Abstract
Allergy and viral respiratory infections have long been recognized as two of the most important risk factors for exacerbations of asthma. These observations have raised questions regarding potential interactions between these two important risk factors. For example, does allergy diminish the antiviral response, thereby promoting exacerbations of asthma? Alternately, do viral respiratory infections potentiate ongoing allergic inflammation in the airway? The answers to these questions are likely to have implications regarding the prevention and treatment of exacerbations of asthma. This article reviews that clinical evidence linking viral infections and allergy to exacerbations of asthma, reviews potential interactions between these two risk factors, and discusses possible application of new insights in virus/allergen interactions to the prevention and treatment of exacerbations of asthma.
Keywords: asthma, rhinovirus, allergy, interferon
Exacerbations of asthma account for a substantial portion of asthma-related morbidity and expenditures. Asthma exacerbations remain one of the most frequent reasons for hospital admissions and incur additional psychological and financial costs related to missed work and school. In the United States, there were 479,000 hospitalizations for asthma in 2009, with a mean length of stay of 4.3 days, and death rates were 0.77/100,000 population (1). Total expenditures for asthma were estimated to be $20 billion per year, and exacerbations account for a large percentage of these costs. Health care costs associated with exacerbations of asthma were recently tallied for patients with moderate to severe asthma in a large U.S. health care database. Patients with asthma exacerbations had approximately double the total health care costs ($9,223 vs. $5,011) and asthma-specific costs ($1,740 vs. $847) compared with matched patients without exacerbations (P < 0.0001) (2). These data indicate that the personal and societal costs of asthma exacerbations are immense and provide an impetus for developing better strategies for prevention and improved treatment.
Risk Factors for Exacerbations of Asthma
Most exacerbations in children and at least half of exacerbations in adults with asthma are associated with a viral respiratory infection (VRI) (3–5). However, studies of school-aged children who provided routine specimens during high-prevalence months demonstrate that VRIs are nearly ubiquitous in children regardless of asthma (6, 7). Many viral infections cause mild or no symptoms, even in children with asthma. Thus, viral infections appear to contribute to most exacerbations of asthma but by themselves usually are not sufficient to cause asthma exacerbations. Indeed, there are risk factors or environmental exposures that can either increase the severity of VRIs or else synergize with VRIs to cause airway obstruction and acute lower respiratory symptoms.
Risk factors for exacerbations of asthma include those related to the individual, environment and infectious agent. Personal risk factors include severity of asthma and level of control, adherence to medical therapy, and allergy. Environmental exposures that can act together with viral infection to promote exacerbations include pollutants, irritants, and, for allergic individuals, allergens (8, 9). Bacterial infections may also contribute to acute wheezing illnesses and exacerbations of asthma (6, 10). It is likely that multiple factors contribute to most exacerbations, and these risk factors are additive and/or synergistic. Among risk factors for exacerbations, allergy and viral infection are most often implicated and are the subject of this review.
Clinical Studies of Allergy and Virus-induced Exacerbations of Asthma
The combination of allergic sensitization and viral illnesses greatly increases the risk of asthma exacerbation and hospitalization. In a series of studies performed at the University of Virginia, children with acute wheezing episodes were evaluated in acute care settings, including emergency departments (11, 12) and a hospital inpatient unit (4). In these studies, allergic sensitization and rhinovirus (RV) infection were significant risk factors for wheezing (odds ratio [OR], 3.2 and 4.4, respectively). Children who had RV infection detected in combination with either sensitization to common aeroallergens or eosinophilia had the strongest odds of wheezing (OR, 17 and 25, respectively) (11). These same relationships hold true for children in Costa Rica, in whom the greatest risk for wheezing was observed among children who were highly sensitized to house dust mite and tested positive for RV (OR for wheezing, 31.5; 95% confidence interval, 8.3–108; P < 0.001) (13). Notably, allergic sensitization increases the risk of wheezing with respiratory viruses even in the preschool years (14, 15). When considered together, these findings indicate that RV infections contribute to most wheezing illnesses during childhood and that allergic sensitization or eosinophilic airway inflammation further increases the risk for wheezing illnesses.
