medication adherence to diabetes control, perhaps a combined approach, such as providing medication adherence information to clinicians and administering MI to patients, is warranted. The primary goal of this study was to assess whether providing AI with or without MI resulted in improved diabetes and lipid control as reflected in glycated hemoglobin (HbA1C) and low-density lipoprotein cholesterol (LDL-C) levels, respectively. Secondary outcomes included changes in oral diabetes medication adherence, changes in lipid-lowering medication adherence, and the proportion of individuals in each study arm experiencing a major atherosclerotic disease (AD) event.
METHODS
Study population and setting
The Multi-arm Intervention Diabetes Adherence Study was approved by the Institutional Review Board of Henry Ford Health System; the study was also in compliance with the health system’s Health Insurance Portability and Accountability Act policy. Data are presented in accordance with the CONSORT guidelines.31 Study participants were members of a large health system serving southeast Michigan and metropolitan Detroit. Between July 1, 2007 and January 1, 2008, potential eligible participants meeting the following criteria were identified: age ≥18 years, a member the health plan with prescription drug coverage in both 2007 and 2008, ≥1 HbA1C measurement with the last value ≥7%, ≥1 LDL-C measurement with the last value ≥100 mg/dL, and ≥1 prescription for both an oral diabetes medication and a lipid-lowering medication. Patients were not eligible to participate if they had been in hospice care or hospitalized ≥90 days, if they were participating in any other study involving diabetes management or medication adherence, or if their primary care provider did not consent to be part of the study. Eligible patients were sent a letter inviting them to participate, and those who refused were not eligible for randomization.
Study design and randomization
Patients receiving treatment for both diabetes and hyperlipidemia were randomized to one of the three following arms: 1) usual care (UC), 2) adherence information provided to clinicians (AI) to discuss with patients, and 3) adherence information provided to clinicians to discuss with patients and motivational interviewing provided directly to patients via an “adherence clinic” (AI + MI). The adherence clinic consisted of nurses and pharmacists trained in providing MI.
In order to meet recruiting goals, two different waves of patient recruitment were implemented on June 19, 2008 and October 13, 2008. Study individuals were randomized within blocks of 3 (i.e., each block of 3 patients had individuals assigned to all 3 arms in random order) within strata defined by the patient’s provider. To achieve a nearly equal distribution of patients assigned to each study arm for each provider, a two-step randomization process was used. A random number generator was first used to randomly sort each participating physician’s list of enrolled patients. The order of treatment arm assignment was then randomly selected for each physician’s patient list of participating patients. The team statistician notified the project coordinator, who provided this information on treatment assignment to the clinical team.
For individuals randomized to arms 2 and 3, medication adherence was calculated (as described below) using electronic prescription and fill information. These adherence measures were then provided as an automated electronic message to the patient’s primary care physicians via the electronic prescription writing application, Rcopia (DrFirst Inc., Rockville, MD). Participating clinicians could view these data, along with the latest HbA1C and LDL-C measurements, at the time of reviewing, writing, and refilling prescriptions. Physicians interested in more information could also view trends in a patient’s medication adherence over time by drug class. Clinicians receiving this information were given instruction on how to interpret and discuss these with their patients.
Participants enrolled in study arm 3 received additional support through an “adherence clinic.” The clinic consisted of nurses and pharmacists (i.e., “coaches”) trained in using MI to promote medication adherence. Training included simulated encounters with standardized patients and didactic lessons. Simulated patient encounters were recorded and an independent reviewer rated each interaction regarding the correct application of MI techniques. Six coaches (3 pharmacists and 3 registered nurses) passed the training; four of these coaches were also certified diabetes educators. Individuals randomized to receive arm 3 had up to six sessions with an adherence coach. The initial session involved either a face-to-face visit or a phone discussion, and the subsequent 5 sessions involved a phone discussion. Follow-up sessions were scheduled to occur every 3 months. In addition to using MI, coaches had delegated authority to make medication adjustments. Because the study design involved interaction between the research team, adherence clinic staff, and patients, none of these groups were blinded to study arm assignment.
