Abstract
Heterogeneity in the development and progression of cigarette smoke–induced lung diseases strongly argues for a need to improve the clinical and phenotypic characterization of patients with chronic obstructive lung disease and emphysema. Smokers with emphysema are at a much higher risk for accelerated loss of lung function, increased cardiovascular morbidity, and development of lung cancer. Recent evidence in human translational studies and animal models suggests that emphysema is associated with activation of specialized antigen-presenting cells and that cigarette smoke can disrupt the induction of immune tolerance in the lungs. Quantitative assessment of cytokines expressed by autoreactive T lymphocytes in response to human lung elastin fragments has shown a strong positive correlation between T helper Type 1 (Th1) and Th17 cells’ immune responses and emphysema. In search of factors that could reduce the threshold for induction of autoimmune inflammation, we have discovered that cleavage of complement protein 3 (C3) generates bioactive molecules (e.g., C3a) and activates lung antigen-presenting cells. The autocrine and paracrine function of C3a and its receptor are required in T cell–mediated inflammatory responses to cigarette smoke in both human and preclinical models of emphysema. Targeting upstream molecules that reduce the potential for generation of autoreactive T cells could lead to the development of novel therapeutics to prevent progression of emphysema in smokers.
Keywords: emphysema, T cells, complement proteins, C3a
Despite substantial advances in the current understanding of the pathogenesis of cigarette smoke–induced chronic lung inflammation and emphysema, disease heterogeneity and the unpredictable rate of disease progression remain a conundrum. In particular, fundamental questions remain regarding how genetic and environmental factors could affect disease severity, age of onset, and disease progression in active and former smokers (1–3). Emerging data from large clinical studies show emphysema phenotype as one of the main factors associated with high morbidity and mortality; accordingly, the importance of phenotype characterization in smokers has become an essential component of observational and interventional studies in recent years (4). However, susceptibility to emphysema varies greatly among asymptomatic smokers, and its prevalence is difficult to estimate because appropriate diagnostic tests are universally delayed until significant respiratory symptoms develop. To date, smoking cessation remains the best intervention to reduce the rapid decline in lung function, whereas medical interventions reduce only the number of disease exacerbations (5). In this report, we describe how critical observations in longitudinal studies in smokers have provided the rationale for subsequent studies on the role of innate and acquired immunity in the pathogenesis of emphysema.
Autoimmunity in Emphysema: Link to Disease Progression
In the Lung Health Study, a large, multicenter longitudinal study in which researchers prospectively assessed the rate of lung function decline in smokers with normal lung function, an increased incidence of FEV1 decline was observed in active smokers; however, a subpopulation of active smokers maintained normal lung function (6). Interestingly, this study also documented a significant decline in FEV1 in a subpopulation of sustained quitters, indicating that rapid loss of lung function could occur in susceptible individuals despite smoking cessation (6). This heterogeneity in susceptibility to cigarette smoke–induced lung destruction supports a role for activation of acquired immune memory responses to cigarette smoke (7, 8).
Development of early-onset emphysema in highly susceptible smokers might be attributable in part to underdetected mutations in the α1-antitrypsin (A1AT) gene locus (e.g., ZZ, MZ, MS). However, in the vast majority of individuals, the genetic factors responsible for increased susceptibility to emphysema remain largely unknown (9). Indeed, several large studies have shown that although multiple genes contribute to increased risk of emphysema, each individual gene shows only a modest and independent effect (10, 11).
