Abstract
Rationale: Survivors of septic shock have impaired functional status. Volume overload is associated with poor outcomes in patients with septic shock, but the impact of volume overload on functional outcome and discharge destination of survivors is unknown.
Objectives: This study describes patterns of fluid management both during and after septic shock. We examined factors associated with volume overload upon intensive care unit (ICU) discharge. We then examined associations between volume overload upon ICU discharge, mobility limitation, and discharge to a healthcare facility in septic shock survivors, with the hypothesis that volume overload is associated with increased odds of these outcomes.
Methods: We retrospectively reviewed the medical records of 247 patients admitted with septic shock to an academic county hospital between June 2009 and April 2012 who survived to ICU discharge. We defined volume overload as a fluid balance expected to increase the subject’s admission weight by 10%. Statistical methods included unadjusted analyses and multivariable logistic regression.
Measurements and Main Results: Eighty-six percent of patients had a positive fluid balance, and 35% had volume overload upon ICU discharge. Factors associated with volume overload in unadjusted analyses included more severe illness, cirrhosis, blood transfusion during shock, and higher volumes of fluid administration both during and after shock. Blood transfusion during shock was independently associated with increased odds of volume overload (odds ratio [OR], 2.65; 95% confidence interval [CI], 1.33–5.27; P = 0.01) after adjusting for preexisting conditions and severity of illness. Only 42% of patients received at least one dose of a diuretic during their hospitalization. Volume overload upon ICU discharge was independently associated with inability to ambulate upon hospital discharge (OR, 2.29; 95% CI, 1.24–4.25; P = 0.01) and, in patients admitted from home, upon discharge to a healthcare facility (OR, 2.34; 95% CI, 1.1–4.98; P = 0.03).
Conclusions: Volume overload is independently associated with impaired mobility and discharge to a healthcare facility in survivors of septic shock. Prevention and treatment of volume overload in patients with septic shock warrants further investigation.
Keywords: fluid therapy, mobility limitation, outcomes, septic shock, water–electrolyte imbalance
Septic shock is common in the intensive care unit (ICU) and often results in organ dysfunction and mortality (1). Over the past several decades, the incidence of septic shock and the severity of illness have increased while the case fatality rate and severity-adjusted mortality rate have decreased. As a result, more patients are surviving more severe illness (2, 3). Many survivors have impaired physical function and are discharged to healthcare facilities rather than to home (2–5). Between 1979 and 2000, the percentage of patients with sepsis discharged to a healthcare facility increased from 16.8% to 31.8% (2). In 2007, this percentage was 35% (3).
The decline in mortality from severe sepsis and septic shock has been attributed partly to improved recognition and protocol-based early intervention (3). Early and aggressive fluid resuscitation is the standard of care in septic shock (6–8). Although it is necessary to address the relative hypovolemia of sepsis, this practice frequently leaves patients with a positive fluid balance (9). Several studies in patients with sepsis have shown an association between positive fluid balance or volume overload and mortality, acute kidney injury (AKI), or need for fluid-related interventions (9–13).
Two studies suggest better outcomes with a negative fluid balance or active diuresis in patients with sepsis and/or acute respiratory distress syndrome (ARDS) (14, 15). In the Fluid and Catheter Treatment Trial, 70% of patients had infection (pneumonia or sepsis) identified as their primary ARDS risk factor, and the reduction in ventilator and ICU days in the conservative fluid strategy group was no different in patients with shock at enrollment (15). There has been considerable attention placed on fluid management and its impact on survival in septic shock. However, little is known about the relationship between fluid management and functional outcome among septic shock survivors.
This study describes contemporary fluid management approaches in ICU patients who survive septic shock. We report the prevalence of and risk factors for volume overload upon ICU discharge and examine associations between volume overload upon ICU discharge, functional outcome, and discharge destination. We hypothesized that volume overload upon ICU discharge would be independently associated with inability to ambulate upon hospital discharge and, in patients admitted from home, discharge to a healthcare facility.
Portions of this material were presented in abstract form at the American Thoracic Society international conferences in 2012 and 2013 (16, 17).
Methods
Patient Selection
We evaluated a retrospective cohort of 247 consecutive patients with septic shock admitted to an academic county hospital between June 2009 and April 2012. Patients had previously been identified in a quality improvement project designed to enhance early identification and treatment of septic shock in the emergency department. Criteria used to identify patients were adapted from guidelines published by Levy and coworkers in 2001 (18). Patients had to have suspected infection and had to meet at least two of four criteria for systemic inflammatory response syndrome, systolic blood pressure less than 90 mm Hg, mean arterial pressure less than 60 mm Hg, or lactate greater than 4 mmol/L, after at least 20 ml/kg fluid resuscitation. The present study included only ICU patients who survived to ICU discharge. All data were abstracted from the patients’ electronic medical records. The institutional review board at the University of Washington approved the study and waived the need for informed consent.