Ascertainment of study outcomes
Laboratory results, medication adherence, and major atherosclerotic disease events were obtained through available electronic data sources, including the electronic medical record, pharmacy claims, and encounter codes (i.e., diagnostic and procedural codes). Glycated hemoglobin levels were reported in both National Glycohemoglobin Standardization Program (NGSP) units and International Federation of Clinical Chemistry (IFCC) units.32 The method of estimating medication adherence using pharmacy claims has been previously described.3 In order to assess medication adherence at the various time points post-randomization (i.e., 6, 12, and 18 months), medication use for the 3 months preceding each of these follow-up time points was assessed. The total days’ supply of medication was obtained from pharmacy claims. To prevent under- and over-counting the total days’ supply, the proportion of a prescription which predated but extended into the 3-month observation period was added in, and the proportion that extended past the 3-month observation block was prorated. Adherence was estimated as the total days’ supply in the 3-month period divided by then number of days of observation (i.e., 90 days).
As previously described,33 major AD events were identified through encounter diagnoses and procedural codes in the electronic medical record, as well as death data from the Michigan Department of Community Health. Individuals were considered to have a history of atherosclerotic disease if they had a prior diagnosis of coronary heart disease (i.e., myocardial infarction, unstable angina, or coronary revascularization), cerebrovascular disease (i.e., a cerebrovascular accident, a transient ischemic attack, or carotid revascularization), or peripheral vascular occlusive disease at any time prior to randomization. Programmers blinded to the treatment assignment of study individuals performed outcome ascertainment using the aforementioned electronic data sources.
Statistical analysis
The primary study outcome was HbA1C levels and LDL-C levels at 18 months following randomization. Secondary outcomes included the following post-randomization: HbA1C and LDL-C levels at 6 and 12 months; adherence to oral diabetes medications at 6, 12, and 18 months; adherence to lipid lowing medications at 6, 12, and 18 months; and major AD events at 18, 24, and 36 months. The composite outcome encompassing major AD events included coronary heart disease (CHD) events (i.e., unstable angina [USA], fatal and non-fatal acute myocardial infarction [AMI], coronary revascularization), cerebrovascular events (i.e., transient ischemic attack [TIA], fatal and non-fatal cerebrovascular accident [CVA], and cerebrovascular revascularization), and peripheral vascular occlusive disease (PVOD) events (i.e., hospitalization, revascularization, amputation, or death).
For samples size calculations, it was assumed that ~50% of individuals in the AI + MI group would not have contact with the adherence clinic. Moreove, it was estimated that 550 individuals in each study arm (with an effective size of 275 individuals in the AI + MI arm) would provide 80% power to detect differences of 0.3% in HbA1C and 6.1 mg/dL in LDL-C between UC and AI and differences of 0.4% in HbA1C and 7.4 mg/dL in LDL-C between UC and AI + MI.
Baseline characteristics of study participants were compared using chi-squared analyses for categorical variables and analysis of variance for normally distributed continuous variables. Analyses of study outcomes were performed according to intention-to-treat. For individuals who were lost to follow-up, the last available values were carried forward. For major AD events, it was assumed that individuals did not have any events following the point at which they were lost to follow-up. Analysis of variance was used to compare differences in HbA1C, LDL-C, adherence to diabetes medications, and adherence to lipid-lowering medications between study arms. The number of atherosclerotic disease events per study arm was compared using a chi-squared test. Logistic regression analysis was used to assess for goal attainment (i.e., the dichotomous characterization of study outcomes – HbA1C <7.0%, LDL-C levels <100 mg/dL, and medication adherence >80%) and for differences in major AD events between study arms.
Several post-hoc analyses were performed. Spearman’s rank correlation and linear regression were used to analyze the relationship between the number of MI sessions completed and study outcomes at the various follow-up time intervals. A “per protocol” analysis was performed including only individuals who continued to be in the study at each follow-up time interval (i.e., without carrying forward data). The primary and secondary outcomes were reanalyzed adjusting for demographic and baseline characteristics. Lastly, the number of MI sessions was analyzed for correlation with changes in study outcomes.
All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).34 Significance thresholds were adjusted to account for number of analyses, and thus, a type-I α of 0.0125 was selected for the analyses of the primary outcomes.