Other studies have identified interactions between VRI, allergy, and allergen exposure in causing wheezing illnesses. In a case-control study of acute exacerbations compared with subjects with stable asthma and children hospitalized for nonrespiratory diagnoses, virus infection, allergy, and exposure to a relevant allergen were all independently associated with exacerbations (8). The combination of these risk factors led to a pronounced risk of hospital admission (OR, 19.4; P < 0.001). Similar findings were reported in adults with asthma (9).
Viral Factors
RVs are most closely associated with exacerbations of asthma in children and adults. Influenza, coronaviruses, parainfluenza viruses, and other viruses are detected in lower frequencies during exacerbations. Whether this close association is related to a particular property of RVs, or merely reflects the very common nature of RV infections, is not totally clear.
RV Species and Virulence
RVs include three species of the Enterovirus genus. The A and B species were first recognized in the 1950s, and the first C species virus was reported in 2006 after detection by molecular techniques (16, 17). Additional studies using similar approaches have since reported more than 50 new C types, as well as a number of additional A and B types (18–20). The long delay in the detection of the C types was due to the inability to culture these viruses, which have been present for as long as the other RV species (21). RV-C viruses can be grown in organ culture of sinus mucosa (22) and in primary cells differentiated at the air–liquid interface (23, 24). In addition, RV-C types can be produced by reverse genetics techniques by transfecting cells with viral RNA transcribed from plasmids (22, 25). Modeling of the RV capsid structure suggests that the receptor binding platform for RV-C is distinct from that of RV-A and RV-B (26), and in fact RV-C types use a unique (and so far unidentified) receptor to enter cells (22). To date, there are no cell lines (other than air–liquid interface cells) that support RV-C infection (References 23 and 24, and our unpublished findings).
The marked genetic diversity among RV types suggests that RV type or species could influence virulence. In case-control studies, RV-C and in some studies RV-A are overrepresented in children with lower respiratory infections compared with children with upper respiratory or no symptoms (21, 27–34). Similarly, RV-B is seven to eight times less likely to cause moderate to severe respiratory illness than other RV species in infants (35). Finally, young children who wheeze with RV-C infection are more likely to develop recurrent wheezing than children who wheeze with other viruses (28).
Why RV-C and RV-A viruses are more likely to cause exacerbations of asthma than RV-B is uncertain and could relate to a number of genetic, structural, and functional differences among the RV species. For example, most RVs grow best at 33 to 35°C, a property that could limit their spread into the small airways in the lung. In contrast, RV-C types grow equally well at 33, 35, and 37°C (23). Growth at warmer temperatures may contribute to the association between RV-C infection and lower respiratory illness. Furthermore, there are differences among RV types and species related to the function of viral proteases, which are important in directing viral replication and inhibiting key metabolic processes of the host cells (36). In addition, in vitro studies demonstrated that RV-B replicates more slowly and is associated with reduced cytopathic effects and induction of cytokine secretion than RV-A and RV-C (37).
Collectively, these data suggest that lower rates of RV-B replication and cytokine induction could contribute to reduced virulence. Species-related biochemistry of the 2A protease corresponds with reduced replication and inflammatory responses. Finally, the relative insensitivity of RV-C to temperature may give this virus an advantage in causing lower airway infections.
How Do RV Infections Cause Exacerbations of Asthma?
VRIs can cause respiratory illness by damaging epithelial cells and other structural cells and by inducing inflammatory responses that secondarily cause dysfunction of the airways and cause symptoms of illness. Because RV infections generally infect a small percentage of airway epithelial cells (38, 39), and cell lysis is generally not extensive, it is likely that the inflammatory response is a major contributor to RV-induced colds and exacerbations of asthma. Even though cell lysis is limited, RV can impair epithelial cell barrier function, leading to increased permeability to macromolecules and promoting secondary infection with airway bacteria (40).
