Abstract
The structural plasticity of dendritic spines is considered to be essential for various forms of synaptic plasticity, learning and memory. The process is mediated by a complex signaling network consisting of numerous species of molecules. Furthermore, the spatiotemporal dynamics of the biochemical signaling is regulated in a complicated manner due to geometrical restrictions from the unique morphology of the dendritic branches and spines. Recent advances in optical techniques have enabled the exploration of the spatiotemporal aspects of the signal regulations in spines and dendrites and have provided many insights into the principle of the biochemical computation that underlies spine structural plasticity.
Introduction
Dendritic spines are tiny postsynaptic protrusions covering the dendrites in most of the principal neurons in the central nervous system. Plasticity of the structure and function of dendritic spines is considered to be important for synaptic plasticity and memory. Each dendritic spine consists of a small bulbous head (~0.1 fL) connected to its parent dendrite through a narrow neck (~0.1 μm in diameter and ~0.5 μm in length). The neck acts as a diffusional barrier and an electrical resistance, isolating the spine head biochemically (Bloodgood and Sabatini, 2005; Svoboda et al., 1996) and electrically (Grunditz et al., 2008; Harnett et al., 2012; Tonnesen et al., 2014) from its parent dendrite. The structure and function of spines are regulated by biochemical reactions mediated by calcium (Ca2+) and numerous signaling molecules. The spatiotemporal dynamics of the biochemical reaction are restricted in a complicated manner due to unique morphology of the spines and dendritic shafts. Imaging studies have demonstrated that some signaling activities are restricted to the spine to maintain synaptic-specificity of long-term potentiation (LTP) (Lee et al., 2009; Sabatini et al., 2002; Yuste and Denk, 1995), while the other signals spread locally along the dendritic shaft and nearby spines (Harvey et al., 2008; Murakoshi et al., 2011; Yasuda et al., 2006a) and distantly even into the nucleus located a few hundred micrometers away from the stimulated spines (Zhai et al., 2013). Thus, the distinct spatiotemporal dynamics of biochemical signaling could have a large impact on the length and time scales of various forms of synaptic plasticity. Here, we review recent findings demonstrating how the biochemical signals are initiated at single spines and how they are transmitted, computed and integrated at the distinct neuronal compartments to regulate functions of the spines and dendrites as well as the nucleus during structural plasticity of the dendritic spines.
Structural plasticity of dendritic spines
Remodeling of neuronal networks through activity-dependent functional modification of synaptic connections and associated structural changes of synapses is hypothesized to be a cellular substrate of learning and memory. Recent studies have revealed that the morphology of spine head, neck and its substructures are dynamically modified during various forms of synaptic plasticity.
Plasticity of spine heads
The volume of a spine head is proportional to the area of the postsynaptic density (PSD) in the spine, the presynaptic area of its synaptic partner, the number of synaptic AMPARs and the amplitude of the AMPAR-mediated currents (Harris and Stevens, 1989; Matsuzaki et al., 2001; Schikorski and Stevens, 1997; Takumi et al., 1999). Thus, the morphology of the spine is tightly coupled with the synaptic function and a change in spine volume has been considered to be an important substrate of synaptic plasticity. Indeed, many studies have demonstrated that LTP and LTD (long-term depression) are associated with spine enlargement and shrinkage, respectively (Desmond and Levy, 1983; Hayama et al., 2013; Matsuzaki et al., 2004; Nagerl et al., 2004; Oh et al., 2013; Okamoto et al., 2004; Van Harreveld and Fifkova, 1975; Zhou et al., 2004). The studies of the spine structural plasticity have been promoted by the development of the 2-photon glutamate uncaging technique. This technique allows one to selectively stimulate a single spine while simultaneously imaging the morphology of the stimulated spine with two-photon microscopy (Matsuzaki et al., 2001). It has been found that repetitive glutamate uncaging under low Mg2+ (nominally zero) condition induces a rapid and transient enlargement of spine head in the first several minutes in the hippocampal CA1 pyramidal neurons. This is followed by a volume change sustained for hours (Lee et al., 2009; Matsuzaki et al., 2004). This time course of the spine enlargement is similar to that induced by high frequency electrical stimulation of Schaffer Collateral axons in the presence of Mg2+ (Matsuzaki et al., 2004). The morphological change of the stimulated spine is associated with an increase in postsynaptic glutamate sensitivity. These morphological and functional changes are observed only in the stimulated spine but not in the neighboring spines, indicating LTP can be induced in an input specific manner at the single spine level (Fig.1a). In this review, we refer this form of spine morphological plasticity as to structural LTP.
Fig.1.
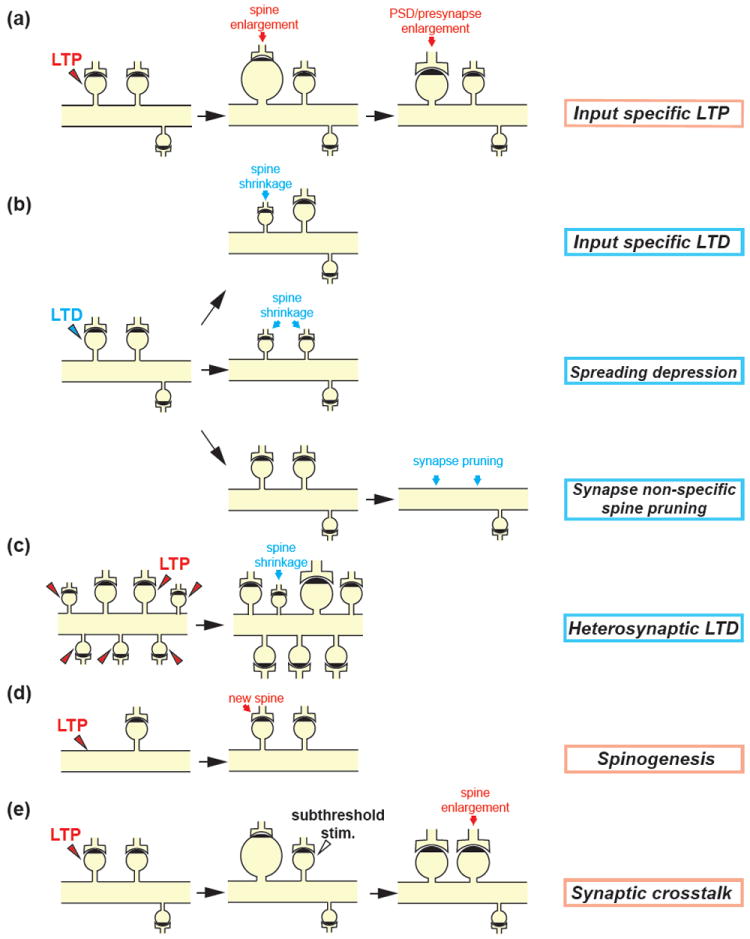
Spine structural plasticity
(a) Structural plasticity during LTP: Repetitive 2-photon uncaging of MINI-glutamate (0.5-2Hz for 1min) at a single spine under low Mg2+ (nominally zero) condition or paired with postsynaptic depolarization induces a rapid enlargement of the spine head in a few minutes. The volume of the enlarged spine gradually decreases over ~5 min to a plateau and sustains more than an hour (Lee et al., 2009; Matsuzaki et al., 2004). The PSD and presynapse increase with a delay of 0.5-3 hours (Bosch et al., 2014; Meyer et al., 2014). Note that the enlargement of the spine volume is restricted to the stimulated spine (input specific LTP).