RESULTS
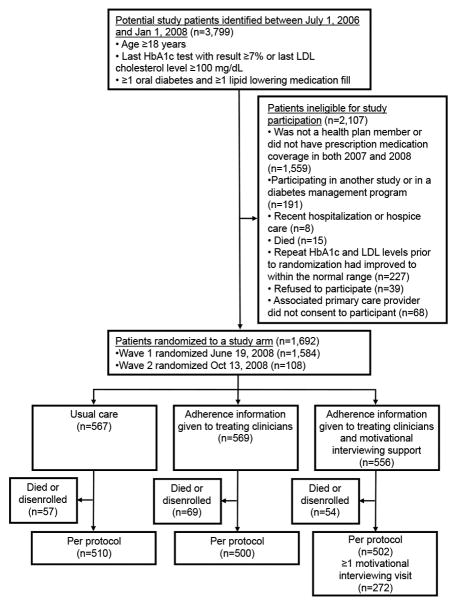
There were 3,799 individuals who met the eligibility criteria between July 1, 2007 and January 1, 2008 (Figure 1). Of these, 2,107 individuals were excluded or were not eligible to participate for predefined reasons. The remaining 1,692 individuals were randomized into one of the 3 treatment arms – UC (n = 567), AI (n = 569), and AI + MI (n = 556).
Figure 1.
Flow diagram detaining patient recruitment, exclusion, enrollment, and study arm randomization for the multi-arm adherence intervention trial. Also shown are the individuals lost to follow-up due to death or health plan disenrollment after randomization. The post hoc per protocol analysis consisted of individuals who remained after removing those lost to follow-up.
The baseline characteristics of study individuals are shown in Table 1. There were small but statistically significant differences in the mean age (P=0.029), baseline HbA1C levels (P=0.036), and baseline high-density lipoprotein cholesterol levels (P=0.033) between study arms. Baseline medication adherence was similar for individuals in the UC, AI, and AI + MI arms at 75.5%, 75.9%, and 74.8%, respectively for oral diabetes medication (P=0.883) and 71.4%, 69.7%, and 69.4%, respectively for lipid-lowering medication (P=0.633).
Table 1.
Baseline characteristics of individuals randomized to each study arm
| Variable | Adherence intervention group | P-value | ||
|---|---|---|---|---|
| UC (n=567) | AI (n=569) | AI + MI (n=556) | ||
| Age (years) – mean ± SD | 64.9 ± 11.5 | 63.3 ± 10.9 | 64.5 ± 10.5 | 0.029 |
| Female – no (%) | 302 (53) | 270 (47) | 266 (48) | 0.092 |
| Race-ethnicity – no (%) | ||||
| African American | 224 (40) | 237 (42) | 238 (43) | 0.726 |
| White | 297 (52) | 294 (52) | 281 (51) | |
| Other or unknown | 46 (8) | 38 (7) | 37 (7) | |
| HbA1C (%) – mean ± SD (in mmole/mole)† | 8.0 ± 1.4 (63.9 ± 15.3) | 8.2 ± 1.4 (66.1 ± 15.3) | 8.0 ± 1.3 (63.9 ± 14.2) | 0.036* |
| LDL-C (mg/dL) – mean ± SD† | 99.9 ± 31.4 | 98.5 ± 33.8 | 97.1 ± 31.5 | 0.362 |
| HDL-C (mg/dL) – mean ± SD† | 46.3 ± 12.2 | 45.1 ± 11.8 | 44.5 ± 11.6 | 0.033* |
| Serum creatinine (mg/dL) – mean ± SD† | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.9 | 0.138 |
| Systolic blood pressure (mmHg) – mean ± SD | 133.6 ± 15.4 | 132.3 ± 15.3 | 133.2 ± 15.8 | 0.457 |
| Diastolic blood pressure (mmHg) – mean ± SD | 74.8 ± 10.0 | 75.6 ± 9.3 | 75.0 ± 9.2 | 0.451 |
| Body mass index (kg/m2) – mean ± SD | 33.6 ± 6.4 | 33.4 ± 6.8 | 33.7 ± 7.2 | 0.846 |
| History of atherosclerotic disease – no (%) | 66 (12) | 59 (10) | 59 (11) | 0.766 |
| Insulin Use – no (%) | 187 (33) | 191 (34) | 192 (35) | 0.860 |
| Oral hypoglycemic medication used – no (%) | ||||
| Biguanide | 335 (59) | 364 (64) | 347 (62) | 0.234 |
| Thiazolidinediones | 78 (14) | 85 (15) | 73 (13) | 0.673 |
| Sulfonylureas | 327 (58) | 341 (60) | 337 (61) | 0.575 |
| Alpha-glucosidase Inhibitors | 5 (1) | 4 (1) | 3 (1) | 0.792 |
| Meglitinides | 8 (1) | 3 (1) | 2 (<1) | 0.094 |
| Lipid-lowering medication used – no (%) | ||||
| Statins | 446 (79) | 452 (79) | 443 (80) | 0.908 |
| Ezetimibe | 73 (13) | 77 (14) | 90 (16) | 0.243 |
| Fibrates | 59 (10) | 51 (9) | 50 (9) | 0.628 |
| Niacin | 7 (1) | 6 (1) | 5 (1) | 0.861 |
| Bile acid sequestrants | 9 (2) | 4 (1) | 3 (1) | 0.147 |
| Adherence – oral diabetes medication – mean ± SD‡ | 75.5 ± 35.4 | 75.9 ± 35.1 | 74.8 ± 36.1 | 0.883 |
| Adherence – lipid-lowering medication – mean ± SD‡ | 71.4 ± 37.0 | 69.7 ± 37.4 | 69.4 ± 37.4 | 0.633 |