Exposures
We considered fluid administration during two distinct time periods: during shock and after shock resolution. Shock resolution was defined as the end of a 12-hour period in the ICU without vasopressors and without sustained hypotension (no more than one MAP reading <60 mm Hg in a 12-h period). Fluid given in the emergency department and the ICU before shock resolution was defined as given during shock. Fluid given after shock resolution and until ICU discharge was defined as given after shock resolution. Crystalloids given at a rate less than 250 ml/h were considered maintenance fluid. The timing and dosage of four diuretics (furosemide, bumetanide, torsemide, and metolazone) were reported if given at any time during the hospitalization.
There is no broadly accepted definition of what positive fluid balance should be considered “overload.” Extrapolating from previous studies, we defined volume overload upon ICU discharge as a fluid balance on the day of ICU discharge that would be expected to increase the patient’s body weight by 10% or more in relation to ICU admission weight (10, 19, 20). For example, if patients’ admission weight was 70 kg and the patient had a positive fluid balance (total fluid in minus total fluid out) of 7 L or more for their ICU stay, they would meet our definition of volume overload. Patients’ admission weight was measured on a bed scale upon arrival at the ICU. Intravenous or oral fluids of any type were considered input. Types of output included urine, ultrafiltrate from renal replacement therapy, stool, gastric drainage, emesis, and surgical or wound drains.
Outcomes
Mobility before hospital discharge was assessed by nursing staff using the Functional Independence Score (FIS) for ambulation, a functional outcome measure developed by the Washington State Department of Health and used in hospitals statewide (21). The FIS is a modified version of the Functional Independence Measure. The Functional Independence Measure was developed by the American Congress of Rehabilitation Medicine and the American Academy of Physical Medicine and Rehabilitation (22, 23). Patients were deemed unable to ambulate independently if they required one or more people for assistance with ambulation or could not ambulate at all (FIS of 1 or 2). Patients were considered able to ambulate independently if they could ambulate by themselves with or without an assistive device (e.g., cane, walker) (FIS of 3 or 4). When an FIS was not documented by nursing staff before discharge, a chart review was performed by study personnel to determine likely FIS upon discharge. Two study personnel agreed on the score independently. A healthcare facility was defined as any acute care, skilled nursing, inpatient rehabilitation, or assisted living facility. Only patients admitted from home were assessed for the outcome of discharge to a healthcare facility.
Other Definitions
Patients with end-stage renal disease (ESRD) were not evaluated for development of AKI. In subjects without ESRD, AKI was defined according to Acute Kidney Injury Network criteria as a rise in creatinine of at least 0.3 mg/dl or 50% or greater within 48 hours (24).
Statistical Analysis
In unadjusted analyses, we evaluated associations between patient characteristics and/or clinical events and volume overload upon ICU discharge, as well as the relationship between volume overload and outcomes of interest. Continuous variables were expressed as mean and SD or median and interquartile range (IQR) and compared using Student’s t test or the Wilcoxon rank-sum test as appropriate. Categorical variables were expressed as proportions and compared using the χ2 test. Multivariable logistic regression was performed to evaluate associations between clinical variables and volume overload upon ICU discharge. We adjusted for markers of illness severity (Acute Physiology and Chronic Health Evaluation II [APACHE II] score, need for vasopressors, receipt of mechanical ventilation, AKI, and need for blood transfusion during shock) and preexisting conditions we believed might be associated with volume overload (chronic kidney disease, cirrhosis, and congestive heart failure).
Relationships between volume overload upon ICU discharge and inability to ambulate or discharge to a healthcare facility were also considered using logistic regression with adjustment variables chosen a priori. For the outcome of inability to ambulate, we chose adjustment variables we felt likely to be confounders in the association between volume overload and inability to ambulate. These included demographic factors (e.g., age and sex) and multiple markers of severity of illness, including APACHE II score, need for mechanical ventilation, need for vasopressors, and duration of shock.
We then performed several sensitivity analyses (1) using Acute Physiology Score and presence or absence of chronic disease as adjustment variables instead of APACHE II score to address possible colinearity between age and APACHE II score, (2) restricting the cohort to patients in whom the FIS was determined by nursing staff to address possible confirmation bias, (3) restricting the cohort to patients admitted from home as a surrogate for mobility status upon admission, and (4) using a composite outcome of inability to ambulate or death before hospital discharge in those who survived their initial ICU stay to evaluate selection bias.