A number of studies have identified RV-induced inflammatory processes that likely contribute to airway dysfunction and symptoms. RV induces acute-phase cytokines (e.g., IL-1β and type I interferons) and mediators (prostaglandins, kinins) that can cause malaise and myalgia (41, 42). RV activates neural pathways that promote sneezing and cough through mechanisms that are likely to involve cellular inflammation and cytokine and mediator release. RV infection induces epithelial cells to secrete chemokines that promote the recruitment of inflammatory cells (43, 44). Epithelial chemokine secretion is potentiated by interferons originating in mononuclear cells (45). During the early phase of a cold, serum fluids and proteins leak into the airway (46), and in turn low-density lipoprotein can stimulate chemokine secretion by epithelial cells (47). During the latter stages of infection, mucus secretion is increased (46).
RV infections were long presumed confined to the upper airway, but there is now conclusive evidence that RV can also infect the lower airways. For example, RV can be the sole pathogen detected in lower respiratory secretions of children with pneumonia (48). After experimental inoculation of seronegative volunteers, RV is detectable in lower airway secretions from about half of the study participants, whether or not asthma is present (39, 49). Upper airway viral shedding peaks 2 to 4 days after inoculation, and in subjects with lower airway infections, lower airway viral shedding typically lags behind by a few days (49, 51). In biopsy specimens, RV-infected cells can be found in large lower airways in a patchy distribution that resembles patterns in upper airways (39). Finally, RV has been detected in lower airway secretions from children with tracheostomies (51). Once RV infects lower airways, the consequences of infection are likely to be similar to those caused by infection of the upper airway and include increased airway secretions, mucosal edema, and cellular inflammatory responses. Collectively, these effects likely contribute to airway obstruction.
Mechanisms of Virus/Allergy Interactions
Does Allergy Diminish the Antiviral Response?
The close association between allergic airway disease and virus-induced exacerbations of asthma suggest that respiratory allergies somehow inhibit antiviral responses. Interferons are one of the cornerstones of early innate antiviral defense to respiratory viruses (52, 53). There is evidence from multiple sources that virus-induced interferon responses of peripheral blood mononuclear cells (54, 55), airway mononuclear cells (57), and plasmacytoid dendritic cells (pDCs) (57) are reduced in asthma. In addition, some studies indicate that asthma is associated with a reduction in virus-induced epithelial cell production of IFN-β and IFN-λ (58, 59). Deficient epithelial interferon responses may be a property of more severe asthma (60), because interferon responses of subjects with mild asthma appear to be normal (49, 61). There is also evidence that deficient antiviral responses in asthma could be restricted to the lung (55), which suggests that type 2 inflammation could inhibit virus-induced interferon responses. Accordingly, some studies of airway epithelial cells studied ex vivo, removed from the potential immune modulating effects of other airway cells (e.g., ILC2 cells and Th2 cells), have demonstrated normal virus-induced interferon responses (62–65). In any case, it is likely that the early virus-induced interferon response is an important determinant of the subsequent course of the infection and illness. Parenthetically, the kinetics of the virus-induced interferon response are likely to be important. Too little interferon during the early phases of infection could lead to unrestrained viral replication, whereas excessive interferon secretion during the peak illness (in response to high-level viral replication) could add to the burden of symptoms (66–68). Two possible mechanisms for allergic inflammation to inhibit antiviral responses have recently been described and are reviewed in the following sections.