UC denotes usual care; AI, adherence information given to treating primary care clinicians; AI + MI, adherence information given to treating primary care clinicians and motivational interviewing administered through an adherence clinic; SD, standard deviation; HbA1C, glycated hemoglobin; mmole/mole, millimole of HbA1C per mole of hemoglobin; and LDL-C, low density lipoprotein cholesterol.
P-value<0.05 for the comparison across all 3 treatment arms.
Represents the last measured laboratory value in the year prior to randomization.
Adherence was measured on a scale of 0–100, representing the estimated proportion of medication taken as prescribed in the 3-month period prior to randomization.
Neither of the primary outcomes, HbA1C and LDL-C levels at 18 months post-randomization, were significantly different for AI or AI + MI when compared with UC (Table 2). Similarly, none of the secondary outcomes were significantly different for AI or AI + MI when compared with UC. These secondary outcomes included HbA1C and LDL-C levels at other time points (i.e., 6 months and 12 months post-randomization), oral diabetes medication adherence and lipid lower medication adherence at 6, 12, and 18 months post-randomization, and the proportion experiencing a major atherosclerotic disease event by 18, 24, and 36 months post-randomization.
Table 2.
Study outcomes among individuals randomized to usual care (UC), adherence information feedback (AI), and adherence information feedback with motivational interviewing (AI + MI)
| Outcome | Adherence intervention group | AI vs. UC P-value | AI + MI vs. UC P-value | ||
|---|---|---|---|---|---|
| UC (n=567) | AI (n=569) | AI + MI (n=556) | |||
| Primary outcomes | |||||
| HbA1C (%) – mean ± SD (in mmole/mole) | |||||
| At 18 months | 7.88 ± 1.53 (62.6 ± 16.7) | 7.91 ± 1.53 (62.9 ± 16.7) | 7.79 ± 1.34 (61.6 ± 14.6) | 0.763 | 0.285 |
| LDL-C (%) – mean ± SD | |||||
| At 18 months | 89.02 ± 32.11 | 87.27 ± 35.67 | 85.56 ± 32.86 | 0.380 | 0.084 |
| Secondary outcomes | |||||
| HbA1C (%) – mean ± SD (in mmole/mole) | |||||
| At 6 months | 7.81 ± 1.42 (61.8 ± 15.5) | 7.90 ± 1.44 (62.8 ± 15.7) | 7.81 ± 1.41 (61.8 ± 15.4) | 0.291 | 0.968 |
| At 12 months | 7.94 ± 1.60 (63.3 ± 17.5) | 7.96 ± 1.54 (63.5 ± 16.8) | 7.84 ± 1.40 (62.2 ± 15.3) | 0.829 | 0.283 |
| LDL-C (%) – mean ± SD | |||||
| At 6 months | 92.92 ± 32.33 | 92.07 ± 36.68 | 91.23 ± 31.17 | 0.671 | 0.399 |
| At 12 months | 90.63 ± 32.41 | 90.70 ± 36.90 | 87.43 ± 32.43 | 0.971 | 0.115 |
| Oral diabetes medication adherence – mean ± SD | |||||
| At 6 months | 0.75 ± 0.35 | 0.74 ± 0.36 | 0.74 ± 0.37 | 0.667 | 0.530 |
| At 12 months | 0.75 ± 0.35 | 0.74 ± 0.36 | 0.74 ± 0.38 | 0.573 | 0.443 |
| At 18 months | 0.75 ± 0.36 | 0.73 ± 0.37 | 0.73 ± 0.38 | 0.469 | 0.369 |
| Lipid-lowering medication adherence – mean ± SD | |||||
| At 6 months | 0.70 ± 0.37 | 0.69 ± 0.36 | 0.69 ± 0.36 | 0.881 | 0.849 |
| At 12 months | 0.70 ± 0.36 | 0.69 ± 0.37 | 0.69 ± 0.37 | 0.779 | 0.733 |
| At 18 months | 0.70 ± 0.37 | 0.70 ± 0.37 | 0.70 ± 0.37 | 0.952 | 0.856 |
| Major atherosclerosis disease event – no. (%) | |||||
| By 18 months | 29 (5.1) | 43 (7.6) | 20 (3.6) | 0.091 | 0.213 |
| By 24 months | 63 (11.1) | 80 (14.1) | 58 (10.4) | 0.134 | 0.714 |
| By 36 months | 101 (17.8) | 112 (19.7) | 99 (17.8) | 0.419 | 0.998 |
HbA1C denotes glycated hemoglobin; SD, standard deviation; mmole/mole, millimole of HbA1C per mole of hemoglobin; and LDL-C, low density lipoprotein cholesterol.