In exploratory analyses for the outcome of inability to ambulate, we considered two additional models, one with fluid balance upon ICU discharge modeled as a continuous predictor and the other with fluid balance divided into quintiles and included as dummy variables. For the outcome of discharge to a healthcare facility in patients admitted from home, we limited our adjustment variables to a subset of the full model (APACHE II score, age, and need for mechanical ventilation) to avoid overadjusting the data. A two-sided P value less than 0.05 was considered statistically significant. The statistical analysis was performed using SAS 9.0 (SAS Institute, Cary, NC) and STATA 13 (StataCorp, College Station, TX) software.
Results
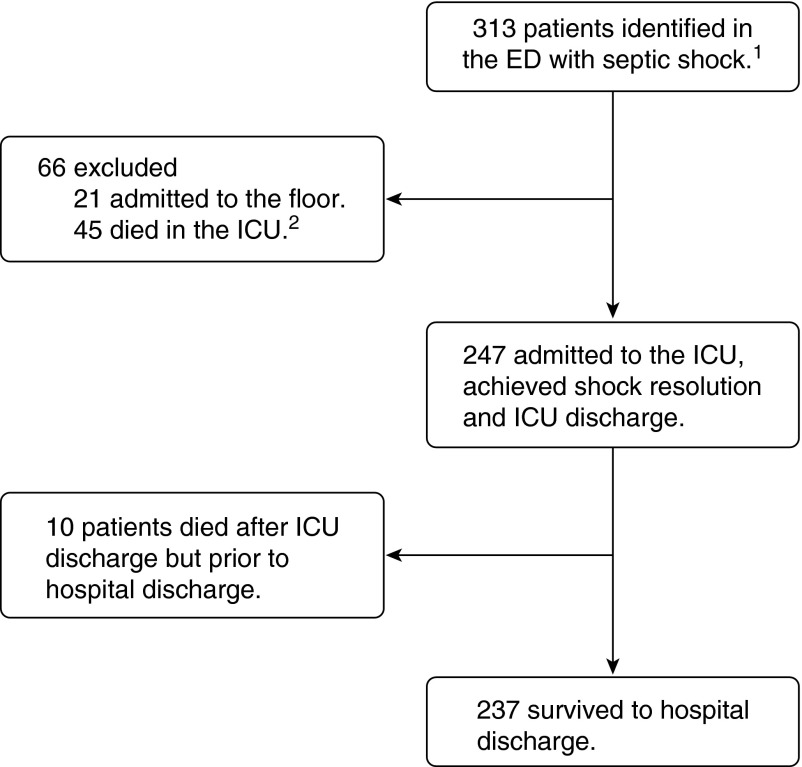
Two hundred forty-seven patients admitted to the ICU with septic shock survived to ICU discharge, and 237 survived to hospital discharge (Figure 1). Ninety (36%) had one or more preexisting chronic conditions, including congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, ESRD, or cirrhosis. The mean duration of shock was 1.7 days, and the mean duration of ICU stay after shock resolution was 4.1 days.
Figure 1.
Patient selection and outcomes. 1In the emergency department (ED), patients met at least two of four criteria for systemic inflammatory response syndrome (body temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or arterial carbon dioxide tension <32 mm Hg, and white blood cell count >12,000/mm3 or <4,000/mm3 or >10% bands) and had suspected infection. After 20 ml/kg crystalloid resuscitation, patients were included if they had systolic blood pressure less than 90 mm Hg, mean arterial pressure less than 60 mm Hg, or lactate greater than 4 mmol/L. 2ICU = intensive care unit.