High-Affinity IgE Receptors and Interferon Responses
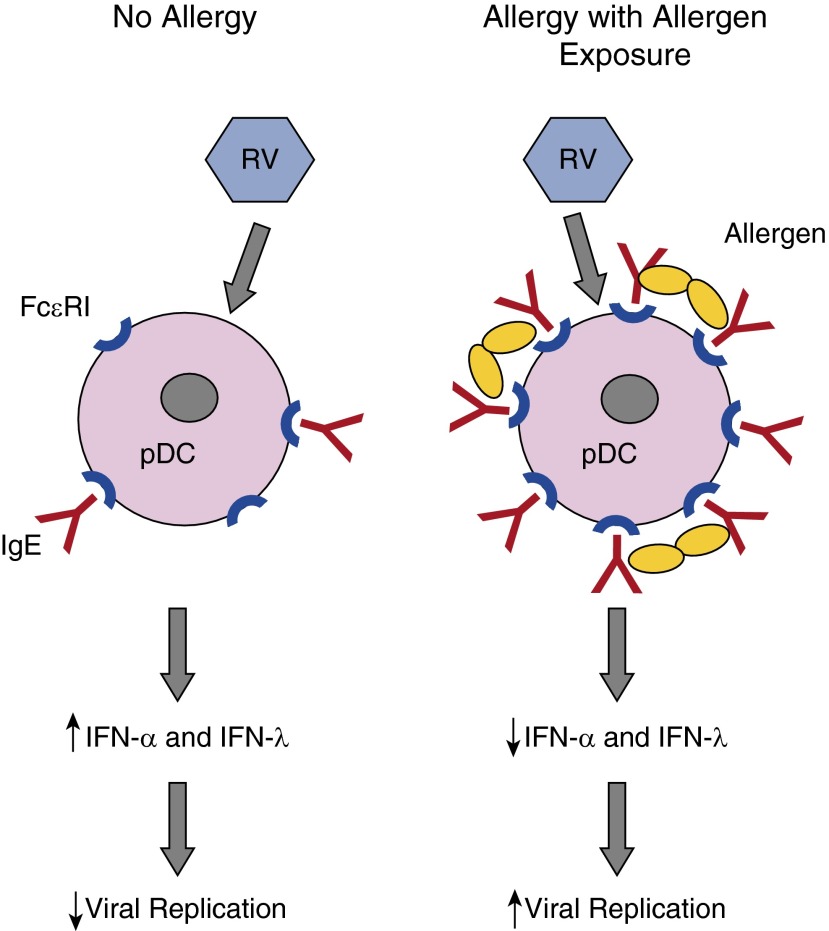
Allergic inflammation can inhibit innate antiviral immunity under some conditions (69). For example, plasmacytoid dendritic cells secrete high levels of type I and III interferons and are important cells in the antiviral response in the lung (70) (Figure 1). Interestingly, these cells express the high-affinity IgE receptor (FcεRI), which can regulate antiviral responses. Expression of FcεRI on the cell surface is inversely related to the virus induction of interferon (57). Furthermore, cross-linking FcεRI markedly impairs type I and type III interferon responses to influenza virus or RV (57, 71). These findings suggest that IgE-mediated allergic inflammation could inhibit antiviral responses in pDCs patrolling the airway epithelium. In turn, pDC interferon secretion during viral infections can inhibit Th2 responses (72) and thus has both antiviral and immunomodulating functions.
Figure 1.
High-affinity IgE receptors (FcεRIs) on plasmacytoid dendritic cells and effects on interferon responses. In the absence of allergy, plasmacytoid dendritic cells (pDCs) express low levels of cell surface FcεRI, and some of these receptors are occupied by IgE. Rhinovirus (RV) infection induces pDCs to secrete IFN-α and IFN-λ, which inhibit viral replication in an autologous and paracrine fashion. In the context of allergy with allergen exposure, FcεRI and IgE are increased, and cross-linking of receptors by allergen can inhibit interferon secretion. The net result in asthma could be increased viral replication, more severe illness, and increased risk for exacerbation of chronic asthma. Figure courtesy of William W. Busse, M.D., University of Wisconsin-Madison.