Redefining outcomes as the likelihood of achieving goal levels for diabetes control (HbA1C <7%), lipid control (LDL-C <100 mg/dL), oral diabetes medication adherence (>80% adherence), and lipid-lowering medication adherence (>80% adherence) showed similar results (Table 3). Both the AI and AI + MI study arms were not significantly different from the UC in the likelihood of achieving treatment goals. Similarly, the likelihood of having a major atherosclerotic disease event was not significantly between arms.
Table 3.
Comparison between study arms for the achievement of treatment goals or the occurrence of major atherosclerotic events.
| Outcome | Comparison of study arms | |
|---|---|---|
| AI vs. UC OR (95% CI) | AI + MI vs. UC OR (95% CI) | |
| Achievement of HbA1C goal of <7% (<53.0 mmole/mole) | ||
| By 6 months | 0.83 (0.64, 1.08) | 0.89 (0.69, 1.16) |
| By 12 months | 1.03 (0.79, 1.33) | 0.97 (0.74, 1.25) |
| By 18 months | 0.98 (0.76, 1.27) | 0.98 (0.76, 1.28) |
| Achievement of LDL-C goal of <100 mg/dL | ||
| By 6 months | 1.09 (0.85, 1.39) | 1.09 (0.85, 1.39) |
| By 12 months | 1.05 (0.82, 1.35) | 1.18 (0.92, 1.51) |
| By 18 months | 0.99 (0.77, 1.28) | 1.04 (0.80, 1.34) |
| Achievement of oral diabetes medication adherence of >80% | ||
| By 6 months | 0.95 (0.75, 1.22) | 1.09 (0.85, 1.39) |
| By 12 months | 0.99 (0.78, 1.26) | 1.07 (0.83, 1.37) |
| By 18 months | 0.95 (0.74, 1.21) | 1.05 (0.82, 1.35) |
| Achievement of lipid-lowering medication adherence of >80% | ||
| By 6 months | 0.94 (0.74, 1.19) | 0.97 (0.76, 1.23) |
| By 12 months | 1.01 (0.80, 1.28) | 1.00 (0.79, 1.26) |
| By 18 months | 1.01 (0.79, 1.29) | 0.99 (0.78, 1.25) |
| Occurrence of a major atherosclerosis disease event | ||
| By 18 months | 1.52 (0.93, 2.47) | 0.69 (0.39, 1.24) |
| By 24 months | 1.31 (0.92, 1.86) | 0.93 (0.64, 1.36) |
| By 36 months | 1.13 (0.84, 1.52) | 1.00 (0.74, 1.36) |
UC denotes usual care; AI, adherence information given to treating primary care clinicians; AI + MI, adherence information given to treating primary care clinicians and motivational interviewing administered through an adherence clinic; OR, odds ratio; CI, confidence interval; HbA1C, glycated hemoglobin; mmole/mole, millimole of HbA1C per mole of hemoglobin; and LDL-C, low density lipoprotein cholesterol.
