Abstract
Objective
To examine the relationship between measures of subclinical atherosclerosis and subsequent cognitive function.
Method
Participants from the Dallas Heart Study (DHS), a population-based multiethnic study of cardiovascular disease pathogenesis, were re-examined 8 years later (DHS-2) with the Montreal Cognitive Assessment (MoCA); N = 1904, mean age = 42.9, range 8–65. Associations of baseline measures of subclinical atherosclerosis (coronary artery calcium, abdominal aortic plaque, and abdominal aortic wall thickness) with MoCA scores measured at follow-up were examined in the group as a whole and in relation to age and ApoE4 status.
Results
A significant linear trend of successively lower MoCA scores with increasing numbers of atherosclerotic indicators was observed (F(3, 1150) = 5.918, p = .001). CAC was weakly correlated with MoCA scores (p = .047) and MoCA scores were significantly different between participants with and without CAC (M = 22.35 vs 23.69, p = 0.038). With the exception of a small association between abdominal AWT and MoCA in subjects over age 50, abdominal AWT and abdominal aortic plaque did not correlate with MoCA total score (p ≥.052). Cognitive scores and atherosclerosis measures were not impacted by ApoE4 status (p ≥.455).
Conclusion
In this ethnically diverse population-based sample, subclinical atherosclerosis was minimally associated with later cognitive function in middle-aged adults.
Keywords: Montreal Cognitive Assessment, MoCA, Cognition, Atherosclerosis, Dallas heart study
1. Introduction
A relationship between vascular risk factors and the onset and progression of cognitive dysfunction is well established, in large part by the well-known Framingham Study [1] and others [2–5]. The most common cause of vascular disease is atherosclerosis [6] and atherosclerotic risk has been traditionally assessed based on the sum of predisposing factors such as hypertension [7], diabetes [8], and increased abdominal fat [9]; however, computerized tomography (CT) and magnetic resonance imaging (MRI) provide direct measurement of atherosclerosis. Carotid atherosclerosis has been associated with the onset, severity, and progression of cognitive dysfunction [10–13]; however, atherosclerosis begins in the abdominal aorta and only later involves the carotid arteries [14]. Therefore, other direct measures may provide a better index of subclinical atherosclerosis [15], such as coronary artery calcium (CAC) [16], abdominal aortic plaque [17], and abdominal aortic wall thickness (AWT) [18], which have been shown to independently predict future adverse cardiovascular events [19].
The extent to which subclinical atherosclerosis is related to cognition is not fully understood, particularly whether these processes exert a detectable effect on cognition in the absence of stroke or prior to the onset of dementing illnesses. The relationship between objective evidence of atherosclerosis and cognition has been studied in convenience samples and studies of the elderly, but has not been widely examined in a population-based sample of adults with a wide age range. Research on subclinical atherosclerosis has recently increased due to advances in imaging, and the majority of this work has utilized carotid measures of atherosclerosis [20,21]. This is the first study to date to simultaneously examine three direct measures of atherosclerosis (CAC, abdominal aortic plaque, abdominal AWT) in relationship to global cognitive function in a large, ethnically diverse community-based sample. Using a large community-based sample, it was hypothesized that higher levels of atherosclerosis as measured by CAC, abdominal aortic plaque, and abdominal AWT would be moderately related to lower cognitive performance at a later point in life and that an increasing number of positive atherosclerotic indicators would have an incremental negative effect on MoCA scores.