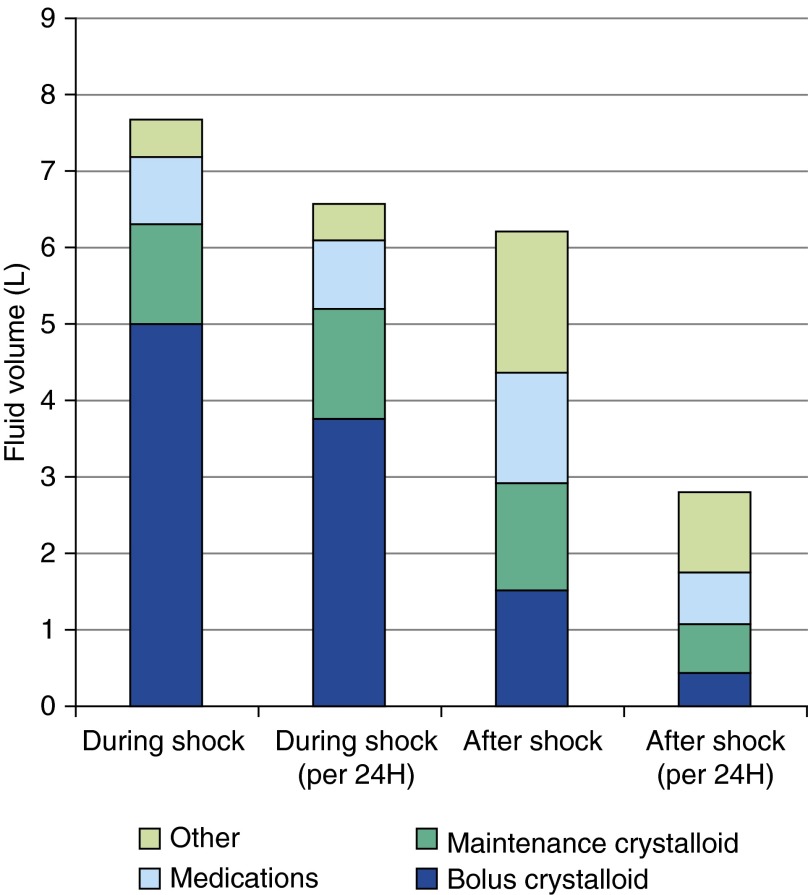
The breakdown of fluid administration during shock and after shock resolution is presented in Figure 2. Of note, 91 patients (37%) received bolus fluids after shock resolution. Two hundred seventeen patients (88%) received maintenance fluids during shock, and 203 (82%) received maintenance fluids after shock resolution. Blood was given to 73 patients (30%) overall, with 61 patients (25%) and 30 patients (12%) getting blood products during shock and after shock resolution, respectively.
Figure 2.
Fluids administered during shock and after shock resolution. Shock resolution was defined as the end of 12 hours in the intensive care unit without vasopressors and without more than one mean arterial pressure reading less than 60 mm Hg. Volumes presented are the median for each fluid category. “Other” fluid consists of albumin, blood, total parenteral nutrition, enteral nutrition, free water, and oral fluids. Crystalloid includes dextrose 10% in water, dextrose 5% in lactated Ringer solution, dextrose 5% in water, dextrose 5% in water with 0.45% NaCl, dextrose 5% with 0.45% NaCl-KCl 20 mEq/L, dextrose 5% with 0.45% NaCl-KCl 40 mEq/L, dextrose 5% with 0.9% NaCl, lactated Ringer solution, and sodium chloride 0.9%. Maintenance crystalloid was composed of any of these fluids given at a rate less than 250 milliliters per hour, and bolus crystalloid was any of these fluids given at a rate of 250 milliliters or more per hour.
Only 103 patients (42%) received at least one dose of a diuretic during their hospitalization. First diuretic administration occurred a median of 2.2 (IQR, 0.9–4.5) days after shock resolution. Of the 103 patients receiving diuretics, 72 (70%) received their first dose of diuretic in the ICU and 31 (30%) received their first dose after transfer to acute care. The characteristics of patients without ESRD who did or did not receive diuretics during their hospitalization are shown in Table E1 in the online supplement.
Two hundred thirteen patients (86%) had a positive fluid balance upon ICU discharge. The median fluid balance upon ICU discharge was +5.2 L (IQR, 2.1–10.3 L) or 6.4% (IQR, 2.6–13.5%) of admission body weight. Among 87 patients (35%) who met criteria for volume overload upon ICU discharge, the median fluid balance was +12.5 L (IQR, 9.2–18.3 L) or 16.5% (IQR, 12.5–22.4%) of admission body weight. The characteristics of patients with versus without volume overload upon ICU discharge are presented in Table 1.
Table 1.