Of the patients randomized to UC, AI, and AI + MI, 57 patients (16 deaths and 41 health plan disenrollments), 69 patients (19 deaths and 50 health plan disenrollments), and 54 patients (23 deaths and 31 health plan disenrollments) were not available for follow-up, respectively. Analogous per protocol analyses to those above showed similar results (Tables E1 and E2 of the online supplement).
Of the 556 individuals in the AI + MI study arm, the mean number of visits by 18 months post-randomization was 1.15 (SD 1.54, range 0–6). Baseline characteristic of individuals in the AI + MI study arm who had one or more visits (n=272) were compared with those who had no visits (n=284) (data not shown). Patients without visits had significantly lower levels of adherence when compared with patients with at least a visit. Given the level of follow-up, a post-hoc analysis of individuals assigned to the AI + MI arm was performed to assess the relationship between number of adherence clinic encounters and the outcomes (Table E3 of the online supplement). There was no significant correlation between the number of adherence clinic encounters at 6, 12, and 18 months post-randomization and either HbA1C or LDL-C levels at these time points, and there was no statistically significant correlation between visits and lipid-lowering medication adherence at any time point. The only positive finding was a weak, albeit statistically significant correlation between the number of visits prior to the 12- and 18-month time points and oral diabetes mediation adherence (Spearman’s correlation coefficient [ρ] = 0.105, P=0.014 and ρ=0.094, P=0.027, respectively).
Adjusting for demographic characteristics and baseline status for each outcome also did not reveal any significant differences (Supplemental Tables E4 and E5). Finally, assessing the relationship between the number of MI sessions and the change in each study outcome did not show any significant correlations (Supplemental Table E6).
DISCUSSION
This large randomized controlled trial assessed the effects of providing patient medication adherence information to providers with and without additional motivational interviewing provided by trained staff. The results showed that neither intervention significantly improved diabetes or lipid control as measured by HbA1C and LDL-C levels, respectively.
Although studies suggest that MI may improve medication adherence35 and promote other positive behavioral changes, such as smoking cessation,36, 37 this study highlights some of the limitations inherent in administering behavior interventions in large patient populations. In particular, MI was offered without cost (and in some cases with a small monetary incentive for participation) to patients by staff trained in MI techniques. Nevertheless, patient participation was poor with slightly less than half (49%) of patients having one of more MI sessions at 18 months following randomization despite multiple attempts to engage the individuals assigned to AI + MI. Moreover, patients who might have benefited the most from the intervention (i.e., individuals with the lowest levels of adherence) were also the ones least likely to participate. Therefore, it is uncertain whether the relationship found between the number of MI sessions and medication adherence among patients in this treatment arm was a result of the behavioral technique or if these findings simply reflect a selection bias for individuals already motivated to change.
Poor patient uptake has been seen in another recent controlled intervention to use MI to improve diabetes control.38 In this study, Lakerveld and colleagues randomly assigned 314 individuals to a lifestyle intervention which included motivational interviewing and problem solving treatment administered through 6 face-to-face counseling session and 3 telephone sessions; the 308 individuals in the control arm received health brochures. Individuals in the intervention group completed a median of 2 face-to-face sessions and 2.3 telephone sessions. After 12 months of follow-up, the mean diabetes and cardiovascular risk was not significantly different between treatment arms. However, even in other well-designed, randomized studies where coaches were frequently evaluated on their MI technique and patient participation with the MI intervention was higher, investigators failed to show demonstrable improvements in diabetes control39 or medication adherence40, 41 as a result of the intervention. These prior studies, as well the current study, question the practicability and real-world effectiveness of MI to evoke robust improvements in diabetes management. On the other hand, there is some evidence to suggest that MI may contribute to improved adherence to highly active antiretroviral therapy among patients with HIV infection.42–45
Similar to an earlier study in this health system population,46 providing patient adherence information to clinicians did result in a marked improvement in medication adherence. Medication adherence information was again provided electronically so that it was visible to primary care physicians at the time of writing and renewing prescriptions. Since nearly all prescriptions were being written electronically, it was expected that this information would be seen at the time of treatment decisions and would elicit contemporaneous patient-physician discussions regarding adherence. Unfortunately, it was beyond the scope of the current study to objectively measure whether such discussions occurred or whether this information was ignored, as has been seen for other non-interruptive electronic alerts.47 Extinction or “alert fatigue” may occur when physicians are exposed to repeated alerts or have to deal with multiple competing demands.48–50 Moreover, it can be concluded that the longer study period of this intervention, as compared with the earlier trial (i.e., adherence measured out to 18 months as compared with 1 year),46 did not result in increases in physician adoption sufficient to evoke greater levels of patient medication adherence.