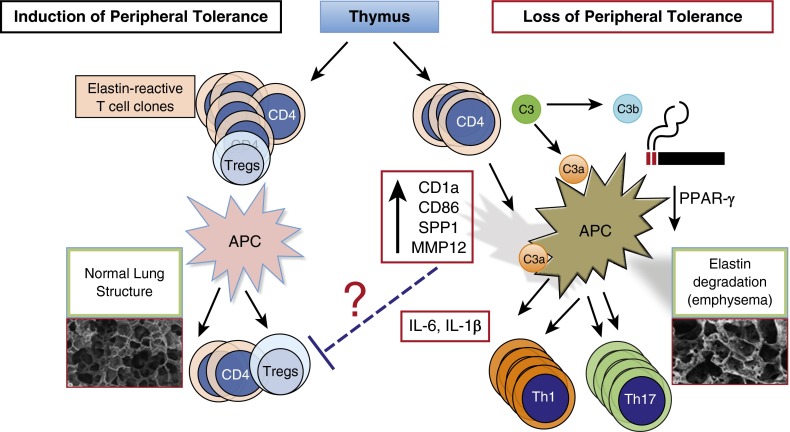
These recent discoveries suggest that the genetic basis for smoke-induced emphysema phenotype is compatible with a heritable multifactorial trait, similar to the genetic basis for autoimmune diseases. We proposed that lung parenchymal destruction in smokers with emphysema may in part be secondary to loss of peripheral tolerance to self-antigens (e.g., lung elastin fragments) or to development of autoimmune responses to neoantigens (8). Under normal conditions, strong autoreactive T lymphocytes are efficiently eliminated in the thymus, whereas immature but committed T cells with low affinity to self could emerge, providing a highly variable number of autoreactive T cells in the circulation. Environmental and genetic factors, as well as interaction with tolerogenic or activating antigen-presenting cells, could govern the state of autoreactive T-cell expansion (self-reactivity) (12). Chronic exposure to cigarette smoke is a potent stimulator of lung antigen-presenting cells (13, 14) that can promote loss of peripheral tolerance (Figure 1) (15–17). Given the finding that activated T and B cells are present in the lungs of former smokers (18, 19), we proposed the hypothesis that activation of lung antigen-presenting cells in susceptible smokers could unleash autoreactive T cells that propagate a vicious cycle and promote lung destruction even in the absence of exposure to smoke (20).
Figure 1.
Schematic diagram of possible mechanisms involved in loss of peripheral tolerance in cigarette smoke–induced emphysema. Under normal conditions, signals from tolerogenic antigen-presenting cells (APCs) in the lungs or regional lymph nodes promote maintenance of peripheral tolerance; self-reactive T cells emerging from the thymus remain nonresponsive to self-antigens (left side). However, cigarette smoke downregulates peroxisome proliferator-activated receptor (PPAR)-γ, which in turn activates SPP1 gene expression and increases several costimulatory molecules (e.g., CD1a, CD86, major histocompatibility complex class II) on lung APCs. In susceptible smokers, possibly with lower thresholds for developing autoimmune T cells (e.g., higher number of self-reactive T cells or exaggerated responses to cigarette smoke activation of APCs), antigen-specific autoreactive helper T Type 1 (Th1) and Th17 cells clonally expand when they come in contact with activated APCs and promote a slow but progressive form of lung destruction (right side). Cigarette smoke induces recruitment of innate immune cells. Enzymes released by activated neutrophils and macrophages (e.g., neutrophil elastase, matrix metalloproteinase 12 [MMP12]) could activate complement proteins (e.g., C3, C3a) and their receptors (e.g., C3aR), thereby accelerating the development of autoreactive Th1 and Th17 cells and lung destruction in smokers. Tregs = regulatory T cells.
Evidence for Elastin-Specific Autoreactive T Cells in Emphysema
In support of the hypothesis that loss of tolerance to self-proteins could direct T-cell–mediated autoimmune inflammation in the lungs, several components of the lung matrix molecules, including collagen and elastin, have been shown to degrade when exposed to potent enzymes released by activated neutrophils and macrophages (21–23). Examination of lung explants from former smokers showed large numbers of activated CD4 and CD8 T cells that were biased to T helper Type 1 (Th1) and Th17 cells expressing IFN-γ and IL-17, respectively (24). An increased relative abundance of Th1 and Th17 cells was found in the lung parenchyma of former smokers with emphysema compared with the lung parenchyma of former smokers without emphysema. Furthermore, lung antigen-presenting cells expressing CD1a and belonging to a myeloid dendritic cell (mDC) lineage were found to express several costimulatory molecules, including CD83, CD86, and major histocompatibility complex class II markers, indicating an activated state (24). Autologous culture of CD1 mDCs isolated from the lungs of smokers with emphysema induced T-cell proliferation and increased IFN-γ and IL-17 expression compared with control mDCs in nonemphysematous lungs (24).
Human elastin is a hydrophobic molecule with repeats of glycine, valine, and proline that form a highly insoluble and durable matrix protein designed to last throughout the lives of most species (25). When we generated 20–amino acid peptide sequences with overlapping 10-mers that spanned the whole human elastin molecule, we found several highly immunogenic segments (26). Employing the in vitro T-cell responses to synthetic 20-mer overlapping elastin peptides, we identified sequences that bound to major histocompatibility complex class II (DRB1) with strong affinity and showed the strongest cognate cytokine secretion in T cells (26). We used a combination of elastin stimulation and dilutional cloning techniques to clone several lines of T cells that were highly reactive to elastin peptides and secreted IL-6 and IFN-γ upon stimulation with elastin fragments (26).