(b) Structural plasticity during LTD: Different protocols for LTD induction have been reported to result in the distinct structural plasticity. Low frequency glutamate uncaging (90 pulses at 0.1Hz) in low extracellular Ca2+ (0.3mM) and Mg2+ (nominally zero) concentration or paired with postsynaptic depolarization induces a spine shrinkage restricted to the stimulated spine (input specific LTD) (Oh et al., 2013) (upper). B-AP paired with subsequent 2-photon glutamate uncaging pulses (~10 ms) at a single spine (80 pulses at 1Hz) shortly after (< 50 ms) GABA uncaging at the adjacent dendritic shaft induces the reduction in the volume of the stimulated spine as well as neighboring non-stimulated spines (spreading depression) (Hayama et al., 2013) (middle). The optogenetic stimulation of presynaptic CA3 pyramidal neurons expressing channelrodopsin-2 (1Hz for 900 light pulses) induces functional LTD but not spine shrinkage in postsynaptic CA1 neurons. However, a few days later, the stimulated spine and many neighboring synapses are removed (synapse-nonspecific spine pruning) (Wiegert and Oertner, 2013) (lower).
(c) Heterosynaptic LTD: LTP stimulation at multiple spines on a single dendritic segment by glutamate uncaging induces shrinkage of nearby unstimulated spines (Oh et al., 2015).
(d) Spinogenesis induced by glutamate uncaging: 2-photon glutamate uncaging (40 pulses at 2Hz) at dendritic shafts triggers rapid de novo spinogenesis in young neurons (Kwon and Sabatini, 2011).
(e) Synaptic crosstalk associated with structural plasticity: Repetitive glutamate uncaging (30 pulses at 0.5Hz, 4ms pulse duration) is applied to a single spine to induce LTP. A subthreshold stimulus (30 pulses at 0.5Hz, 1ms pulse duration), which by itself does not trigger LTP, is then applied to a nearby spine. This induces a sustained structural and functional LTP in the weakly stimulated spine (Harvey and Svoboda, 2007; Harvey et al., 2008).
Similar to the functional LTP, there are two distinct temporal stages in structural LTP : protein synthesis-independent, early phase of LTP (E-LTP) and protein synthesis-dependent late phase of LTP (L-LTP) (Bosch et al., 2014; Govindarajan et al., 2011). L-LTP can be induced in single spines by glutamate uncaging paired with postsynaptic depolarization or the bath application of BDNF or forskolin (an activator of cAMP signaling) (Govindarajan et al., 2011; Tanaka et al., 2008a). Notably, structural and functional plasticity share, at least part of, their signaling pathways: they both require Ca2+ influx through postsynaptic NMDARs, activation of CaMKII and small GTPases, and actin polymerization (Harvey et al., 2008; Kim et al., 2014; Lee et al., 2009; Matsuzaki et al., 2004; Murakoshi et al., 2011). Although structural and functional plasticity can be dissociated under some condition (Kopec et al., 2007; Sdrulla and Linden, 2007; Wang et al., 2007), these results suggest substantial overlap between the mechanisms underlying LTP and spine enlargement.
In addition to LTP, two protocols to induce LTD and spine shrinkage using 2-photon glutamate uncaging have also been found (Hayama et al., 2013; Oh et al., 2013). In the first protocol, low frequency glutamate uncaging (0.1 Hz) in low Ca2+ (0.3mM) extracellular solution under postsynaptic depolarization or nominally zero Mg2+ can induce spine-specific LTD and spine shrinkage (Fig.1b). The second protocol is much more complicated: GABA uncaging at the dendritic shaft ~10 ms prior to back-propagating action potentials (b-APs) followed by glutamate uncaging at single spines has been found to induce LTD and spine shrinkage. Interestingly, this protocol induces volume shrinkages in the surrounding, non-stimulated spines located within ~15 μm from the stimulated spines as well as in the stimulated spines (Fig.1b). This form of LTD requires suppression of bAP-evoked Ca2+ transients by GABA uncaging. GABA signaling does not appear to encode precise timing information, since simple pharmacological activation of GABA receptors can replace GABA uncaging.
Plasticity of spine necks
It has been speculated that spines serve as electrical compartments because of the resistance at the necks (Segev and Rall, 1988). The electrical compartmentalization amplifies local excitatory postsynaptic potentials (EPSPs) within the spine and produces a voltage gradient between spines and the dendritic shaft, reducing dendritic and somatic excitatory postsynaptic potentials (EPSPs) compared to that in the spine. The voltage may be further amplified in spines with voltage dependent conductance (Bywalez et al., 2015; Grunditz et al., 2008; Yuste, 2013). Indirect estimates of the spine neck resistance, based on the cable theory or calculations from the measured diffusional fluxes, vary greatly (Bloodgood and Sabatini, 2005; Harris and Stevens, 1989; Svoboda et al., 1996; Yuste, 2011). However, recent evidences have supported the idea of the electrical compartmentalization by the spine neck. A study using whole cell recordings with glutamate uncaging at individual spines revealed that stimulation of spines with longer necks produces smaller EPSPs at the soma in layer 5 pyramidal neurons (Araya et al., 2006; Araya et al., 2014), although no such correlation was observed in olfactory bulb granule neurons (Bywalez et al., 2015). In addition, Ca2+ transients within spines through NMDARs and voltage sensitive calcium channels (VSCCs) are evoked by subthreshold synaptic stimulation, to the degree consistent with the voltage amplification by spine necks (Bloodgood et al., 2009; Grunditz et al., 2008; Kovalchuk et al., 2000; Yuste and Denk, 1995). Voltage gated sodium channels were also shown to be locally activated within spines stimulated with glutamate uncaging, which opens high-voltage-activated Ca2+ channels in olfactory bulb granule neurons (Bywalez et al., 2015). Furthermore, the ratio of the EPSPs in the spine and the dendrite was measured by comparing Ca2+ elevation in response to voltage changes at the spine and that induced by glutamate uncaging that cause the equivalent voltage changes in the presence of NMDA receptor inhibitor. The experiments revealed that spines exhibit a high neck resistance which amplifies the local synaptic depolarization by ~50-fold (Harnett et al., 2012). Although the exact neck resistance is still unknown, these results suggest that spines can act as electrical compartments.
Importantly, the diffusion across the spine neck is dynamically regulated by neuronal activities (Bloodgood and Sabatini, 2005; Grunditz et al., 2008; Tonnesen et al., 2014). Diffusional coupling between spine and dendrite has been measured with fluorescence recovery after photo-bleaching (FRAP) of fluorescent proteins or fluorescence decay after photo-activation of photoactivatable GFP (PA-GFP). These studies revealed that the coupling time increases in response to 2-photon glutamate uncaging at the spines paired with b-APs or a postsynaptic depolarization for a few minutes (Bloodgood and Sabatini, 2005; Grunditz et al., 2008), suggesting that high neuronal activity can cause higher neck resistance. In contrast, a study found that protein-synthesis dependent LTP, induced by 2-photon glutamate uncaging paired with b-APs, is coupled with widening of the spine neck (Tanaka et al., 2008a). Furthermore, a recent study using super-resolution imaging based on stimulated-emission depletion (STED) demonstrated that the spine necks become wider and shorter after LTP induced with 2-photon glutamate uncaging (Tonessen et al., 2014) (Fig.1a). Thus, it appears that LTP induction leads to the lowering of spine-neck resistance. However, since the neck widening counteracts the increased biochemical compartmentalization by head enlargement, the degree of the diffusional coupling between spine and dendrite appears to be not altered during structural LTP. The reduction of the neck resistance should decrease the voltage amplification in spines and thus may reduce the probability of further induction of LTP. On the other hand, shorter and wider neck may facilitate the transport of resources from the dendrites into the spines undergoing LTP (Tonnesen et al., 2014).
Since the spine-neck plasticity can change electrical filtering by the neck, it could be one mechanism to change EPSPs at the soma during synaptic plasticity (Araya et al., 2014). However, according to a mathematical simulation using measured spine morphology with superresolution microscopy, the spine-neck plasticity has relatively minor effects on the amplitude of somatic EPSPs in the passive regime (Tonnesen et al., 2014). Thus, the roles of the spine-neck plasticity appear to be mainly regulations of the local voltage amplification in spines and biochemical compartmentalization.