Cohort characteristics and ICU clinical variables by volume overload on ICU discharge
| Patient Characteristics | No Volume Overload | Volume Overload | P Value |
|---|---|---|---|
| Number of patients | 160 | 87 | |
| Age (yr), mean (SD) | 54.6 (17.3) | 55 (14.7) | 0.87 |
| Males, n (%) | 105 (65.6%) | 51 (58.6%) | 0.28 |
| Weight on admission (kg), mean (SD) | 84.7 (28.8) | 76.9 (26.7) | 0.03 |
| APACHE II score, mean (SD) | 21.7 (9.2) | 27 (8.7) | <0.01 |
| Congestive heart failure, n (%) | 30 (18.9%) | 13 (14.9%) | 0.44 |
| End-stage renal disease on hemodialysis, n (%) | 7 (4.4%) | 7 (8.0%) | 0.23 |
| Chronic kidney disease (not on dialysis), n (%) | 11 (6.9%) | 7 (8.0%) | 0.74 |
| Chronic obstructive pulmonary disease, n (%) | 17 (10.6%) | 10 (11.5%) | 0.83 |
| Cirrhosis, n (%) | 9 (5.6%) | 14 (16.1%) | <0.01 |
| Admitted from healthcare facility, n (%) | 41 (25.6%) | 26 (29.9%) | 0.47 |
| Clinical variables | |||
| Shock days (n), mean (SD) | 1.3 (1.3) | 2.4 (2.4) | <0.01 |
| Days in ICU after shock resolution (n),* mean (SD) | 2.7 (3.1) | 6.8 (10.1) | <0.01 |
| Fluid in shock (L), median [IQR] | 5.8 [3.3–10] | 16.1 [7.5–22.9] | <0.01 |
| Fluid/24 h in shock (L), median [IQR] | 5.8 [3.7–7.9] | 8.3 [5.8–11.9] | <0.01 |
| Fluid/24 h in ICU after shock (L), median [IQR] | 2.7 [1.8–3.3] | 3.3 [2.2–4.1] | <0.01 |
| Received maintenance fluids during shock,† n (%) | 139 (86.9%) | 78 (89.7%) | 0.52 |
| Received maintenance fluids after shock resolution, n (%) | 126 (78.8%) | 77 (88.5%) | 0.06 |
| Received albumin during shock, n (%) | 1 (0.6%) | 6 (6.9%) | 0.01 |
| Received blood products during shock, n (%) | 24 (15.0%) | 37 (42.5%) | <0.01 |
| Required vasopressors, n (%) | 59 (36.9%) | 53 (60.9%) | <0.01 |
| Had central venous catheter, n (%) | 104 (65.0%) | 62 (71.3%) | 0.32 |
| Had arterial line, n (%) | 46 (28.8%) | 48 (55.2%) | <0.01 |
| Had Foley catheter, n (%) | 127 (79.4%) | 81 (93.1%) | 0.01 |
| Received diuretics in first ICU stay,‡ n (%) | 43 (28.1%) | 29 (36.3%) | 0.2 |
| Acute kidney injury in the ICU,‡,§ n (%) | 32 (20.9%) | 27 (33.8%) | 0.03 |
| Received mechanical ventilation, n (%) | 70 (43.8%) | 57 (65.5%) | <0.01 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
Shock resolution was defined as the end of a 12-h period in the ICU without vasopressor use and with no more than one mean arterial pressure reading <60 mm Hg.
Maintenance fluid was defined as any crystalloid administered at a rate <250 ml/h.
Percentages are of the 233 patients without a preexisting diagnosis of end-stage renal disease.
Acute kidney injury was defined according to Acute Kidney Injury Network criteria (24) for increase in creatinine (increase in creatinine≥0.3 mg/dl or ≥50% within 48 h).
In a multivariable logistic regression model adjusting for preexisting conditions and markers of severity of illness (Table 2), blood product administration during shock was significantly associated with increased odds of volume overload upon ICU discharge (odds ratio [OR], 2.65; 95% confidence interval [CI], 1.33–5.27; P = 0.01). In the same model, congestive heart failure was inversely associated with volume overload (OR, 0.43; 95% CI, 0.19–0.98; P = 0.04).
Table 2.
Associations between patient characteristics, clinical variables, and volume overload upon intensive care unit discharge
| Exposure Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| APACHE II score | 1.04 | 1–1.09 | 0.06 |
| Acute kidney injury in the ICU | 1.12 | 0.5–2.54 | 0.78 |
| Blood transfusion during shock | 2.65 | 1.33–5.27 | 0.01 |
| Mechanical ventilation | 1.12 | 0.53–2.33 | 0.77 |
| Vasopressors | 1.71 | 0.9–3.23 | 0.1 |
| Chronic kidney disease | 1 | 0.33–3.03 | 1 |
| Congestive heart failure | 0.43 | 0.19–0.98 | 0.04 |
| Cirrhosis | 2.24 | 0.86–5.86 | 0.1 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
Of the 237 subjects who survived to hospital discharge, 197 had an FIS for mobility assigned by nursing staff a mean of 24 hours before discharge and 40 had an FIS assigned by study personnel. Of 105 subjects (43.4%) unable to ambulate upon hospital discharge, 51 (82.3%) had been admitted from a healthcare facility and 54 (30%) had been admitted from home. All patients admitted from a healthcare facility were discharged to a healthcare facility. Of those admitted from home, 46 (25.6%) were discharged to a healthcare facility. Volume overload upon ICU discharge was associated with inability to ambulate independently upon hospital discharge (OR, 2.29; 95% CI, 1.24–4.25; P = 0.01), independent of age, sex, APACHE II score, duration of shock, need for vasopressors, and receipt of mechanical ventilation (Table 3). The results were similar when we used Acute Physiology Score and chronic disease in the model instead of APACHE II score (Table E2) and also when we studied only the 197 patients who had their mobility status determined by nursing staff before hospital discharge (OR, 2.11; 95% CI, 1.08–4.12; P = 0.03).