The presence of autoreactive T cells in smokers with emphysema was highly correlated with decline in 6-minute walk distance over the course of 5 years and with the degree of lung function abnormality (i.e., FEV1%, diffusion capacity of carbon monoxide, quantitative measurement of emphysema on imaging studies) (26). Therefore, discovery and cloning of elastin-specific autoreactive T cells in smokers with emphysema provides the first experimental evidence in support of the hypothesis that loss of tolerance to self-antigens or neoantigens may be an important causative factor in emphysema development in a subset of smokers. It should be noted, however, that the presence of autoreactive T and B cells against self-antigens in smokers with emphysema does not prove causality and could represent an epiphenomenon. Nonetheless, our cloning of elastin-specific T cells in the peripheral blood that secrete IL-6 and IFN-γ in response to their cognate antigen (e.g., elastin fragments) represents immunological evidence for the possible functional significance of autoreactive immune responses in smokers with emphysema (26).
Chronic Obstructive Pulmonary Disease Exacerbation: Activation of Complement Proteins in Emphysema
We and others have searched for factors that could potentially lower the threshold for the loss of peripheral tolerance to self-antigens or neoantigens in the lungs during disease exacerbation. There is direct and indirect evidence for perturbations in factors associated with complement proteins and their regulatory elements that can activate T cells (27, 28). Prior work showed that an increased concentration of proinflammatory cytokines (e.g., IL-6, IL-1β, IL-17) is among the most important hallmarks of smoke-mediated inflammation in the lung. Decreased expression or function of peroxisome proliferator-activated receptor (PPAR)-γ has been associated with a number of autoimmune diseases (29–31), and lack of this transcription factor in antigen-presenting cells (14, 32) or macrophages (33) results in spontaneous development of lung inflammation and emphysema. We recently showed that cigarette smoke decreases the expression of PPAR-γ, an antiinflammatory nuclear receptor transcription factor (13, 14). Our group was the first to show that, after mice develop emphysema and despite continued exposure to cigarette smoke, intranasal administration of ciglitazone (an agonist of PPAR-γ) can reduce lung inflammation and emphysema (14). The molecular mechanisms by which induction of PPAR-γ results in lung repair, as well as how cigarette smoke reduces expression and/or function of PPAR-γ, remains unclear; however, this is an active area of investigation. Furthermore, IL-17 plays a critical role in smoke-induced emphysema in humans and in mouse models of the disease (13, 34–37). Adoptive transfer of lineage-negative CD11b+CD11c+ mDCs isolated from the lungs of smoke-exposed mice to naive mice recapitulates smoke-induced lung disease, indicating a direct causal relationship between this cell population and emphysema (13).
In an animal model of emphysema, we show that, in contrast to lung mDCs isolated from wild-type mice exposed to chronic smoke, lung mDCs from C3- and C3ar-deficient mice exposed to chronic smoke have attenuated IL-6 expression and fail to induce IL-17 in CD4 T cells. These findings provide direct evidence that the mechanism underlying the attenuated response to smoke in C3−/− and C3aR−/− mice involves failure of lung mDCs to induce proinflammatory and acquired immune responses. The prominent deposition of C3 in the lungs of smokers with emphysema and increased C3 concentration in the plasma of smokers with emphysema further support a role for complement-mediated activation of immune responses in the development of emphysema (28). Increased expression of C3a receptor (C3aR) on mDCs is required for effective activation and clonal expression of autoreactive Th17 cells in the lungs. Collectively, in addition to the loss of self-tolerance in smokers with emphysema, we propose a new protease-dependent pathway for C3a generation: proteinases secreted by innate immune cells (e.g., neutrophil elastase, cathepsin G, matrix metalloproteinase 12) cleave C3 to release C3a, which acts on mDCs to upregulate cell surface expression of its own receptor (e.g., C3aR) (Figure 1).