The molecular mechanisms underlying spine neck plasticity is unknown, but there are several proteins identified to be localized at spine necks. In particular, septins, highly conserved family of GTPases, are known to assemble into a hetero-oligomeric complex and higher-order structures such as filaments, rings and gauzes. Interestingly, it has been reported that septin-7 forms a complex with septin-5/11, localizes at the base of spine necks (Tada et al., 2007; Xie et al., 2007) and serves as diffusion barrier of membrane proteins including GluA2 (Ewers et al., 2014). Since septins regulate compartmentalization of the yeast plasma membrane during mitosis by forming rings at the bud necks (Barral et al., 2000; Takizawa et al., 2000), it may also play an important role in regulating the width of the spine neck. Further, a recent study has demonstrated that Ankyrin-G, a protein that acts as an adapter to connect trans-membrane proteins to the underlying spectrin-actin cytoskeleton, forms distinct nanodomains within spine heads and necks (Smith et al., 2014). Interestingly, the nanodomain confines AMPARs in spines, possibly acting as a diffusion barrier. In addition, the presence of Ankyrin-G at the spine neck is tightly associated with the larger head volume. When the 190kDa isoform of Ankyrin-G, a major isoform in spines, are overexpressed, the neck width as well as the head volume is significantly increased. These results suggest that septin and Ankyrin-G may regulate the morphology and function of spine necks during spine structural plasticity.
Plasticity of PSDs and presynapses
Spine head volume is tightly correlated with the size of the presynaptic active zone and PSD (Harris and Stevens, 1989; Schikorski and Stevens, 1997; Takumi et al., 1999). Therefore, spine growth associated with LTP should be accompanied with a growth in active zone, PSD and potentially other spine substructures. Indeed, recent studies have revealed that this is the case at the single spine level. Within the initial several minutes after LTP induction, the actin cytoskeleton grows and actin and actin binding proteins such as profilin and cofilin are rapidly accumulated into the stimulated spine (Bosch et al., 2014). In contrast, the amount of PSD proteins and the PSD size do not increase at this temporal stage (Bosch et al., 2014; Steiner et al., 2008). However, with a delay over a few hours, PSD scaffold proteins such as Homer1b, PSD-95 and shank1b slowly accumulate and the size of PSD increases (Bosch et al., 2014; Meyer et al., 2014) (Fig. 2a). These changes are found to be followed by the slow growth of the presynaptic terminals, suggesting the existence of dynamic retrograde signaling during glutamate-uncaging evoked LTP (Meyer et al., 2014). These results suggest that overall synaptic structures are gradually re-scaled over a few hours following LTP induction (Fig.1a).
Fig.2.
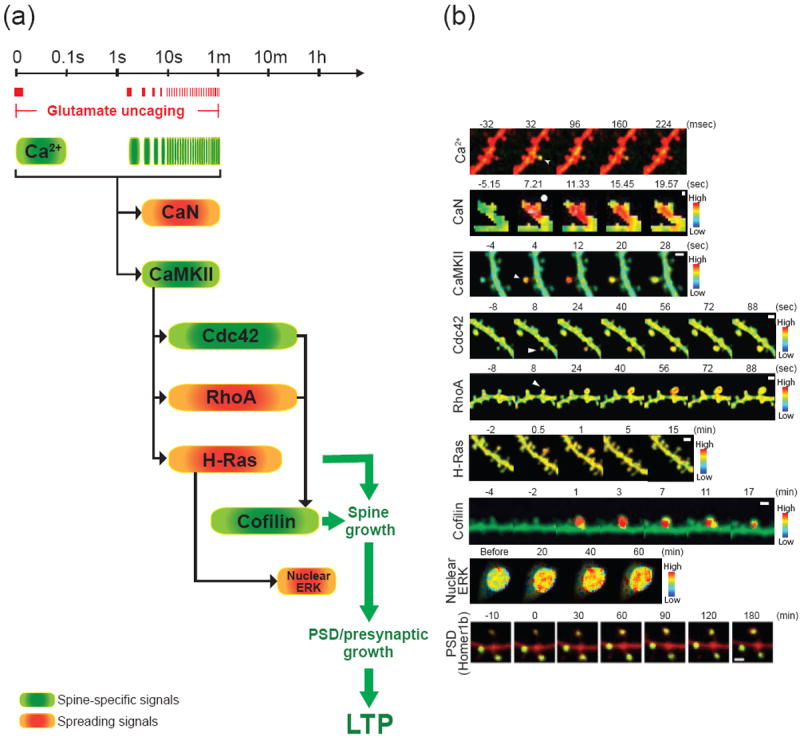
The spatiotemporal dynamics of signaling activities during structural LTP
(a) The timescale of signaling activities during structural LTP induced by 2-photon glutamate uncaging (0.5 - 20 Hz). Spine specific signals and spreading signals are indicated in green and orange, respectively. The timings of glutamate uncaging of a typical LTP induction protocol (0.5 Hz) are shown in red bars.
(b) Ca2+ elevation, activities of CaN, CaMKII, cdc42, RhoA, H-Ras, cofilin, nuclear ERK and the accumulation of Homer1b during structural LTP induced at a single spine or 7 spines (ERK). The arrows and circles show the spines stimulated with glutamate uncaging. Scale bars (white) are 10 μm for ERK and 1 μm for others. The images are adopted and modified from (Zhai et al., 2013) for Ca2+ and ERK, (Fujii et al., 2013) for CaN, (Lee et al., 2009) for CaMKII, (Murakoshi et al., 2011) for RhoA and cdc42, (Harvey et al., 2008) for H-Ras and (Bosch et al., 2014) for cofilin and Homer1b with permission. The Ca2+ elevation is visualized with a Ca2+ indicator Fluo-4FF (green) and Alexa-594 (red). Note that Ca2+ elevation in response to the first uncaging pulse during LTP induction protocol (1 Hz, 60 pulses) is displayed. CaMKII, cdc42, RhoA, H-Ras, cofilin and nuclear ERK activities are imaged with 2pFLIM combined with FRET sensors. CaN activities are visualized with dual FRET with optical manipulation (dFOMA). The accumulation of Homer1b in spines is visualized with GFP tagged-Homer1b and RFP (cell fill). Note that the Ca2+ elevation and the activations of CaMKII, cdc42, and cofilin are restricted to the stimulated spines, whereas the activation of CaN, RhoA and H-Ras spread into the dendritic shafts and the nearby spines.
Interestingly, SynGAP, one of the most abundant proteins in PSD (Cheng et al., 2006), is dissociated from PSD within a few minutes of chemical LTP induction (Araki et al., 2015). This dispersion state of SynGAP is sustained for more than half an hour. Since SynGAP is an inactivator of Ras, a signaling protein required for maintenance of LTP (Harvey et al., 2008; Zhu et al., 2002), de-localization of SynGAP may help to increase Ras activity in the stimulated spines, thereby stabilizing LTP. While it is not clear whether the amount of SynGAP in the PSD eventually follows the size of the PSD or not, the protein contents of PSD and spines appear to change dramatically during spine structural plasticity.
Spine formation and elimination
In addition to changes in the structure of preexisting spines, LTP induction is also found to be associated with the formation of new spines and filopodia (Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999; Nagerl et al., 2004; Nagerl et al., 2007; Toni et al., 1999). A study demonstrated that 2-photon glutamate uncaging at dendritic shafts in young layer 2/3 pyramidal neurons in the cortex is sufficient to induce rapid de novo spinogenesis within a few micrometers of the stimulated spot and within several tens of seconds during the glutamate uncaging stimuli (Kwon and Sabatini, 2011) (fig.1d). The newly formed spines are functional since they respond to synaptically evoked glutamate release through AMPARs and NMDARs. This study also demonstrated that spine formation does not necessarily require an intermediate filopodia stage, and glutamate is sufficient to assemble the machinery required for nucleating spine formation. The long-term stabilization of newly formed spines appears to require further potentiation (Hill and Zito, 2013).