Cell Signaling, Allergy, and Antiviral Responses
Intracellular signaling mechanisms may also contribute to allergy-induced suppression of antiviral responses. Suppressor of cytokine signaling (SOCS) 1 is expressed by airway epithelial cells and can suppress interferon and other cytokines. SOCS1 is increased in the nuclei of airway epithelial cells in asthma, and this effect may contribute to reduced virus-induced interferon responses (73). In addition, in epithelial cells, allergic inflammation and RV infection can induce mucus metaplasia through mechanisms that involve induction of the transcription factors forkhead box protein A3 (FOXA3) and SAM pointed domain containing ETS transcription factor (SPDEF) (74). FOXA3 expression in epithelial cells can also inhibit type I interferons and other antiviral responses and enhances expression of proallergic factors such as thymic stromal lymphopoietin (75). During acute viral infections, the function of FOXA3 may be to limit inflammatory responses as the infection resolves, but chronic expression in the context of allergic inflammation could instead inhibit antiviral responses, potentially leading to more severe illness (75).
Do RV Infections Potentiate Ongoing Allergic Airway Inflammation?
In a longitudinal study of cohabitating couples who were discordant for asthma, asthma was associated with similar RV-induced upper respiratory symptoms but worse lower respiratory symptoms (76). This finding suggests that differences in the lower airway environment may account for enhanced illness severity in asthma. Several features of the lower airway could contribute to increased severity of RV illnesses. Structural and physiologic changes associated with allergic airway inflammation include goblet cell hyperplasia, hyperemia, airway hyperresponsiveness, and airway narrowing (77, 78). Accordingly, RV stimulation of mucus secretion could be worse in airways with greater numbers of mucus-secreting cells, asthma-related hyperemia might increase virus-induced edema and transudate, and bronchospasm could be accentuated in airways that are hyperreactive. These effects together could promote airway narrowing and closure, leading to reduced airflow and increased risk of exacerbation.
The epithelium has a number of innate antiviral defense mechanisms and an intact, well-differentiated epithelium is relatively resistant to RV infection (79). Allergic airway inflammation in asthma can impair epithelial barrier function, and in vitro studies indicate that RV replication is enhanced when apical cells of well-differentiated epithelial cell cultures are either damaged or stripped away (80). Thus, reduced barrier function in asthma could lead to more severe RV infections.
Implications for Prevention and Treatment
Standard asthma treatment regimens incompletely control exacerbations, and new approaches are needed. Most of the time, exacerbations are multifactorial, and the combination of viral infection and allergy are the two most common contributors. This observation suggests that controlling allergic inflammation and enhancing antiviral responses might both be effective approaches to reducing the frequency and severity of asthma exacerbations.
Omalizumab binds to the Fc portion of IgE to prevent cell surface binding to block activation of cells through the high-affinity IgE receptor FcεRI. In a randomized placebo-controlled trial of guidelines-based asthma treatment compared with omalizumab added to standard therapy, omalizumab prevented seasonal peaks of exacerbations during the fall and spring (81). Nasal secretions obtained during exacerbations were analyzed for viruses, and these data confirmed that the treatment group had fewer viral as well as nonviral exacerbations. Results from this interventional study indicate that IgE-mediated inflammation contributes to virus-induced exacerbations of asthma. The findings also raise the possibility that other drugs targeting type-2 inflammation (e.g., mepolizumab and IL-5 [82]) might also inhibit virus-induced exacerbations.
Another new approach has been to use inhaled IFN-β to boost antiviral responses in the airway mucosa. Volunteers with persistent asthma and a history of exacerbations with colds were randomized to treatment with either nebulized IFN-β or placebo within 24 hours of the onset of cold symptoms (83). IFN-β treatment did not reduce asthma symptom scores (primary outcome) in the intent-to-treat population; however, three positive outcomes were noted. First, IFN-β was well tolerated and induced markers of antiviral defenses in the blood and sputum Second, IFN-β treatment led to increased peak expiratory flow compared with placebo. Finally, in study subjects with more severe asthma (British Thoracic Society Step 4 and 5), IFN-β reduced the probability that colds would lead to exacerbations. If these findings are confirmed, inhaled IFN-β could be useful for preventing asthma exacerbations induced by common colds in patients with more severe asthma.