Other study limitations must be considered, as well. First, this study was carried out at a single, large integrated health system. Therefore, the clinical culture or patient population may not reflect those at other locations. Second, significant baseline differences were noted between patients randomized to each of the study arms. However, these differences were not likely to be clinical significant or overly influential on the study results, since adjusting for these characteristics did not result in significant outcome differences between study arms. Lastly, the measurement and frequency of the primary, laboratory outcome measures (i.e., HbA1C and LDL-C) relied on both patients seeing their PCP and physicians ordering these tests as part of routine care. While this lack of standardization may have resulted in missed opportunities to assess glycemic and lipid control, we did not observe differences in the frequency of these measurements between treatment arms.
SUMMARY AND IMPLICATIONS
This study shows that neither providing AI to physicians nor combining AI to physicians with motivational interviewing support to patients had significant impacts on diabetes and lipid control when compared with usual care. Although poor participation precluded assessing the true efficacy of the intervention as designed, the intervention was ineffective on a population level despite the allocation of considerable resources.12
Therefore, the effectiveness of MI by diabetes educators as a general method to promote better disease management remains unproven. It is important to note, however, that the collective assessment of treatment effectiveness can overshadow beneficial effects in subgroups of individuals.51 It is possible that some individuals may benefit from MI and/or repeated discussions about medication adherence, as suggested by the observed relationship number of MI sessions and diabetes medication adherence. However, preemptively identifying individuals who will benefit clinically from MI will be a challenge, since patients at highest risk (i.e., those with the lowest levels of medication adherence) appear to be the most reluctant to participate. This suggests that either additional resources (e.g., patient incentives) are needed or a different approach is required to evoke positive behavioral changes. Clearly, adherence is a complex problem with potentially multiple “phenotypes,”52 so the challenge remains to find interventions that can be tailored to individual needs, have high retention rates, result in improved clinical outcomes, and are both feasible and sustainable within the reality of current health care.12
Supplementary Material
Acknowledgments
FUNDING SOURCES
This project was made possible through funding from the National Institute of Diabetes and Digestive and Kidney (R01DK064695 to Drs. Pladevall and Williams), the National Institute of Allergy and Infectious Diseases (R01AI079139 to Dr. Williams), and the National Heart Lung and Blood Institute (R01HL079055 and R01HL118267 to Dr. Williams), National Institutes of Health and the Fund for Henry Ford Hospital (to Drs. Pladevall and Williams). The aforementioned funding organizations did not influence the study design, collection or analysis of results, interpretation of findings, or the decision to publish.
Footnotes
The authors report no relevant conflict of interest with the subject matter of this manuscript.
References
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25(6):1015–1021. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW. Cardiovascular morbidity associated with nonadherence to statin therapy. Pharmacotherapy. 2005;25(8):1035–1043. doi: 10.1592/phco.2005.25.8.1035. [DOI] [PubMed] [Google Scholar]
- 7.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Adler DA, Irish J, McLaughlin TJ, et al. The work impact of dysthymia in a primary care population. Gen Hosp Psychiatry. 2004;26(4):269–276. doi: 10.1016/j.genhosppsych.2004.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 10.Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care. 2011;49(4):378–384. doi: 10.1097/MLR.0b013e31820292d1. [DOI] [PubMed] [Google Scholar]
- 11.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 12.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Schectman JM, Schorling JB, Nadkarni MM, Voss JD. Can prescription refill feedback to physicians improve patient adherence? Am J Med Sci. 2004;327(1):19–24. doi: 10.1097/00000441-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Onyirimba F, Apter A, Reisine S, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–415. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 17.Boren SA, Puchbauer AM, Williams F. Computerized prompting and feedback of diabetes care: a review of the literature. J Diabetes Sci Technol. 2009;3(4):944–950. doi: 10.1177/193229680900300442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bambauer KZ, Adams AS, Zhang F, et al. Physician alerts to increase antidepressant adherence: fax or fiction? Arch Intern Med. 2006;166(5):498–504. doi: 10.1001/archinte.166.5.498. [DOI] [PubMed] [Google Scholar]
- 19.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. New York: Guilford; 2002. [Google Scholar]