In the opposite direction, it has been known that LTD induction can cause eliminations of preexisting spines in a NMDAR dependent manner (Fig. 1b) (Bastrikova et al., 2008; Nagerl et al., 2004; Okamoto et al., 2004; Zhou et al., 2004). A recent study followed the fate of spines up to 7 days after LTD induction in organotypic hippocampal slices (Wiegert and Oertner, 2013). By combining the optogenetic stimulation of presynaptic CA3 pyramidal neurons expressing channelrodopsin-2 with calcium imaging of spines in postsynaptic CA1 neurons expressing G-CaMP3, individual synapses were optically stimulated and their activities were monitored with Ca2+ responses. In this paradigm, they observed reductions of the success rate and the amplitude of postsynaptic Ca2+ transients after LTD induction, suggesting that this form of LTD is induced by both postsynaptic and presynaptic mechanisms. Interestingly, after a few days of LTD induction, these depressed synapses and their neighbors were eliminated (Fig.1b). The delayed elimination of depressed synapses seems not to be correlated with the degree of the initial LTD, but rather the synapses with initially low probability of neurotransmitter release (measured before LTD induction) tend to be more selectively eliminated. Surprisingly, the potency of the remaining synapses was recovered from depression at 7 days after LTD induction. Thus, over days after LTD, the stimulated neurons change the way to regulate synaptic strength from an “analog” regulation in the potency of each synapse to a “digital” regulation in the number of synapses.
Heterosynaptic plasticity in dendritic segments
While individual synapses can serve as independent computational units, it has been reported that there is heterosynaptic spreading of functional plasticity (Abraham, 2008). Recent studies, by utilizing 2-photon glutamate uncaging, have shown several forms of heterosynaptic plasticity can occur even at the single spine level. For example, LTP induction at a single spine with glutamate uncaging lowers the threshold for LTP induction at surrounding spines (Harvey and Svoboda, 2007; Harvey et al., 2008) (Fig.1e). The reduction in the threshold for LTP induction, or “cross-talk” of synaptic plasticity, lasts ~10 min and spreads over ~10 μm along the dendritic shaft. In addition, the induction of protein-synthesis-dependent LTP induced by glutamate uncaging combined with bath application of forskolin can reduce the threshold for LTP induction at surrounding spines (Govindarajan et al., 2011). This heterosynaptic facilitation occurs within ~70 μm from the stimulated spine and last ~90 min. Moreover, in young neurons, repetitive glutamate uncaging at single spines reduce the induction threshold for glutamate-induced spinogenesis in the surrounding area for at least a few minutes (Kwon and Sabatini, 2011). In addition to these positive effects of LTP on surrounding synapses, it has been known that LTP induction in one set of synapses causes LTD in the other set of synapses on the same cell (Abraham et al., 1994; Doyere et al., 1997). Similar to this so-called “heterosynaptic LTD”, it was recently revealed that the LTP induction of multiple spines on single dendritic segment can cause spine shrinkage and synaptic weakening of nearby unstimulated spines located within a few micrometers (Fig. 1c) (Oh et al., 2015).
These results strongly suggest that intracellular signaling factors can spread from the stimulated spines and have a large impact on the surrounding dendritic spines. The physiological meanings of these heterosynaptic plasticity and synaptic crosstalk are still unclear but may contribute to clustered plasticity, in which the accumulation of potentiated synapses in the same dendritic branches leads the formation of long-term memory engrams through bidirectional synaptic weight changes among synapses within a dendritic branch (Govindarajan et al., 2006).
Biochemical computation in dendritic branches for structural plasticity
In the past decades, signaling pathways leading to LTP and LTD have been extensively studied with pharmacological, genetic and biochemical tools. These studies have revealed that Ca2+ influx through synaptic NMDARs triggers a variety of signaling pathways, which in turn induce the long lasting changes in the postsynaptic sensitivity to glutamate and the probability of glutamate release from presynaptic terminals (Bliss and Collingridge, 2013; Bredt and Nicoll, 2003; Enoki et al., 2009; Huganir and Nicoll, 2013; Zakharenko et al., 2001). Imaging techniques based on Förster resonance energy transfer (FRET) enable the measurement of spatiotemporal dynamics of biochemical signaling activity in live cells. However, these techniques have been difficult to implement due to small fluorescence from the tiny volume of spines, and strong light scattering by brain tissue. The development of 2-photon fluorescence lifetime imaging microscopy (2pFLIM) in combination with highly optimized FRET based biosensors has overcome these limitations and allowed researchers to directly monitor biochemical signal transduction at a single spine resolution (Yasuda, 2006a, 2006b, 2012). Using this and other imaging techniques, the detailed spatiotemporal dynamics of signal transduction during synaptic plasticity have been revealed.
Calcium sensing
Synaptic stimulation produces a short Ca2+ transient largely restricted to the stimulated spines (Mainen et al., 1999; Noguchi et al., 2005; Sobczyk and Svoboda, 2007; Yuste and Denk, 1995). The Ca2+ transient lasts only ~0.1s and, when repeated, initiates biochemical signal transduction crucial for LTP and LTD (Fig.2). When Ca2+ flows into the spine through NMDARs, it binds to calmodulin (CaM) and activates Ca2+/calmodulin-dependent kinase II (CaMKII). It has been well established that this Ca2+-CaM-CaMKII signaling cascade is the first reaction necessary for the LTP induction (Lisman et al., 2012) (Fig.2a). The kinetics of CaMKII activation during structural LTP was determined by imaging of CaMKII activities using 2pFLIM in combination with FRET based CaMKII sensor (Lee et al., 2009; Takao et al., 2005). It has been revealed that the induction of LTP with glutamate uncaging in CA1 pyramidal neurons triggers a rapid CaMKII activation restricted to the stimulated spine. This activity decays with a time constant of ~10 s (Fig.2b). These results suggest that CaMKII serve as a relay to extend the short Ca2+ transient at a time scale of milliseconds to the signal at a time scale of seconds (Fig. 2). Thus, downstream signaling molecules are required to further extend signals for the persistence of LTP over the course of minutes or hours.
In addition to CaMKII, calcineurin (CaN), a calcium-dependent phosphatase, was found to be activated during spine enlargement in dissociated neurons (Fujii et al., 2013). In this study, the authors developed dual FRET system and simultaneously imaged activities of CaMKII and CaN in response to glutamate uncaging at single spines. They reported that strong uncaging stimuli can activate both CaMKII and CaN with similar temporal dynamics with an activation time window of 1 min (Fig. 2a). However, the spatial profiles of their activations are distinct. While CaMKII activation is compartmentalized within the stimulated spines, CaN activity spreads over several micrometers and invade adjacent spines (Fig.2b). When stimulation is weak, only CaN is activated and the activation is restricted to the stimulated spines. The spreading of CaN from stimulated spines may be important for heterosynaptic LTD (Oh et al., 2015) since this form of LTD depends on CaN.
Regulation of actin cytoskeleton
Actin filaments constitute the major cytoskeleton of dendritic spines and therefore an important determinant of spine morphology. Actin monomers in the cytoskeleton in spines undergo continuous and rapid turnover due to their dynamic cycles between monomeric G-actin and filamentous F-actin called treadmilling (Chazeau et al., 2014; Frost et al., 2010; Honkura et al., 2008; Star et al., 2002). Because the equilibration is more shifted toward F-actin in one end (barbed end) than the other end (pointed end), each actin monomer undergoes a cycle of binding to the barbed end, moving toward the pointed end and unbinding at the pointed end. Thus, the flow of actin monomers due to treadmilling indicates the direction of the filaments. The dynamics of treadmilling within a spine has been observed using photoactivation of paGFP tagged actin, and these studies revealed a retrograde flow of actin monomers from the tip to the base of spines (Frost et al., 2010; Honkura et al., 2008). However, recent super resolution imaging studies based on single-particle tracking combined with photoactivated localization microscopy (PALM) have demonstrated that the direction of actin flow is highly inhomogeneous and unoriented in spine heads (Chazeau et al., 2014; Frost et al., 2010). In contrast, the flow is more oriented, directing toward the dendritic shaft in the spine neck (Frost et al., 2010). The unoriented flow of actin is consistent with relatively unorganized structure of actin cytoskeleton in spines observed in electron microscopy (Korobova and Svitkina, 2010).