Conclusions
Clinical studies have identified allergy and viral infection as major risk factors for exacerbations of asthma. These findings have led to new approaches to prevention of exacerbations based on blocking allergic mechanisms and redoubled efforts to boost antiviral defenses in the airways. Understanding how allergy and other risk factors promote virus-induced exacerbations of asthma remains a worthwhile goal. Advances in this area could provide additional insights into the pathogenesis of asthma exacerbations and successively lead to better approaches to the prevention or treatment of asthma exacerbations.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Institutes of Health/National Heart, Lung, and Blood Institute. Bethesda, MD: 2012. 2012 Chart Book on Cardiovascular, Lung and Blood Diseases. [Google Scholar]
- 2.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, Gangnon RE, Bochkov YA, Jackson DJ, Lemanske RF, Jr, Gern JE. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307.e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF, Jr, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006, e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, Custovic A. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763–766A. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, Aniscenko J, Kebadze T, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 12.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, Platts-Mills TAE, Heymann PW. Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics. 1993;92:535–540. [PubMed] [Google Scholar]
- 13.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–1505.e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jartti T, Kuusipalo H, Vuorinen T, Söderlund-Venermo M, Allander T, Waris M, Hartiala J, Ruuskanen O. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21:1008–1014. doi: 10.1111/j.1399-3038.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, Gern JE, Lemanske RF., Jr Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, Dean A, St George K, Briese T, Lipkin WI. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Schnurr D, Ganem D, DeRisi JL, Boushey HA. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Jr, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linder JE, Kraft DC, Mohamed Y, Lu Z, Heil L, Tollefson S, Saville BR, Wright PF, Williams JV, Miller EK. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131:69–77.e1–6. doi: 10.1016/j.jaci.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochkov YA, Palmenberg AC, Lee WM, Rathe JA, Amineva SP, Sun X, Pasic TR, Jarjour NN, Liggett SB, Gern JE. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011;17:627–632. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf S, Brockman-Schneider R, Bochkov YA, Pasic TR, Gern JE. Biological characteristics and propagation of human rhinovirus-C in differentiated sinus epithelial cells. Virology. 2013;436:143–149. doi: 10.1016/j.virol.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao W, Bernard K, Patel N, Ulbrandt N, Feng H, Svabek C, Wilson S, Stracener C, Wang K, Suzich J, et al. Infection and propagation of human rhinovirus C in human airway epithelial cells. J Virol. 2012;86:13524–13532. doi: 10.1128/JVI.02094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griggs TF, Bochkov YA, Nakagome K, Palmenberg AC, Gern JE. Production, purification, and capsid stability of rhinovirus C types. J Virol Methods. 2015;217:18–23. doi: 10.1016/j.jviromet.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basta HA, Sgro JY, Palmenberg AC. Modeling of the human rhinovirus C capsid suggests a novel topography with insights on receptor preference and immunogenicity. Virology. 2014;448:176–184. doi: 10.1016/j.virol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DW, Bizzintino J, Ferrari G, Khoo SK, Zhang G, Whelan S, Lee WM, Bochkov YA, Geelhoed GC, Goldblatt J, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 30.Mak RK, Tse LY, Lam WY, Wong GW, Chan PK, Leung TF. Clinical spectrum of human rhinovirus infections in hospitalized Hong Kong children. Pediatr Infect Dis J. 2011;30:749–753. doi: 10.1097/INF.0b013e31821b8c71. [DOI] [PubMed] [Google Scholar]
- 31.Drysdale SB, Alcazar M, Wilson T, Smith M, Zuckerman M, Lauinger IL, Tong CY, Broughton S, Rafferty GF, Johnston SL, et al. Respiratory outcome of prematurely born infants following human rhinovirus A and C infections. Eur J Pediatr. 2014;173:913–919. doi: 10.1007/s00431-014-2262-1. [DOI] [PubMed] [Google Scholar]
- 32.Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14:1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arden KE, Chang AB, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J Med Virol. 2010;82:1458–1461. doi: 10.1002/jmv.21819. [DOI] [PubMed] [Google Scholar]