Several studies have shown increased lung remodeling in response to acute cigarette smoke exposure (38, 39). Unclear, however, is whether activation of complement proteins or accumulation of neutrophils and macrophages in chronic inflammatory conditions induce fibrosis in the lungs. In response to 4 months of cigarette smoke exposure, chest computed tomography and mean linear intercept examination of lung showed reduced lung density and increased lung volume consistent with physiological and histological features of emphysema (13). Further, immunization with elastin peptides results in activation of the acquired immune responses and destruction of the elastin-rich organs (40). Similarly, human emphysematous lungs harbor a distinct subset of Th17 cells and elastin-specific B cells, indicating that acquired immune responses are activated in the lungs of smokers with emphysema (20, 24, 41).
Conclusions
Development and progression of emphysema in current or former smokers is a complex process with an expanding list of several known and unknown contributors that include age, race, sex, genetics, and environmental factors. Experimental and translational research has shown that each of these factors could contribute to some facet of smoke-induced lung disease and that aging and its effect on acquired immune responses may play a more prominent role in the progression of emphysema and merits future investigation. In addition to innate immune cells (e.g., neutrophils and macrophages) that are recruited to the lung in response to cigarette smoke, recent human association studies, as well as preclinical models of smoke-induced emphysema, have confirmed that acquired immune cells (T and B cells) and lung antigen-presenting cells are also activated in smoke-induced lung inflammation (14, 24, 42, 43). Ever-smokers with emphysema harbor elastin-specific autoreactive T cells in their peripheral blood long after smoking cessation, and the degree of activated T cells correlates with loss of lung function (26). Although memory T cells that are reactive to self-antigens or neoantigens are present in former smokers, it is unclear whether there are mechanisms by which immune tolerance could be restored and/or autoreactive T cells could be removed.
Whether activation of C3 in the lungs by innate immune cells via their secreted proteinase is a bystander consequence of the increase in inflammation driven by smoke, as well as whether genetic factors regulate this process, remains unknown. Given that few treatment options are in the pipeline that provide symptomatic relief for smokers with emphysema, there is a strong unmet need for delivering new biologics to slow down disease progression in the right target population. Activation of the C3a/C3aR complement cascade appears to play an important role in the pathogenesis of emphysema, which suggests that targeting C3 cleavage and/or suppressing the active fragments could be used to treat this disease. These findings introduce a new field of investigation related to the mechanism and kinetics of T-cell activation and the consequences of the smoke-induced activation of complement protein in the lungs.
Footnotes
Supported by National Institutes of Health Grants HL117181 and HL110883 and U.S. Department of Veterans Affairs merit review awards (F.K., D.C.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35:1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Agustí A, Vestbo J. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:507–513. doi: 10.1164/rccm.201103-0405PP. [DOI] [PubMed] [Google Scholar]
- 3.Roca J, Vargas C, Cano I, Selivanov V, Barreiro E, Maier D, Falciani F, Wagner P, Cascante M, Garcia-Aymerich J, et al. Chronic obstructive pulmonary disease heterogeneity: challenges for health risk assessment, stratification and management. J Transl Med. 2014;12(Suppl 2):S3. doi: 10.1186/1479-5876-12-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestbo J, Agusti A, Wouters EFM, Bakke P, Calverley PMA, Celli B, Coxson H, Crim C, Edwards LD, Locantore N, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Study Investigators. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189:1022–1030. doi: 10.1164/rccm.201311-2006PP. [DOI] [PubMed] [Google Scholar]
- 5.Vestbo J, Lange P. COPD drugs: the urgent need for innovation. Lancet Respir Med. 2014;2:14–15. doi: 10.1016/S2213-2600(13)70278-9. [DOI] [PubMed] [Google Scholar]
- 6.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 7.Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med. 1999;160(Suppl):S21–S25. doi: 10.1164/ajrccm.160.supplement_1.7. [DOI] [PubMed] [Google Scholar]
- 8.Kheradmand F, Shan M, Xu C, Corry DB. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol. 2012;8:285–292. doi: 10.1586/eci.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene CM, Hassan T, Molloy K, McElvaney NG. The role of proteases, endoplasmic reticulum stress and SERPINA1 heterozygosity in lung disease and α-1 anti-trypsin deficiency. Expert Rev Respir Med. 2011;5:395–411. doi: 10.1586/ers.11.20. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Kowgier M, Loth DW, Soler Artigas M, Joubert BR, Hodge E, Gharib SA, Smith AV, Ruczinski I, Gudnason V, et al. Large-scale genome-wide association studies and meta-analyses of longitudinal change in adult lung function. PLoS One. 2014;9:e100776. doi: 10.1371/journal.pone.0100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzie ME, Crawford M, Cho JH, Orellana R, Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122–131. doi: 10.1136/thoraxjnl-2011-200089. [DOI] [PubMed] [Google Scholar]