Since functional and structural LTP require actin reorganization (Kim and Lisman, 1999; Krucker et al., 2000; Lang et al., 2004; Matsuzaki et al., 2004; Okamoto et al., 2004), signaling pathways associated with actin polymerization and depolymerization have been intensively studied. Among them, small GTPases including Ras, Rho, cdc42 and Rac and their downstream molecules are known to play critical roles in actin reorganization, spine morphogenesis and LTP. 2pFLIM imaging of small GTPase activities including H-Ras, Cdc42 and RhoA activities showed that the induction of LTP at single spines similarly activates these small GTPases within ~1 min of LTP induction (Harvey et al., 2008; Murakoshi et al., 2011; Oliveira and Yasuda, 2014). However, interestingly, their activation profiles are very different: The activities for Cdc42 and RhoA, but not H-Ras, are sustained for more than ~30min. Notably, Cdc42 activity is restricted to the stimulated spine, whereas H-Ras and RhoA activities are not compartmentalized and spread over ~5-10 μm of dendrite and invade nearby spines (Fig.2b). Inhibition of CaMKII using KN62 or autocamtide CaMKII inhibitor peptide (AIP2) inhibited the activations of H-Ras, Cdc42 and RhoA, indicating these molecules are downstream of CaMKII (Harvey et al., 2008; Murakoshi et al., 2011). Furthermore, pharmacological inhibition of p21-activated kinase (PAK) and Rho kinase (ROCK), which are the effectors for Cdc42 and RhoA, respectively, inhibited structural LTP (Murakoshi et al., 2011). Thus, Ca2+-CaMKII–Cdc42 pathway constitutes the spine-specific signal transduction spanning from the timescale of milliseconds to more than half an hour to cause synapse specific plasticity (Fig.2a).
Activation of small GTPases is known to lead to the activation of actin binding proteins including cofilin (Fig. 3). A recent study showed that cofilin is rapidly and persistently accumulated at the stimulated spine after LTP induction with 2-photon glutamate uncaging (Bosch et al., 2014). Imaging cofilin-actin and cofilin-cofilin interaction with 2pFLIM showed sustained increases of these interactions in the stimulated spines (Fig.2b). These results suggest that LTP induces the formation of stable actin-cofilin complex restricted to the potentiated spine. Pharmacological analysis suggests that the cofilin activation requires several kinases including LIM kinase (LIMK), PAK, and ROCK (Fig.3). Thus, overall, cofilin seems to be one of the most important factors that intermediate small GTPase signaling and structural LTP. Interestingly, cofilin also plays an important role in AMPAR trafficking (Gu et al., 2010), further supporting the important role of cofilin in LTP and spine enlargement. In addition to cofilin, Arp2/3 is highly enriched in dendritic spines and generates de novo actin filaments of a branched architecture found in the spine head (Korobova and Svitkina, 2010; Racz and Weinberg, 2008). Loss of Arp2/3, which is activated by the downstream molecules of Rac and Cdc42, completely blocks structural LTP, but not LTD (Kim et al., 2013). Thus both cofilin and Arp2/3 seem to converge downstream of small GTPases to regulate structural LTP via actin remodeling (Fig. 3).
Fig.3.
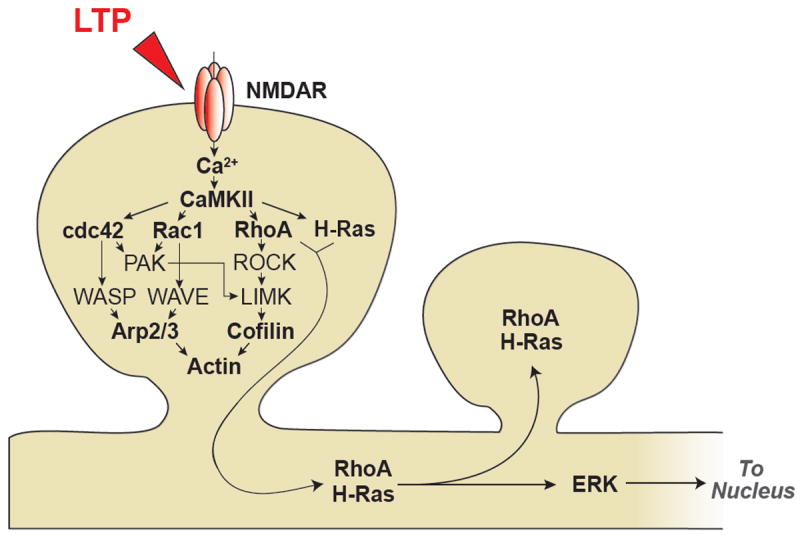
Schematic diagram of signaling pathways associated with structural LTP
Mechanisms and roles of signal spreading
The fact that biochemical signals can spread from stimulated spines to their parent dendritic shafts is not surprising, since diffusion is extremely efficient at micrometer length scale. For cytosolic and membrane bound proteins, it takes only ~0.3 and 5 s to diffuse out of the spine, respectively (Bloodgood and Sabatini, 2005; Harvey et al., 2008; Murakoshi et al., 2011). For diffusible trans-membrane proteins like AMPAR, it takes about ~30 s (Borgdorff and Choquet, 2002; Patterson et al., 2010). On the other hand, the sustained compartmentalization of signaling in spines requires specific mechanisms. For example, Cdc42 is as mobile as H-Ras and RhoA, yet only Cdc42 activation is highly restricted in the stimulated spine. One possible mechanism is to inactivate the molecules rapidly before their spreading (Yasuda and Murakoshi, 2011).
Spreading signals likely play important roles in many forms of heterosynaptic plasticity such as the facilitation and inhibition of LTP in the surrounding area (see above). In this respect, it is interesting that many subcellular compartments required for synaptic plasticity exist outside of the spine. For example, recycling endosomes containing AMPARs are often found in dendritic shafts and translocated into the spines during LTP (Park et al., 2006). Also, the protein synthesis machinery is located in dendritic shaft (Buxbaum et al., 2014; Ostroff et al., 2002; Steward and Levy, 1982). This arrangement seems to be optimized for signal spreading over several micrometers. The spread of signaling may also contribute to the induction of plasticity in a clustered fashion and create local accumulation of synaptic inputs that in turn results in functional compartmentalization of dendritic segments (Branco and Hausser, 2010; Govindarajan et al., 2006; Larkum and Nevian, 2008). The clustered plasticity has been found in several paradigms in vivo. For example, sensory deprivation by whisker trimming can induce the accumulation of SEP-GluA1 in spines located within a short stretch (~10 μm) of dendritic branches of layer 2/3 pyramidal neurons in the somatosensory cortex (Makino and Malinow, 2011). Similarly, acute whisker stimulation leads to an increase in the intensity of SEP-GluA1 in spines and adjacent dendritic shafts in a subset of dendrites of layer 2/3 pyramidal neurons in the somatosensory cortex (Zhang et al., 2015). Further, motor learning-dependent spinogenesis in layer 5 pyramidal neurons in the motor cortex appears to be clustered in dendritic branches and shows a spatial correlation over ~1 μm (Fu et al., 2012). Also, spontaneous activities of adjacent spines are frequently synchronized in CA3 pyramidal neurons in organotypic hippocampal slice cultures (Kleindienst et al., 2011; Takahashi et al., 2012). Further studies will be required to reveal if heterosynaptic plasticity and synaptic crosstalk is associated with the clustered plasticity and input synchronization.