Table 3.
Relationships between volume overload upon ICU discharge and inability to ambulate upon hospital discharge as estimated by multivariable logistic regression
| Exposure Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Volume overload | 2.29 | 1.24–4.25 | 0.01 |
| Age (decades) | 1.49 | 1.22–1.82 | <0.01 |
| Male | 0.69 | 0.38–1.25 | 0.22 |
| APACHE II score | 1.02 | 0.97–1.06 | 0.45 |
| Mechanical ventilation | 0.71 | 0.33–1.51 | 0.37 |
| Duration of shock (d) | 1.01 | 0.84–1.21 | 0.95 |
| Vasopressors | 1.8 | 0.9–3.57 | 0.1 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
After restricting this analysis to patients admitted from home, we found that the association between volume overload and inability to ambulate remained statistically significant (OR, 2.19; 95% CI, 1.01–4.75; P = 0.05). The association between volume overload and the composite outcome of inability to ambulate or death before hospital discharge in patients who survived their initial ICU stay was slightly stronger (OR, 2.62; 95% CI, 1.44–4.78; P < 0.01) than the association when we used the outcome of inability to ambulate alone (Table E3). In exploratory analyses with fluid balance modeled as a continuous predictor or divided into quintiles, the association between higher fluid balance and inability to ambulate persisted (Tables E4 and E5). Among patients admitted from home, volume overload was independently associated with discharge to a healthcare facility (OR, 2.34; 95% CI, 1.1–4.98; P = 0.03) (Table 4).
Table 4.
Relationship between volume overload upon ICU discharge and discharge to a healthcare facility in patients admitted from home as estimated by multivariable logistic regression
| Exposure Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| APACHE II score | 1.02 | 0.97–1.07 | 0.5 |
| Age | 1.03 | 1.01–1.06 | 0.02 |
| Volume overload | 2.34 | 1.10–4.98 | 0.03 |
| Mechanical ventilation | 2.01 | 0.75–5.42 | 0.17 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
In unadjusted analyses in which we looked at secondary outcomes of interest, we found that patients with volume overload upon ICU discharge were more likely to have had a longer duration of mechanical ventilation in the ICU (4.4 [2.0–7.2] d vs. 1.8 [1.0–4.5] d; P < 0.01), to have been readmitted to the ICU (14.9% vs. 5.6%; P = 0.03), to have had a Foley catheter for more days after ICU discharge (8.3 [2.6–20.7] d vs. 3.8 [1.5–6.8] d; P = 0.003), to have had a longer hospital stay after ICU discharge (9.6 [3.4–22.6] d vs. 3.1 [1.2–7.8] d; P < 0.01), and to have died before hospital discharge (9.2% vs. 1.3%; P = 0.01).
Discussion
In this single-center cohort study of septic shock survivors, nearly all patients had positive fluid balance upon ICU discharge, over one-third met our definition of volume overload, and only 42% received a diuretic at any time during their hospitalization. Volume overload upon ICU discharge was preceded by more severe illness, higher volumes of fluid resuscitation and blood transfusion during shock, and longer duration of ICU stay, and it was followed by poor outcomes, including mortality after ICU discharge but before hospital discharge, longer hospital stay, and readmission to the ICU. Among patients admitted from home, almost one-third were unable to ambulate upon hospital discharge and one-fourth were discharged to a healthcare facility. Volume overload upon ICU discharge was independently associated with inability to ambulate upon hospital discharge and, in patients admitted from home, discharge to a healthcare facility.
Comparison with Previous Studies
In major randomized controlled trials of protocol-based resuscitation in sepsis, 72-hour mean or median total fluid administration has ranged from 7.7 L to 13.4 L (7, 8, 25, 26). In 2011 Boyd and colleagues reported a mean fluid balance at 4 days of 11 ± 8.9 L in a cohort of patients with septic shock (12), whereas 5 years earlier the European Sepsis Occurrence in Acutely Ill Patients study investigators reported a 72-hour mean fluid balance of 1.8 ± 5 L (10). The authors of a recent single-center study reported a 44% prevalence of diuretic use in patients with clinical evidence of volume overload at Day 3 after admission for septic shock (9). Although there appears to be variability in practice over time, based on geographic location, and between studies, our results are within the range of what might be expected on the basis of previous reports.