2. Method
2.1. Participants
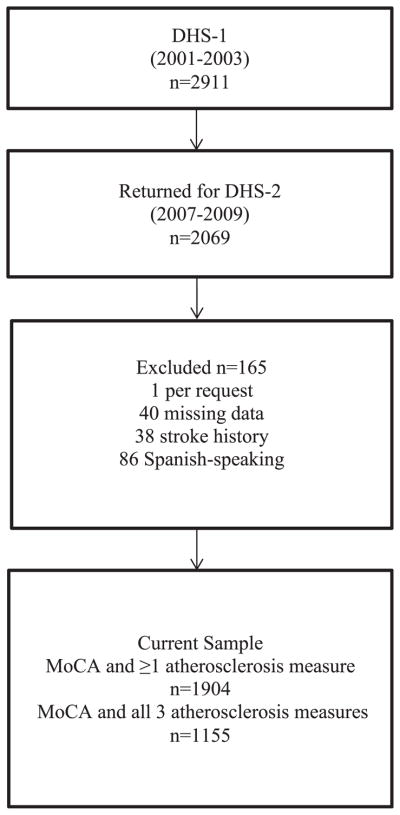
This investigation was conducted as part of the Dallas Heart Study (DHS), a longitudinal, population-based, multi-ethnic cohort study of factors contributing to cardiovascular disease [22]. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. The first phase of DHS (DHS-1) was initiated in 1999, and participants were drawn from those who returned for follow-up in DHS-2 (n = 2069), which gathered data from September 2007 to January 2010 (see Fig. 1).
Fig. 1.
Flowchart of sample selection.
All participants met the following inclusion criteria: 1) English-speaking and 2) completed a valid Montreal Cognitive Assessment (MoCA) test. Thirty-eight individuals were excluded due to positive stroke history and 1 person was removed due to unclear stroke history. One participant was excluded because he requested that his data not be used. Forty entries were deleted due to missing items. Primarily Spanish-speaking participants who were not fluent in English (n = 86) were excluded. Following these exclusions, 1904 participants with valid MoCA scores were available for the current study. Of those, 1154 had DHS-1 data for all three selected measures of atherosclerosis. The baseline characteristics of the 1154 subjects with full data did not significantly differ from the smaller subset of the sample with available MoCA scores but incomplete atherosclerosis measures (n = 750).
2.2. Procedures
During DHS-1, demographic and health-related information, physical examination, and blood and urine sampling were obtained. Participants underwent EBCT and abdominal MRI. In DHS-2, this information was gathered with the addition of cognitive testing using the MoCA. The MoCA was administered by trained personnel, scored in the conventional manner but without the suggested 1-point correction for ≤12 years of education. The de-identified MoCA data were entered item-by-item in the DHS-2 database using only the subject identifier.
2.3. Measures
2.3.1. Atherosclerosis
Coronary calcification was assessed for participants 30 years of age or older with electron beam computed tomography (EBCT) using a previously described protocol [23]. A calcium score was expressed in Agatston units [24] and the mean of the two consecutive scans was used as the final CAC score. Individuals with a mean EBCT score >10 Agatston units were classified as CAC “positive” [23].
Abdominal aortic plaque scores and abdominal AWT were determined by MRI using a previously described protocol [25]. Areas of increased signal intensity, luminal protrusion, and focal wall thickening were identified as atherosclerotic plaque and categorized as “present” or “absent” [17]. Since there is no widely accepted clinical cut-off for AWT, a dichotomous threshold at the 75th percentile was retrospectively defined using the overall DHS-1 sample of approximately 6000 participants, as has been done by other DHS investigators [26]. The 75th percentile cut-off was stratified by gender and age and defined as “normal” or “elevated.”
2.3.2. Cognition
The MoCA is a commonly used 30-point cognitive screening tool that requires approximately 10 min to administer and evaluates aspects of attention, orientation, language, verbal memory, visuo-spatial, and executive function. The MoCA items have been described in detail elsewhere [27]. The proposed cut-off score for mild cognitive impairment is [26,27] although whether this cutoff is appropriate for all sociodemographic groups has been questioned [28,29].
2.3.3. Other measures
Hypertension was defined as average systolic blood pressure 140 mm Hg and/or diastolic blood pressure 90 mm Hg or use of antihypertensive medication. Diabetes was defined by a fasting glucose level 126 mg/dl or use of any hypoglycemic medication. Hypercholesterolemia was defined as low-density lipoprotein cholesterol ≥160 mg/dl, total cholesterol ≥240 mg/dl, or the use of statin medication. Waist circumference was measured in centimeters on a horizontal plane 1 cm above the iliac crest. The National Cholesterol Education Program obesity threshold was used to determine abdominal obesity; 88 cm and 102 cm for women and men, respectively [30]. Apolipoprotein E genotyping was conducted by a previously established method [31].