- 12.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;4:117ra9. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan M, You R, Yuan X, Frazier MV, Porter P, Seryshev A, Hong JS, Song LZ, Zhang Y, Hilsenbeck S, et al. Agonistic induction of PPARγ reverses cigarette smoke-induced emphysema. J Clin Invest. 2014;124:1371–1381. doi: 10.1172/JCI70587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res. 2013;55:48–57. doi: 10.1007/s12026-012-8347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Núñez B, Sauleda J, Antó JM, Julià MR, Orozco M, Monsó E, Noguera A, Gómez FP, Garcia-Aymerich J, Agustí A PAC-COPD Investigators. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–1031. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 18.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 21.Kucich U, Christner P, Lippmann M, Fein A, Goldberg A, Kimbel P, Weinbaum G, Rosenbloom J. Immunologic measurement of elastin-derived peptides in human serum. Am Rev Respir Dis. 1983;127:S28–S30. doi: 10.1164/arrd.1983.127.2P2.S28. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso WV, Sekhon HS, Hyde DM, Thurlbeck WM. Collagen and elastin in human pulmonary emphysema. Am Rev Respir Dis. 1993;147:975–981. doi: 10.1164/ajrccm/147.4.975. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro SD, Campbell EJ, Welgus HG, Senior RM. Elastin degradation by mononuclear phagocytes. Ann N Y Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- 24.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 25.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, Hesselbacher S, Tsai CL, Shan M, Spitz M, Scheurer M, Roberts L, Perusich S, Zarinkamar N, Coxson H, et al. Autoreactive T cells in human smokers is predictive of clinical outcome. Front Immunol. 2012;3:267. doi: 10.3389/fimmu.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity. Immunol Res. 2012;54:247–253. doi: 10.1007/s12026-012-8327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan X, Shan M, You R, Frazier MV, Hong MJ, Wetsel RA, Drouin S, Seryshev A, Song LZ, Cornwell L, et al. Activation of C3a receptor is required in cigarette smoke-mediated emphysema. Mucosal Immunol. 2015;8:874–885. doi: 10.1038/mi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, et al. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor γ is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- 31.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor γ is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 32.Nencioni A, Grünebach F, Zobywlaski A, Denzlinger C, Brugger W, Brossart P. Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor γ. J Immunol. 2002;169:1228–1235. doi: 10.4049/jimmunol.169.3.1228. [DOI] [PubMed] [Google Scholar]
- 33.Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Deletion of PPARγ in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182:5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- 34.Chang Y, Al-Alwan L, Audusseau S, Chouiali F, Carlevaro-Fita J, Iwakura Y, Baglole CJ, Eidelman DH, Hamid Q. Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2014;306:L132–L143. doi: 10.1152/ajplung.00111.2013. [DOI] [PubMed] [Google Scholar]
- 35.Kurimoto E, Miyahara N, Kanehiro A, Waseda K, Taniguchi A, Ikeda G, Koga H, Nishimori H, Tanimoto Y, Kataoka M, et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Chu S, Zhong X, Lao Q, He Z, Liang Y. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int Immunopharmacol. 2013;15:58–66. doi: 10.1016/j.intimp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol. 2011;23:212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- 38.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008;294:L612–L631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 39.Liesker JJ, Ten Hacken NH, Zeinstra-Smith M, Rutgers SR, Postma DS, Timens W. Reticular basement membrane in asthma and COPD: similar thickness, yet different composition. Int J Chron Obstruct Pulmon Dis. 2009;4:127–135. doi: 10.2147/copd.s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob MP, Hornebeck W, Lafuma C, Bernaudin JF, Robert L, Godeau G. Ultrastructural and biochemical modifications of rabbit arteries induced by immunization with soluble elastin peptides. Exp Mol Pathol. 1984;41:171–190. doi: 10.1016/0014-4800(84)90034-0. [DOI] [PubMed] [Google Scholar]
- 41.Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 42.Churg A, Zhou S, Wright JL. Matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 43.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, et al. An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:51–61. doi: 10.1164/rccm.201401-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]