In 2010, Iwashyna and colleagues reported that, among patients with no functional limitations before admission for sepsis, 40% were unable to walk at the time of the first survey after hospital discharge (median, 0.9 yr after discharge) (4). Our findings are consistent with that report. Several previous studies have shown an association between positive fluid balance or volume overload and poor outcomes, including mortality in patients with septic shock and/or AKI (9–13, 20, 27–29). One study showed an association between a conservative fluid management strategy and cognitive impairment in survivors of acute lung injury (30). Multiple plausible mechanisms have been proposed for the associations between volume overload, organ dysfunction, and mortality. It is unclear whether volume overload is a mediator or a marker of illness severity in these relationships.
Experimental models, however, suggest volume overload may be responsible for some of this morbidity. Edema, positive fluid balance, and/or high central venous pressure have been shown in human and/or experimental models to have a negative impact on biological processes, including microvascular perfusion, renal perfusion, sodium and water excretion, intraabdominal pressure, cardiac function, and pulmonary gas exchange (31–36). Intestinal edema and edema of the skin may contribute to impaired epithelial integrity and risk of bacterial translocation and/or infection (37). Animal models of septic shock have shown capillary leak in the vessels serving skeletal muscle, along with endomysial edema (38). It has been theorized that the formation of endomysial edema, and an associated increase in diffusion distance from blood vessel to muscle fibers, may contribute to muscle fiber damage in patients with septic shock (38). In observational and experimental models, hyperchloremia or chloride-rich fluids have been associated with fluid retention, reduced renal perfusion, AKI, and mortality (39–44). If any of these effects on biological systems are clinically significant, it is plausible that one or a combination of volume-related complications could result in a prolonged hospital stay, impaired mobility, and/or need for discharge to a healthcare facility.
Significance of the Study Findings
These results suggest that avoidance and treatment of volume overload may improve outcomes in survivors of septic shock. In this study, patients with volume overload upon ICU discharge spent a mean of 6.8 days in the ICU after shock resolution and a median of 9.6 days in the hospital after ICU discharge. Although other clinical factors impact the appropriateness of fluid restriction and/or diuresis after shock resolution, these postshock days may represent an opportunity to reduce the prevalence of volume overload upon ICU discharge. The relative lack of diuresis after ICU discharge is also striking in this cohort and may represent an opportunity for improved communication regarding fluid balance and need for diuresis during the important transition from ICU care to acute care.
Strengths and Limitations
The largest threat to the validity of this study is the potential for confounding by indication. Volume overload is associated with greater severity of illness in this cohort and patients with volume overload spent a mean of 4.1 days longer in the ICU after shock resolution than patients without volume overload. The direction of these associations is not entirely clear. Despite the strong association between severity of illness and volume overload in unadjusted analyses, our multivariable model did not detect an independent association between markers of severity of illness and volume overload. This may be because no association exists after adjusting for the other covariates, but it could also be due to colinearity between severity of illness and fluid balance or to small sample size.
We hypothesize, however, that a portion of the prolonged ICU stay seen in our patients with volume overload is due to volume-related complications or organ dysfunction. A conservative fluid strategy has been shown to decrease the length of ICU stay by a mean of 2.2 days in patients with sepsis and ARDS (15). Preventing or treating volume overload in patients with sepsis might also reduce ICU length of stay via reduction in other edema-related complications or organ dysfunction. We have attempted to account for this by adjusting for multiple markers of severity of illness, but we recognize the potential for residual confounding. The small number of outcomes in our analysis of discharge to a healthcare facility limited our ability to control for multiple confounders.
Our choice to look at only survivors may have introduced selection bias. If larger-volume fluid resuscitation conveys a survival advantage in patients with similar illness severity, this could result in a type I error. In a sensitivity analysis using an outcome of death before hospital discharge or inability to ambulate upon hospital discharge in all 247 patients who survived their ICU stay, we found a stronger association between volume overload and the composite outcome.
It is possible, owing to inaccurate records of urine output, that there was misclassification of volume overload in our cohort. However, patients without Foley catheters, who would have been misclassified in the volume overload group owing to unmeasured output, were likely to be healthier, nonventilated, and unsedated. The inclusion of these patients in our volume overload group would therefore bias our results toward the null.
Finally, this was a single-center study at an urban academic hospital with a relatively young and ethnically diverse population. The results, fluid management practices, and prevalence of volume overload in this cohort may not be representative of other populations or other centers.
Despite these limitations, we believe this study is an important and novel contribution to the literature on fluid management of septic shock and outcomes of volume overload. We provide detailed information on fluid administration in patients with septic shock both during and after shock resolution. To our knowledge, this is the first study in which the prevalence of volume overload and the prevalence and timing of diuretic administration have been reported in septic shock survivors, as well as the first study to show an association between volume overload and poor functional outcome in survivors of septic shock. As the number of septic shock survivors continues to rise, it becomes increasingly important to identify modifiable risk factors for poor functional outcome in these patients. In the context of previously published literature showing an association with fluid balance and mortality in patients with septic shock, this study provides observational evidence that supports an interventional trial comparing “usual care” to a protocol aimed at prevention and treatment of volume overload in patients admitted with septic shock.