2.4. Statistical analysis
Statistical analyses were conducted using SPSS version 18.0 (SPSS, Inc., Chicago IL). The level of significance was set at p ≤ 0.05. To assist with the determination of clinical relevance, correlation coefficients were designated as small (.10–.29), medium (.30–.49), and large (>.50) using standard criteria [32]. Participants with missing data were compared to those with complete data, with no significant differences noted. Markedly skewed variables (CAC, abdominal aortic plaque, and CRP) were log10 transformed to meet assumptions of parametric statistical testing when used as continuous variables; for clarity, we report these results as antilogs. Although the transformation did not fully normalize the distribution of these variables, additional ANCOVA analyses were conducted using rank order transformations and results were similar to those with the log-transformed data.
To determine if there was a relationship between direct measures of atherosclerosis and global cognition, partial Spearman correlations between the MoCA scores and CAC scores, abdominal aorta plaque, and AWT were performed, controlling for education and age because these variables were correlated with MoCA scores in this study and have a well-established effect on cognitive test performance. In order to determine if having more than one positive indicator of atherosclerosis (e.g. CAC>10) resulted in a stronger relationship to cognitive function, participants with a greater number of positive risk factors were compared to those without. For this analysis, only participants with data for all 3 indicators were used and those with no positive indicators (n = 517) were excluded due to unequal sample size. The mean MoCA score among these groups (1, 2, or 3 positive indicators) was compared using ANCOVA, controlling for education and age, and a test for trend was also conducted. To determine if the presence of the ApoE4 allele related to cognitive performance, mean MoCA scores for participants with and without an E4 allele were also compared using ANCOVA.
3. Results
3.1. Sample characteristics
Descriptive information is presented in Table 1. The mean MoCA score for the entire sample was 23.36 (4.03) points. Education was associated with MoCA performance (r = .43, p < .001), as was age (r = −.20, p ≤.001). After controlling for education and age, there was no difference in MoCA scores by sex (M, SD male: 23.29, 3.95 and female: 23.42, 4.08).
Table 1.
Sample Characteristics (Total n = 1904).
| % | Range | M (SD) | |
|---|---|---|---|
| b Age at DHS 2, yrs | 26–74 | 50.87 (10.39) | |
| c Education, yrs | 1–20 | 13.59 (2.69) | |
| Female | 58 | ||
| African American | 54 | ||
| White | 33 | ||
| Hispanic | 11 | ||
| Othera | 2 | ||
| MoCA total score (n = 1904) | 7–30 | 23.36 (4.03) | |
| CAC (Agatston units; n = 1414) | 0–7444 | 52.55 (310.4) | |
| % CAC Positive (>10 AU) | 15 | ||
| Abdominal Aortic Plaque (mm2; n = 1284) | 0–588 | 23.12 (59.4) | |
| % Present Plaque | 37 | ||
| Abdominal AWT (mm; n = 1287) | 1–3.47 | 1.68 (0.30) | |
| % Elevated AWT (≥75th percentile) | 25 | ||
| Waist Circumference (cm) | 56–164 | 99.55 (17.04) | |
| Female | 56–162 | 98.03 (18.36) | |
| Male | 69–164 | 101.62 (14.84) | |
| >88 cm for females | 56 | ||
| >102 cm for males | 40 | ||
| Diabetes | 8 | ||
| Hypertension | 30 | ||
| Hypercholesterolemia | 10 | ||
| ApoE4 (1 or 2 alleles) | 32 | ||
| ApoE Status | |||
| E2/E2 | 1 | ||
| E2/E3 | 10 | ||
| E2/E4 | 3 | ||
| E3/E3 | 48 | ||
| E3/E4 | 22 | ||
| E4/E4 | 4 |
Note. DHS = Dallas Heart Study; MoCA = Montreal Cognitive Assessment; CAC = coronary artery calcium; AWT = aortic wall thickness.
Excluded from further group analyses due to small sample size; analyses did not differ.