New protein synthesis in dendrites
It is known that local translation of mRNAs in dendrites plays an important role in maintaining L-LTP and L-LTD (Costa-Mattioli et al., 2009; Huber et al., 2000; Kang and Schuman, 1996; Sutton and Schuman, 2006). Because of the significance of protein synthesis in the maintenance of synaptic plasticity, several sensors for the visualization of newly synthesized proteins have been developed. For example, newly synthesized proteins can be imaged using destabilized GFP (dGFP) regulated by the UTR of the mRNA encoding a target protein (Aakalu et al., 2001). Since the lifetime of dGFP is short (~2 h), only newly synthesized proteins are visible. However, this method cannot be used for fused proteins since the stability of dGFP may be changed by the fusion. Theoretically, fluorescence recovery after photoconversion of photoconvertible fluorescent proteins or FRAP fused with a target protein should report newly synthesized proteins. However, it appears that these procedures cause a significant phototoxcity when applied over entire neurons (Lin et al., 2008). These limitations were overcome by the development of TimeSTAMP techniques. TimeSTAMP uses an engineered protease that degrades an epitope or a fluorescent protein fused to a target protein (Butko et al., 2012; Lin et al., 2008). When a specific inhibitor for the protease is applied, the epitope tag or the fluorescent protein starts to be formed. A more recent version of TimeSTAMP is made of split YFP linked with the protease and protease recognition sites linked together. This efficiently degrades YFP before it forms fluorescent barrel, decreasing the background signal. The PSD-95 coding sequence including 3′-UTR fused with fluorescent TimeSTAMP revealed that PSD-95 is, indeed, newly synthesized in response to local dendritic stimulation of BDNF and mGluR5 and preferentially localized to stimulated synapse (Butko et al., 2012).
While the functional significance of dendritic translation is evident, how the local translation is regulated by synaptic activity has remained elusive. A recent study revealed a new mechanism that regulates dendritic translation by visualizing single endogenous β-actin mRNA molecules with single molecule fluorescence in situ hybridization (FISH) (Buxbaum et al., 2014). It was shown that ~50% of the dendritic β-actin mRNA molecules are masked by forming complexes with RNA granules containing densely packed ribosomes. β-actin mRNA and ribosomal RNA in these complexes are inaccessible by the FISH probes and presumably inactive for translation. Chemically induced LTP increased the number of β-actin mRNA and ribosomal RNA molecules that can be probed with FISH, suggesting mRNA unmasking occurred in dendrites. The same stimulation also increased the mobility of ribosomes and β-actin mRNA molecules in dendrites, indicating mRNA and ribosomes are released from the complex. These results suggest that RNA granules containing mRNAs and ribosomes exist in a suppressed state along the dendrites and LTP induction could prompt disassembly of the complexes, releasing mRNA and ribosomes to induce local translation in dendrites.
Biochemical computation between spine and nucleus for structural plasticity
Several forms of LTP and memory that last longer than several hours require gene transcription as well as translation (Bliss and Collingridge, 1993; Costa-Mattioli et al., 2009; Cracco et al., 2005; Kelleher et al., 2004; Sutton and Schuman, 2006). Gene-transcription requires NMDAR mediated Ca2+ influx, which activates protein kinase cascades including the CaMKK-CaMKIV, Ras–Raf–MEK–ERK and cAMP-PKA pathways. Activation of these kinases lead to the phosphorylation of transcription factors such as cAMP-responsive element-binding (CREB) and Elk-1 to produce new mRNAs required for L-LTP (Alberini, 2009). Conversely, CaN is also activated by neuronal activity, which dephosphorylates and activates the transcription factor MEF2 (Myocyte enhancer factor 2), leading to the elimination of excitatory synapses required for memory formation (Barbosa et al., 2008; Cole et al., 2012; Flavell et al., 2006; Pulipparacharuvil et al., 2008). Thus, activity-dependent transcription can regulate the persistence of synaptic plasticity as well as the structural refinement of synaptic connections. However, little is known about the mechanisms of the long-distance signaling from the synapse to the nucleus and the nucleus back to the synapse. To couple synaptic activities with changes in gene expression, there must be some mechanism that links local synaptic events in individual spines and signals to the nucleus. This may be mediated by somatic membrane depolarization caused by activation of a population of synapses (Adams and Dudek, 2005) or the propagation of regenerative Ca2+-waves from the stimulated synapses to the nucleus mediated by endoplasmic reticulum (ER) (Ch’ng and Martin, 2011). Additionally, recent studies demonstrated that the signaling between synapse and nucleus can be mediated by biochemical cascades.
Signal spreading from single spines to the nucleus
In response to single-spine stimulation, signaling mediated by RhoA, CaN and H-Ras spreads over ~5-10 μm (Fujii et al., 2013; Harvey et al., 2008; Murakoshi et al., 2011), which is far from the nucleus (Fig. 2b). The spreading of biochemical signaling may be further extended to much longer distances to activate signaling in the nucleus. This possibility was recently explored using 2pFLIM and FRET sensor for ERK activity (Zhai et al., 2013). Since ERK is a downstream effector molecule of H-Ras, the diffusion of H-Ras could cause long-distance spreading of ERK activity. It was demonstrated that the induction of LTP at only a few (3-7) spines is sufficient to activate ERK in the nucleus (Fig.2b). Furthermore, immunostaining showed that downstream transcription factors including CREB and Elk1 are also activated in response to stimulation of a few spines in an ERK-dependent manner. These results suggest that the activation of a small number of spines has a profound impact on the activation of nuclear signaling that regulates gene transcription. The onset of nuclear ERK activation is 5-30 min following the stimulation and shows a greater delay when distal dendrites are stimulated. The delay is consistent with the diffusion of cytosolic proteins from the spine to the nucleus, suggesting that the diffusion of ERK may be an important factor for the process. The signal can be integrated over surprisingly long times (more than 30 min) and space (~80 μm). Furthermore, the spatially dispersed inputs over multiple branches activated nuclear ERK much more efficiently than clustered inputs over one branch. The preference of sparse inputs over multiple dendrites appears to be caused by saturation of ERK activation in response to stimulation of a few dendritic spines on a branch. In this situation, stimulating more than a few spines in one branch will not increase signaling to the nucleus. Instead, the number of stimulated branches is critical for increasing signals in the nucleus. Thus, the dendritic branch seems to act as a biochemical computation unit and super-sensitive integration site in which each branch plays an important role in controlling the synapse-to-nucleus signaling.
In addition to signal spreading via diffusion, energy-dependent transport via motor proteins seems to play important roles in the synapse-to-nucleus signaling. One proposed mechanism is the transmission of signals via molecular messengers that are dissociated from the stimulated synapse and delivered to the nucleus. Interestingly, synapses contain various proteins with a nuclear localization signal (NLS) that are localized both in synapses and the nucleus (Jordan and Kreutz, 2009). Importin α is one such proteins and it functions as an adaptor that binds a NLS-containing cargo and forms a heterotrimeric complex with importin β1 to facilitate the transport of this complex into the nucleus following LTP-inducing stimuli (Goldfarb et al., 2004; Jeffrey et al., 2009; Thompson et al., 2004). Importantly, several transcriptional regulators including CREB, NFκB and Jacob were shown to be translocated into nucleus in response to synaptic activities via the importin-dependent pathway (Jordan and Kreutz, 2009; Karpova et al., 2013). For example, Jacob is found to be a synapto–nuclear messenger containing NLS. Following synaptic, but not extrasynaptic NMDAR activation, Jacob is phosphorylated by ERK, which causes the dissociation of Jacob from spines, leading to its translocation into the nucleus in an importin α-dependent manner. The presence of phosphorylated Jacob in the nucleus increases CREB phosphorylation, inducing the expression of the CRE-dependent genes (Karpova et al., 2013). Furthermore, CREB-regulated transcriptional coactivator 1 (CRTC1) is also translocated from the synapses to the nucleus and binds to CREB to upregulate the CRE-dependent transcription (Kovacs et al., 2007; Zhou et al., 2006). The CRTC1 nuclear translocation requires the Ca2+-CaN pathways and the persistent accumulation of CRTC1 in the nucleus requires the cAMP pathway (Ch’ng et al., 2012; Nonaka et al., 2014).