Conclusions
Volume overload is common in survivors of septic shock and is associated with poor mobility upon hospital discharge and discharge to a healthcare facility in patients admitted from home. Diuretic administration is uncommon and is delayed after shock resolution. A randomized trial of prevention and treatment of volume overload is needed to clarify the role of volume overload in the functional outcome of septic shock survivors.
Footnotes
Supported by a Pulmonary and Critical Care Medicine Research Training grant (T32HL007287) from the National Institutes of Health.
Author Contributions: conception and design of the study: K.H.M. and C.L.H.; acquisition of data: K.H.M., D.C., and E.C.; analysis of data: K.H.M., E.C., and C.L.H.; interpretation of data: K.H.M., P.J.L., and C.L.H.; contributed to conception of study: J.H.; drafting of the manuscript: K.H.M.; revision of the manuscript and final approval of the version to be published: K.H.M., D.C., E.C., P.J.L., J.H., and C.L.H.; and agree to be accountable for all aspects of the work in terms of accuracy and integrity: K.H.M., D.C., E.C., P.J.L., J.H., and C.L.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, et al. Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 7.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 8.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43:68–73. doi: 10.1097/SHK.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, Machado FR. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30:97–101. doi: 10.1016/j.jcrc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 13.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117:1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 15.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell KH, Ray TD, Carlbom DH, Hough CL. Fluid management of sepsis after shock resolution: an analysis of intensive care unit volume status in relation to mobility on hospital discharge [abstract] Am J Respir Crit Care Med. 2013;187(Meeting Abstracts):A3951. [Google Scholar]
- 17.Mitchell KH, Watkins TR, Hough CL. Fluid management of sepsis after shock resolution: an analysis of practice variation and risk factors [abstract] Am J Respir Crit Care Med. 2012;185(Meeting Abstracts):A1124. [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 21.Washington State Department of Health Washington state hospital data dictionary, version CVW4 Olympia, WA: Washington State Department of Health; 201167–88.[accessed 2015 Oct 8]. http://www.doh.wa.gov/Portals/1/Documents/Pubs/530124.pdf [Google Scholar]
- 22.Granger CV, Gresham GE. Baltimore: Williams & Wilkins; 1984. Functional assessment in rehabilitation medicine. [Google Scholar]
- 23.Uniform Data System for Medical Rehabilitation (UDSMR) The FIM Instrument: its background, structure, and usefulness Buffalo, NY: UDSMR; 2012[accessed 2015 Oct 8]. http://www.udsmr.org/Documents/The_FIM_Instrument_Background_Structure_and_Usefulness.pdf [Google Scholar]
- 24.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 26.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, et al. ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 27.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, Forfori F, Pelaia P, Rocco M, Ronco C, et al. NEFROlogia e Cura INTensiva (NEFROINT) investigators. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt S, Regueira T, Bracht H, Porta F, Djafarzadeh S, Takala J, Gorrasi J, Borotto E, Krejci V, Hiltebrand LB, et al. Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care. 2009;13:R186. doi: 10.1186/cc8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daugherty EL, Hongyan Liang, Taichman D, Hansen-Flaschen J, Fuchs BD. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J Intensive Care Med. 2007;22:294–299. doi: 10.1177/0885066607305247. [DOI] [PubMed] [Google Scholar]
- 33.D’Orio V, Mendes P, Carlier P, Fatemi M, Marcelle R. Lung fluid dynamics and supply dependency of oxygen uptake during experimental endotoxic shock and volume resuscitation. Crit Care Med. 1991;19:955–962. doi: 10.1097/00003246-199107000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol. 2013;13:17. doi: 10.1186/1471-2253-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21. doi: 10.1186/s13613-014-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauptmann S, Klosterhalfen B, Weis J, Mittermayer C, Kirkpatrick CJ. Skeletal muscle oedema and muscle fibre necrosis during septic shock: observations with a porcine septic shock model. Virchows Arch. 1994;424:653–659. doi: 10.1007/BF00195781. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury AH, Cox EF, Francis ST, Lobo DN.A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers Ann Surg 201225618–24.[Published erratum appears in Ann Surg 2013;258:1118.] [DOI] [PubMed] [Google Scholar]
- 41.Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 42.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 43.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 44.Neyra JA, Canepa-Escaro F, Li X, Manllo J, Adams-Huet B, Yee J, Yessayan L Acute Kidney Injury in Critical Illness Study Group. Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med. 2015;43:1938–1944. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]