Significantly associated with MoCA scores (r = −.20, p < .001).
Significantly associated with MoCA scores (r = .43, p < .001).
3.2. Relationship to cognition
A partial Spearman’s correlation controlling for age and education showed a weak but statistically significant correlation between MoCA Total Scores and CAC [rho (1409) =−.06, p = .047], whereas abdominal AWT [rho (1283) = −.04, p = .187] and abdominal aortic plaque [rho (1280) = −.06, p = .052] did not correlate with MoCA Total Score. When stratified by sex or ethnicity, the relationships between these direct measures of atherosclerosis and MoCA did not differ (p ≥ .114 for each). When correlational analyses were conducted with only those participants greater than 50 years of age, who were thought to be more vulnerable to the effects of atherosclerosis, a small but significant relationship was found between abdominal AWT and MoCA (n = 355, rho = −.16, p = .004).
Direct measures of atherosclerosis were also examined as categorical variables (CAC >10 Agatston units, AWT ≥ 75th percentile, and present aortic plaque). After controlling for age and education, small but significant differences in MoCA scores were observed between participants with and without CAC (M = 22.35 vs 23.69, p = 0.038). No differences in MoCA were observed based on the presence or absence of abdominal aortic plaque (23.03 vs 23.95, p = .120) or AWT (23.41 vs 23.68, p = .894), see Table 2. Results did not change when race and other vascular risk factors (hypertension, hypercholesterolemia, diabetes, waist circumference) were included as covariates.
Table 2.
MoCA total score by atherosclerosis measure.
| Coronary artery calcium | Abdominal aortic plaque | Abdominal aortic wall thickness | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Positive N = 287 |
Negative N = 1127 |
Positive 471 |
Negative 813 |
Positive 325 |
Negative 962 |
|
| MoCA M(SD) | 22.35 (4.40) | 23.69 (3.87) | 23.03 (4.15) | 23.95 (3.78) | 23.41 (4.13) | 23.68 (3.88) |
| p-value | .038a | .120 | .894 | |||
Note. Positive denotes that the atherosclerotic measure was above the established cut-off or present (e.g. CAC >10 Agatston units). Negative denotes that the atherosclerotic measure was below the established cut-off or absent.
Small but significantly different, p ≤ 0.05.
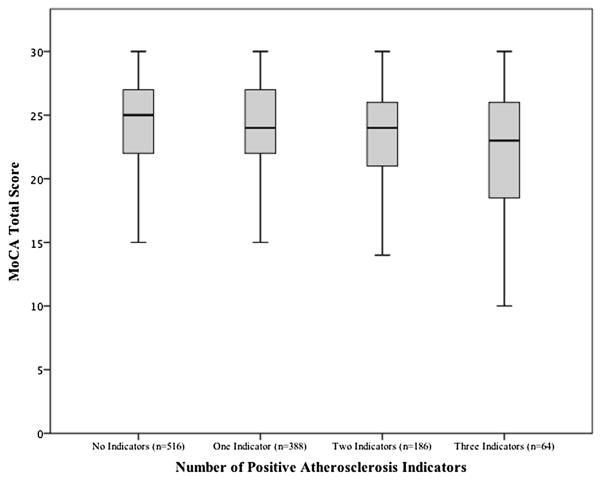
Incrementally lower MoCA scores were observed based on the number of positive atherosclerotic indicators (see Fig. 2), and those with no elevated/present measures of atherosclerosis had slightly but significantly better MoCA scores compared to their counterparts with all three positive atherosclerotic indicators [F(3, 1153) = 5.92, p = .001], though this finding was attenuated by age and education (p = .150). There was a significant linear trend, F(3, 1150) = 5.918, p = .001, indicating that as the number of atherosclerotic indicators increased, MoCA scores decreased proportionally.
Fig. 2.
MoCA Total Score by Number of Atherosclerosis Measures (Positive CAC, Present Aortic Plaque, Elevated AWT). Significant linear trend, F(3, 1150) = 5.918, p = .001.