It is likely that neither simple diffusion nor active transport is efficient enough to alter transcription in the nucleus. The volume of the nucleus is several thousand times bigger than a single spine, and thus the impact of each spine should be very small. Since only a few spines can drive nuclear signal activation, there must be some mechanisms to amplify signal by orders of magnitude. A signaling cascade with multiple steps (for example, the classical Ras-Raf-MEK-ERK pathway) may be able to amplify signaling dramatically. More robust mechanism would be the regenerative signal amplification by positive feedback. For example, a computational model predicts that the duration of LTD in cerebellar Purkinje cells is prolonged by a positive feedback loop consisting of protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phospholipase A2 (PLA2) (Kuroda et al., 2001). Later, it was shown that the reciprocal activations of PKC to MAPK and MAPK to PKC are required for cerebellar LTD and this positive feedback loop causes PKC to be active more than 20min (Tanaka and Augustine, 2008). This kind of mechanisms could also be used for amplifying signal at single spines to affect gene transcription in the nucleus.
Biochemical signaling from nucleus back to spine
Given that L-LTP is specific to stimulated spines, the newly transcribed and synthesized proteins must function specifically in the activated spines. Thus, there must be specific interactions between the newly synthesized proteins and the activated spines during L-LTP. This can be explained by the synaptic tag and capture hypothesis. In this mechanism, LTP induction generates a protein ‘tag’ (or state of molecules) only at potentiated synapses, which can capture newly synthesized plasticity-related protein/products (PRPs) specifically induced by L-LTP (Frey and Morris, 1997; Redondo and Morris, 2011). Although the molecular identity is largely unknown, this hypothesis has provided a framework to account for the protein synthesis-dependent synaptic plasticity. PRPs that have been implicated in synaptic plasticity include Homer1a, Arc and GluA1 (Redondo and Morris, 2011). Among these proteins, Homer1a, a postsynaptic scaffolding protein and a major immediate early gene, was shown to be specifically recruited from the soma to the stimulated spine with synaptic activities, supporting the synaptic tag hypothesis (Okada et al., 2009). The synaptic tag can be a temporary state of the synapse which is represented by multiple proteins and their interactions like the structure of actin cytoskeleton (Redondo and Morris, 2011). For example, it is known that LTP causes formation of a stable pool of F-actin (Honkura et al., 2008; Okamoto et al., 2004), which potentially exists as cofilin-actin co-helices (Bosch et al., 2014). This newly formed pool of F-actin can act as a synaptic tag (Okamoto et al., 2009; Okamoto et al., 2004; Ramachandran and Frey, 2009). Interestingly, synaptic tagging appears to occur not only at the stimulated spines but also at the non-stimulated spines. Following LTP, Arc, an immediate early gene that is necessary for spatial learning and fear memory (Guzowski et al., 2000; Plath et al., 2006; Ploski et al., 2008), is accumulated in non-stimulated spines and excluded from potentiated spines (Okuno et al., 2012). The amount of synaptic Arc was negatively correlated with the amount of surface GluA1 in the synapses, consistent with previous studies suggesting that Arc weakens synapses by promoting endocytosis of AMPARs (Chowdhury et al., 2006). Thus, the inverse synaptic tagging by Arc may help to maintain the contrast of synaptic weight changes between active and inactive synapses during L-LTP by removing the surface AMPARs.
Concluding remarks
We have described the mechanisms and roles of spatiotemporal regulation of biochemical signaling in neurons during spine structural plasticity. The recent advances in optical techniques have revealed new mechanisms of biochemical computation that underlies various forms of synaptic plasticity. 2-photon uncaging of neurotransmitter have enabled researchers to study the spatiotemporal regulation of homo-and heterosynaptic plasticity and synaptic crosstalk at the level of single spines (Fig. 1). Imaging of signal transduction with FRET/FLIM techniques has allowed the spatiotemporal pattern of biochemical signaling initiated at single spines to be accessed directly. These studies have collectively provided many insights into the dynamic regulation of biochemical signaling in neuronal compartments during structural plasticity. In the temporal axis, it has been found that the signaling is transmitted in multiple stages during structural LTP (Figs. 2, 3). First, short Ca2+ signal (~0.1 s) is integrated by CaMKII activation over seconds to ~1 min. Second, the transient CaMKII signal is further relayed to several small GTPases and their downstream kinases, which leads to actin remodeling over the course of minutes to hours. Finally, PSDs and presynaptic structures are reorganized over hours. On the spatial axis, it has been revealed that biochemical computation occurs in multiple length scales from a single spine to a short stretch of dendrite around the spine and a whole dendritic branch (Figs. 2, 3). Biochemical signaling can further spread into the nucleus and regulate the gene transcription.
To understand more complicated aspects of signal transduction, including positive and negative feed-forward and feed-back loops, it is necessary to manipulate signals with high spatiotemporal resolution while imaging signal transduction. In this area, various tools to regulate protein activities with light have been developed (Kennedy et al., 2010; Lee et al., 2014; Levskaya et al., 2009; Tyszkiewicz and Muir, 2008; Wu et al., 2009; Yazawa et al., 2009; Zhou et al., 2012). By combining FRET/FLIM imaging with optical manipulation of protein activities, the mechanisms underlying the spatiotemporal signal regulation in neurons may be clarified. Another future challenge will be to find how the operating principles of signal transduction during synaptic plasticity in vitro can be applied to learning and memory of animals in vivo. Imaging of spine structural plasticity during learning and memory in vivo has been performed by several groups (Holtmaat et al., 2006; Lai et al., 2012; Moczulska et al., 2013; Xu et al., 2009; Yang et al., 2009). Applying FRET-FLIM imaging in vivo will allow us to link findings based upon controlled stimulation in slices with molecular mechanisms of learning and memory. Continued development of optical techniques will help to elucidate the operating principles of biochemical computation mediated by complicated signaling networks in neurons.