- 20.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 21.Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the Eat for Life trial. Am J Public Health. 2001;91(10):1686–1693. doi: 10.2105/ajph.91.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14(2):52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 23.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–283. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halterman JS, Riekert K, Bayer A, et al. A pilot study to enhance preventive asthma care among urban adolescents with asthma. J Asthma. 2011;48(5):523–530. doi: 10.3109/02770903.2011.576741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins RK, McNeil DW. Review of Motivational Interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbek Minet LK, Wagner L, Lonvig EM, Hjelmborg J, Henriksen JE. The effect of motivational interviewing on glycaemic control and perceived competence of diabetes self-management in patients with type 1 and type 2 diabetes mellitus after attending a group education programme: a randomised controlled trial. Diabetologia. 2011;54(7):1620–1629. doi: 10.1007/s00125-011-2120-x. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich E, Candel MJ, Schaper NC, de Vries NK. Effect evaluation of a Motivational Interviewing based counselling strategy in diabetes care. Diabetes Res Clin Pract. 2010;90(3):270–278. doi: 10.1016/j.diabres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Lakerveld J, Bot SD, Chinapaw MJ, et al. Motivational interviewing and problem solving treatment to reduce type 2 diabetes and cardiovascular disease risk in real life: a randomized controlled trial. Int J Behav Nutr Phys Act. 2013;10:47. doi: 10.1186/1479-5868-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight K, Badamgarav E, Henning JM, et al. A systematic review of diabetes disease management programs. Am J Manag Care. 2005;11(4):242–250. [PubMed] [Google Scholar]
- 30.Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive-based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3(8):e002749. doi: 10.1136/bmjopen-2013-002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 33.Habib ZA, Tzogias L, Havstad SL, et al. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):437–447. doi: 10.1002/pds.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SAS/STAT Users Guide Version 9.2 ed. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 35.Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;(1):CD006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control. 2010;19(5):410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakerveld J, Bot SD, Chinapaw MJ, et al. Motivational interviewing and problem solving treatment to reduce type 2 diabetes and cardiovascular disease risk in real life: a randomized controlled trial. Int J Behav Nutr Phys Act. 2013;10:47. doi: 10.1186/1479-5868-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbek Minet LK, Wagner L, Lonvig EM, Hjelmborg J, Henriksen JE. The effect of motivational interviewing on glycaemic control and perceived competence of diabetes self-management in patients with type 1 and type 2 diabetes mellitus after attending a group education programme: a randomised controlled trial. Diabetologia. 2011;54(7):1620–1629. doi: 10.1007/s00125-011-2120-x. [DOI] [PubMed] [Google Scholar]
- 40.Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–483. doi: 10.1001/archinternmed.2011.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barkhof E, Meijer CJ, de Sonneville LM, Linszen DH, de Haan L. The effect of motivational interviewing on medication adherence and hospitalization rates in nonadherent patients with multi-episode schizophrenia. Schizophr Bull. 2013;39(6):1242–1251. doi: 10.1093/schbul/sbt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care. 2012;24(5):583–592. doi: 10.1080/09540121.2011.630354. [DOI] [PubMed] [Google Scholar]
- 43.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence-enhancing interventions for highly active antiretroviral therapy in HIV-infected patients - a systematic review. HIV Med. 2013;14(10):583–595. doi: 10.1111/hiv.12051. [DOI] [PubMed] [Google Scholar]
- 44.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rueda S, Park-Wyllie LY, Bayoumi AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;(3):CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams LK, Peterson EL, Wells K, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126(2):225–231. doi: 10.1016/j.jaci.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009;16(1):66–71. doi: 10.1197/jamia.M2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duke JD, Li X, Dexter P. Adherence to drug-drug interaction alerts in high-risk patients: a trial of context-enhanced alerting. J Am Med Inform Assoc. 2013;20(3):494–498. doi: 10.1136/amiajnl-2012-001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee EK, Mejia AF, Senior T, Jose J. Improving Patient Safety through Medical Alert Management: An Automated Decision Tool to Reduce Alert Fatigue. AMIA Annu Symp Proc. 2010;2010:417–421. [PMC free article] [PubMed] [Google Scholar]
- 50.Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493. doi: 10.1136/amiajnl-2012-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5(3):178–187. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- 52.Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA. 2013;309(20):2105–2106. doi: 10.1001/jama.2013.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.