3.3. ApoE4, cognition, and atherosclerosis
The proportion of ApoE4 was 0.15, which is comparable to the 0.14 observed in the general U.S. population [33]. The frequency was greater in African Americans (0.19) compared to white participants (0.13), consistent with prior reports [34,35]. There was no difference in MoCA Total Scores between participants with (M = 23.40, SD = 3.99) or without at least one E4 allele (M = 23.35, SD = 4.03); F(1, 1643) = .074, p = .780. The influence of E4 on direct atherosclerosis measures was also examined, with no differences in CAC, AWT, or aortic plaque (p ≥ .455). Given that ApoE4 frequency varied by race, these analyses were also examined for African Americans and Whites separately, and results did not differ (p ≥.180).
4. Discussion
Much of the evidence for an association between cognitive function and atherosclerosis comes from studies of select populations, such as the elderly and those with advanced atherosclerosis, and the majority of this work has utilized carotid measures [13,36–38]. This is the first study to simultaneously examine three direct measures of atherosclerosis (coronary artery calcium, abdominal aortic wall thickness, and aortic plaque) in relation to global cognitive function in a population-based study of middle-aged adults. In this sample, we detected a small but significant relationship between measures of subclinical atherosclerosis and MoCA scores obtained approximately 8 years later.
Cognitive performance had a negative association with age and was more strongly influenced by education. A small correlation between CAC and MoCA was observed, as was a similarly weak association between abdominal AWT and MoCA in subjects over age 50. There was no relationship between abdominal AWT and abdominal aortic plaque with MoCA scores, and the relationship between cognitive performance and atherosclerosis did not differ by gender or race. When MoCA scores were compared between positive and negative CAC, present versus absent aortic plaque, and AWT above or below the 75th percentile, results were in the expected direction, with lower cognitive performance in groups with positive atherosclerotic measures and a significant difference (but less than 1 point in MoCA Total Score) between positive and negative CAC. The few prior studies of CAC and cognition have had mixed outcomes, with greater CAC volume associated with worse performance on neuropsychological tests of information processing speed and motor speed, risk of dementia, and brain MRI abnormalities, albeit in elderly samples [39–41], while in a cross-sectional study of middle-to-older aged adults with subclinical atherosclerosis, CAC was not associated with lower neuropsychological performance [11]. The CARDIA study, a larger cohort of middle-aged, healthy adults, demonstrated that a greater amount of both CAC and abdominal aortic plaque was associated with worse performance on three cognitive tests examining psycho-motor speed, verbal memory, and executive function [42]. It is possible that this study saw a relationship in part due to the use of more sensitive, domain-specific neuropsychological tests, while our study was limited to a single global screening measure. The relationship between these direct measures of atherosclerosis and cognition has otherwise been largely unexamined. In this study, the presence of ApoE4 did not significantly impact cognitive performance, similar to prior work suggesting that the E4 allele may not contribute to detectable cognitive decline in a non-dementia context [43].
We did observe a significant trend of incrementally lower MoCA scores with successively greater number of positive indicators of atherosclerosis (e.g. CAC ≥10 and presence of aortic plaque, etc.). Thus, even though the relationships between individual factors and cognition were not significant, a combination of factors or the cumulative effects of these burdens may contribute to cognitive vulnerability over time, though differences were marginal after considering demographic factors. It may be that the levels of CAC, aortic plaque, and abdominal AWT in this relatively young sample had not yet reached a threshold that would result in notable decrements in cognition. It remains to be seen if early-life development or prolonged elevations of these atherosclerotic measures are associated with the development or earlier expression of cognitive impairment with advancing age.
The mechanism by which atherosclerosis contributes to cognitive decline may primarily be through stroke, and as such there may not be a direct, early, consistent link between atherosclerosis and cognition before stroke onset. However, studies using the carotid artery have shown an inverse relationship between subclinical carotid atherosclerosis and cognitive function, mostly in elderly samples [44] but also in middle-aged cohorts [38] and stroke-free cohorts [45]. Atherosclerosis is a gradual, systemic process that starts early in life and may have a pattern of development that is influenced by a variety of modifiers and may affect vessel beds differentially [41,46,47]. Although atherosclerosis begins in the abdomen [14], it may not exert significant cognitive risk until it is present in the carotid arteries due to proximity to the brain and critical role in brain blood supply [47,48].