Acknowledgments
We thank the member of the Yasuda lab for discussion, T. Yasuda S. Soderling and Y. Hayashi and for critical reading and H. Bito and Y. Hayashi for Fig.2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature reviews Neuroscience. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Christie BR, Logan B, Lawlor P, Dragunow M. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10049–10053. doi: 10.1073/pnas.91.21.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JP, Dudek SM. Late-phase long-term potentiation: getting to the nucleus. Nature reviews Neuroscience. 2005;6:737–743. doi: 10.1038/nrn1749. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological reviews. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Zeng M, Zhang M, Huganir RL. Rapid Dispersion of SynGAP from Synaptic Spines Triggers AMPA Receptor Insertion and Spine Enlargement during LTP. Neuron. 2015;85:173–189. doi: 10.1016/j.neuron.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Vogels TP, Yuste R. Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2895–2904. doi: 10.1073/pnas.1321869111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Molecular cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Molecular brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS biology. 2009;7:e1000190. doi: 10.1371/journal.pbio.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Current opinion in neurobiology. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Butko MT, Yang J, Geng Y, Kim HJ, Jeon NL, Shu X, Mackey MR, Ellisman MH, Tsien RY, Lin MZ. Fluorescent and photo-oxidizing TimeSTAMP tags track protein fates in light and electron microscopy. Nature neuroscience. 2012;15:1742–1751. doi: 10.1038/nn.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum AR, Wu B, Singer RH. Single beta-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science. 2014;343:419–422. doi: 10.1126/science.1242939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywalez WG, Patirniche D, Rupprecht V, Stemmler M, Herz AV, Palfi D, Rozsa B, Egger V. Local Postsynaptic Voltage-Gated Sodium Channel Activation in Dendritic Spines of Olfactory Bulb Granule Cells. Neuron. 2015 doi: 10.1016/j.neuron.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Ch’ng TH, Martin KC. Synapse-to-nucleus signaling. Current opinion in neurobiology. 2011;21:345–352. doi: 10.1016/j.conb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Uzgil B, Lin P, Avliyakulov NK, O’Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell. 2012;150:207–221. doi: 10.1016/j.cell.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazeau A, Mehidi A, Nair D, Gautier JJ, Leduc C, Chamma I, Kage F, Kechkar A, Thoumine O, Rottner K, et al. Nanoscale segregation of actin nucleation and elongation factors determines dendritic spine protrusion. The EMBO journal. 2014;33:2745–2764. doi: 10.15252/embj.201488837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Molecular & cellular proteomics : MCP. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CJ, Mercaldo V, Restivo L, Yiu AP, Sekeres MJ, Han JH, Vetere G, Pekar T, Ross PJ, Neve RL, et al. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nature neuroscience. 2012;15:1255–1264. doi: 10.1038/nn.3189. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15:551–556. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Synaptic correlates of associative potentiation/depression: an ultrastructural study in the hippocampus. Brain research. 1983;265:21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- Doyere V, Srebro B, Laroche S. Heterosynaptic LTD and depotentiation in the medial perforant path of the dentate gyrus in the freely moving rat. Journal of neurophysiology. 1997;77:571–578. doi: 10.1152/jn.1997.77.2.571. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Enoki R, Hu YL, Hamilton D, Fine A. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron. 2009;62:242–253. doi: 10.1016/j.neuron.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Ewers H, Tada T, Petersen JD, Racz B, Sheng M, Choquet D. A Septin-Dependent Diffusion Barrier at Dendritic Spine Necks. PloS one. 2014;9:e113916. doi: 10.1371/journal.pone.0113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Inoue M, Okuno H, Sano Y, Takemoto-Kimura S, Kitamura K, Kano M, Bito H. Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIalpha and calcineurin. Cell reports. 2013;3:978–987. doi: 10.1016/j.celrep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends in cell biology. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang SY, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nature reviews Neuroscience. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Grunditz A, Holbro N, Tian L, Zuo Y, Oertner TG. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13457–13466. doi: 10.1523/JNEUROSCI.2702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nature neuroscience. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012;491:599–602. doi: 10.1038/nature11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GC, Matsuzaki M, Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nature neuroscience. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TC, Zito K. LTP-induced long-term stabilization of individual nascent dendritic spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:678–686. doi: 10.1523/JNEUROSCI.1404-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey RA, Ch’ng TH, O’Dell TJ, Martin KC. Activity-dependent anchoring of importin alpha at the synapse involves regulated binding to the cytoplasmic tail of the NR1-1a subunit of the NMDA receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15613–15620. doi: 10.1523/JNEUROSCI.3314-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Kreutz MR. Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends in neurosciences. 2009;32:392–401. doi: 10.1016/j.tins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Bera S, Bar J, Reddy PP, Behnisch T, Rankovic V, Spilker C, Bethge P, Sahin J, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152:1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nature methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, Wetsel WC, Soderling SH. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Wang H, Soderling SH, Yasuda R. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. eLife. 2014;3 doi: 10.7554/eLife.02839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Molecular biology of the cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca(2+) signals in spines of hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Schweighofer N, Kawato M. Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5693–5702. doi: 10.1523/JNEUROSCI.21-15-05693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Current opinion in neurobiology. 2008;18:321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, Heo WD. Reversible protein inactivation by optogenetic trapping in cells. Nature methods. 2014;11:633–636. doi: 10.1038/nmeth.2940. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MZ, Glenn JS, Tsien RY. A drug-controllable tag for visualizing newly synthesized proteins in cells and whole animals. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7744–7749. doi: 10.1073/pnas.0803060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature reviews Neuroscience. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature neuroscience. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Moczulska KE, Tinter-Thiede J, Peter M, Ushakova L, Wernle T, Bathellier B, Rumpel S. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18315–18320. doi: 10.1073/pnas.1312508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Kostinger G, Anderson JC, Martin KA, Bonhoeffer T. Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8149–8156. doi: 10.1523/JNEUROSCI.0511-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Kim R, Fukushima H, Sasaki K, Suzuki K, Okamura M, Ishii Y, Kawashima T, Kamijo S, Takemoto-Kimura S, et al. Region-specific activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron. 2014;84:92–106. doi: 10.1016/j.neuron.2014.08.049. [DOI] [PubMed] [Google Scholar]
- Oh WC, Hill TC, Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E305–312. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Parajuli LK, Zito K. Heterosynaptic Structural Plasticity on Local Dendritic Segments of Hippocampal CA1 Neurons. Cell reports. 2015;10:162–169. doi: 10.1016/j.celrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada D, Ozawa F, Inokuchi K. Input-specific spine entry of soma-derived Vesl-1S protein conforms to synaptic tagging. Science. 2009;324:904–909. doi: 10.1126/science.1171498. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature neuroscience. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AF, Yasuda R. Neurofibromin is the major ras inactivator in dendritic spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:776–783. doi: 10.1523/JNEUROSCI.3096-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Organization of the Arp2/3 complex in hippocampal spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5654–5659. doi: 10.1523/JNEUROSCI.0756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran B, Frey JU. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nature reviews Neuroscience. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdrulla AD, Linden DJ. Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nature neuroscience. 2007;10:546–548. doi: 10.1038/nn1889. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Computational study of an excitable dendritic spine. Journal of neurophysiology. 1988;60:499–523. doi: 10.1152/jn.1988.60.2.499. [DOI] [PubMed] [Google Scholar]
- Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, Myczek K, Radulovic J, Swanson GT, Penzes P. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84:399–415. doi: 10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Svoboda K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron. 2007;53:17–24. doi: 10.1016/j.neuron.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature neuroscience. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Current biology : CB. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y. Locally synchronized synaptic inputs. Science. 2012;335:353–356. doi: 10.1126/science.1210362. [DOI] [PubMed] [Google Scholar]
- Takao K, Okamoto K, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nature neuroscience. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008a;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008b;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KR, Otis KO, Chen DY, Zhao Y, O’Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Katona G, Rozsa B, Nagerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nature neuroscience. 2014;17:678–685. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz AB, Muir TW. Activation of protein splicing with light in yeast. Nature methods. 2008;5:303–305. doi: 10.1038/nmeth.1189. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Experimental neurology. 1975;49:736–749. doi: 10.1016/0014-4886(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Wang XB, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Oertner TG. Long-term depression triggers the selective elimination of weakly integrated synapses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4510–4519. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Current biology : CB. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Harvey CD, Zhong H, Sobczyk A, van Aelst L, Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nature neuroscience. 2006a;9:283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Current opinion in neurobiology. 2006b;16:551–561. doi: 10.1016/j.conb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Murakoshi H. The mechanisms underlying the spatial spreading of signaling activity. Current opinion in neurobiology. 2011;21:313–321. doi: 10.1016/j.conb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R. Studying signal transduction in single dendritic spines. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE. Induction of protein-protein interactions in live cells using light. Nature biotechnology. 2009;27:941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71:772–781. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Electrical compartmentalization in dendritic spines. Annual review of neuroscience. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nature neuroscience. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- Zhai S, Ark ED, Parra-Bueno P, Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cudmore RH, Lin DT, Linden DJ, Huganir RL. Visualization of NMDA receptor-dependent AMPA receptor synaptic plasticity in vivo. Nature neuroscience. 2015 doi: 10.1038/nn.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou XX, Chung HK, Lam AJ, Lin MZ. Optical control of protein activity by fluorescent protein domains. Science. 2012;338:810–814. doi: 10.1126/science.1226854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wu H, Li S, Chen Q, Cheng XW, Zheng J, Takemori H, Xiong ZQ. Requirement of TORC1 for late-phase long-term potentiation in the hippocampus. PloS one. 2006;1:e16. doi: 10.1371/journal.pone.0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]