Despite the large sample size and unselected nature of this cohort, the present study has limitations. The DHS sample was relatively well educated and had a majority of minority participants, so these findings may not generalize to less educated populations or those with a different ethnic composition. While the DHS was longitudinal over two phases, this study utilized data from each, but not both, phases and is thus neither longitudinal nor strictly cross-sectional. An important limitation is the lack of baseline cognitive testing and the 8–10 year interval between measurement of atherosclerosis and MoCA testing. It is possible that less healthy participants dropped out during that interval, which would bias in favor of a negative outcome. It is also possible that concurrent biologic measurements and/or change scores over time may show a greater relationship with cognitive functioning than what was seen in the present study, and those data are forthcoming. Finally, while the MoCA has been reported to be more sensitive than other brief tests such as the Mini-Mental State Examination [49] in detecting mild cognitive impairment [50], it is a screening tool for global cognition and may not be sensitive enough to show the cognitive effects of subtle biological processes. This is supported by the fact that prior studies reporting significant relationships between atherosclerosis and cognition have utilized traditional neuropsychological tests rather than a general screening measure [11,38,42,51].
Subclinical atherosclerosis was examined in relationship to cognitive function measured 8 years later with the MoCA, a brief index of global cognitive function. These results were derived from a large, ethnically diverse, community-based sample and thus may have epidemiologic implications, though the findings were of modest size. We found a weak but significant negative relationship between CAC and MoCA and observed a significant trend of incrementally lower MoCA scores with successively greater number of positive indicators of atherosclerosis. No relationship was found between abdominal plaque or abdominal AWT and cognitive function. Presence of the ApoE4 allele did not impact the relationship between atherosclerosis and global cognitive function. Although CAC, abdominal aortic plaque, and abdominal aortic wall thickness were only minimally associated with later cognitive function in middle-aged adults, it is not yet known if early-life development or prolonged elevations of these biological measures may play a role in the development or earlier expression of cognitive impairment with advancing age.
Acknowledgments
This work was conducted in part with support from the Wallace, Barbara and Kelly King Charitable Foundation and the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest
None.
References
- 1.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 5.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azen SP, Mack WJ, Cashin-Hemphill L, et al. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the cholesterol lowering atherosclerosis study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 7.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 8.Kumari M, Marmot M. Diabetes and cognitive function in a middle-aged cohort: findings from the Whitehall II study. Neurology. 2005;65:1597–1603. doi: 10.1212/01.wnl.0000184521.80820.e4. [DOI] [PubMed] [Google Scholar]
- 9.Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 10.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid artery plaque progression and cognitive decline: the Tromso study 1994–2008. Eur J Neurol. 2012;19:1318–1324. doi: 10.1111/j.1468-1331.2012.03728.x. [DOI] [PubMed] [Google Scholar]
- 11.Gatto NM, Henderson VW, St John JA, et al. Subclinical atherosclerosis is weakly associated with lower cognitive function in healthy hyper-homocysteinemic adults without clinical cardiovascular disease. Int J Geriatr Psychiatry. 2009;24:390–399. doi: 10.1002/gps.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestrini M, Gobbi B, Pasqualetti P, et al. Carotid atherosclerosis and cognitive decline in patients with Alzheimer’s disease. Neurobiol Aging. 2009;30:1177–1183. doi: 10.1016/j.neurobiolaging.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224:506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGill HC, Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S–1315S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 15.Davis PH, Dawson JD, Blecha MB, Mastbergen RK, Sonka M. Measurement of aortic intimal-medial thickness in adolescents and young adults. Ultrasound Med Biol. 2010;36:560–565. doi: 10.1016/j.ultrasmedbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janowitz WR. CT imaging of coronary artery calcium as an indicator of atherosclerotic disease: an overview. J Thorac Imaging. 2001;16:2–7. doi: 10.1097/00005382-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Jaffer FA, O’Donnell CJ, Larson MG, et al. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22:849–854. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]
- 18.Li AE, Kamel I, Rando F, et al. Using MRI to assess aortic wall thickness in the multiethnic study of atherosclerosis: distribution by race, sex, and age. AJR Am J Roentgenol. 2004;182:593–597. doi: 10.2214/ajr.182.3.1820593. [DOI] [PubMed] [Google Scholar]
- 19.Maroules CD, Rosero E, Ayers C, Peshock RM, Khera A. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: the Dallas heart study. Radiology. 2013;269:84–91. doi: 10.1148/radiol.13122707. [DOI] [PubMed] [Google Scholar]
- 20.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Jain T, Peshock R, McGuire DK, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.de Lemos JA, Zirlik A, Schonbeck U, et al. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:2192–2196. doi: 10.1161/01.ATV.0000182904.08513.60. [DOI] [PubMed] [Google Scholar]
- 26.de Lemos JA, Morrow DA, Sabatine MS, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77:1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein IH, Lacritz L, Barlow CE, Weiner MF, DeFina LF. Psychometric evaluation of the Montreal Cognitive Assessment (MoCA) in three diverse samples. Clin Neuropsychol. 2011;25:119–126. doi: 10.1080/13854046.2010.533196. [DOI] [PubMed] [Google Scholar]
- 30.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Emi M, Wu LL, Robertson MA, et al. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3:373–379. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 33.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 34.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 35.Hallman DM, Boerwinkle E, Saha N, et al. The apolipoprotein E polymorphism: a comparison of allele frequencies and effects in nine populations. Am J Hum Genet. 1991;49:338–349. [PMC free article] [PubMed] [Google Scholar]
- 36.Cardenas VA, Reed B, Chao LL, et al. Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke. 2012;43:2865–2870. doi: 10.1161/STROKEAHA.112.659722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendell CR, Waldstein SR, Ferrucci L, O’Brien RJ, Strait JB, Zonderman AB. Carotid atherosclerosis and prospective risk of dementia. Stroke. 2012;43:3319–3324. doi: 10.1161/STROKEAHA.112.672527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects – the Tromso study. Cerebrovasc Dis. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 39.Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr, Newman AB. Coronary artery calcium: associations with brain magnetic resonance imaging abnormalities and cognitive status. J Am Geriatr Soc. 2005;53:609–615. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- 40.Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke. 2010;41:891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bos D, Vernooij MW, Elias-Smale SE, et al. Atherosclerotic calcification relates to cognitive function and to brain changes on magnetic resonance imaging. Alzheimers Dement. 2012;8:S104–S111. doi: 10.1016/j.jalz.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Reis JP, Launer LJ, Terry JG, et al. Subclinical atherosclerotic calcification and cognitive functioning in middle-aged adults: the CARDIA study. Atherosclerosis. 2013;231:72–77. doi: 10.1016/j.atherosclerosis.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintas JL, Souza VC, Henriques AD, et al. Lack of association between apolipoprotein E genotypes and cognitive performance in the non-demented elderly. Psychogeriatrics. 2014;14:11–16. doi: 10.1111/psyg.12029. [DOI] [PubMed] [Google Scholar]
- 44.Sander K, Bickel H, Forstl H, et al. Carotid- intima media thickness is independently associated with cognitive decline. The INVADE study. Int J Geriatr Psychiatry. 2010;25:389–394. doi: 10.1002/gps.2351. [DOI] [PubMed] [Google Scholar]
- 45.Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromso Study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- 46.Galili O, Herrmann J, Woodrum J, Sattler KJ, Lerman LO, Lerman A. Adventitial vasa vasorum heterogeneity among different vascular beds. J Vasc Surg. 2004;40:529–535. doi: 10.1016/j.jvs.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey RJ, Vemuganti R, Varghese T, Hermann BP. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery. 2010;67:484–493. doi: 10.1227/01.NEU.0000371730.11404.36. discussion 493–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston SC, O’Meara ES, Manolio TA, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- 49.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 50.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal Cognitive Assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 51.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]