- 34.Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, Poovorawan Y. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59:115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee WM, Lemanske RF, Jr, Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watters K, Palmenberg AC. Differential processing of nuclear pore complex proteins by rhinovirus 2A proteases from different species and serotypes. J Virol. 2011;85:10874–10883. doi: 10.1128/JVI.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagome K, Bochkov YA, Ashraf S, Brockman-Schneider RA, Evans MD, Pasic TR, Gern JE. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134:332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser AG, Brockman-Schneider RA, Amineva SP, Burchell L, Sedgwick JB, Busse WW, Gern JE. Similar frequency of rhinovirus-infectable cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 39.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 40.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naclerio RM, Proud D, Lichtenstein LM, Kagey-Sobotka A, Hendley JO, Sorrentino J, Gwaltney JM. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 42.Seymour ML, Gilby N, Bardin PG, Fraenkel DJ, Sanderson G, Penrose JF, Holgate ST, Johnston SL, Sampson AP. Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis. 2002;185:540–544. doi: 10.1086/338570. [DOI] [PubMed] [Google Scholar]
- 43.Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, Juhlin KD, Fulmer AW, Ho BY, Walanski AA, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178:962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 44.Lewis TC, Henderson TA, Carpenter AR, Ramirez IA, McHenry CL, Goldsmith AM, Ren X, Mentz GB, Mukherjee B, Robins TG, et al. Nasal cytokine responses to natural colds in asthmatic children. Clin Exp Allergy. 2012;42:1734–1744. doi: 10.1111/cea.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konno S, Grindle KA, Lee WM, Schroth MK, Mosser AG, Brockman-Schneider RA, Busse WW, Gern JE. Interferon-gamma enhances rhinovirus-induced RANTES secretion in human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:594–601. doi: 10.1165/ajrcmb.26.5.4438. [DOI] [PubMed] [Google Scholar]
- 46.Yuta A, Doyle WJ, Gaumond E, Ali M, Tamarkin L, Baraniuk JN, Van Deusen M, Cohen S, Skoner DP. Rhinovirus infection induces mucus hypersecretion. Am J Physiol. 1998;274:L1017–L1023. doi: 10.1152/ajplung.1998.274.6.L1017. [DOI] [PubMed] [Google Scholar]
- 47.Gern JE, Brockman-Schneider R, Bhattacharya S, Malter JS, Busse WW. Serum and low-density lipoprotein enhance interleukin-8 secretion by airway epithelial cells. Am J Respir Cell Mol Biol. 2003;29:483–489. doi: 10.1165/rcmb.2002-0306OC. [DOI] [PubMed] [Google Scholar]
- 48.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, et al. New Vaccine Surveillance Network. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, Hazel E, Bork JA, Kakumanu S, Sorkness R, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–252. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons E, Schroth MK, Gern JE. Analysis of tracheal secretions for rhinovirus during natural colds. Pediatr Allergy Immunol. 2005;16:276–278. doi: 10.1111/j.1399-3038.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoo JK, Kim TS, Hufford MM, Braciale TJ. Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol. 2013;132:1263–1276. [Quiz, p. 1277.]. doi: 10.1016/j.jaci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002;32:537–542. doi: 10.1046/j.0954-7894.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- 55.Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 56.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129:1506–1514.e6. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 57.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 60.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, Saglani S, Sykes A, Macintyre J, Davies J, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, Kon OM, McHale M, Johnston SL. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69:240–246. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390.e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, Lee WM, Bochkov YA, Vrtis RF, Platts-Mills T, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med. 2014;189:532–539. doi: 10.1164/rccm.201310-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, Moore ML, Peebles RS, Jr, Castro M, Sumino K, et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014;134:1402–1412.e7. doi: 10.1016/j.jaci.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gern JE. Interferon-λ1 and viral wheeze in asthma: a Gothic duality? Am J Respir Crit Care Med. 2012;185:468–470. doi: 10.1164/rccm.201112-2195ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwantes EA, Manthei DM, Denlinger LC, Evans MD, Gern JE, Jarjour NN, Mathur SK. Interferon gene expression in sputum cells correlates with the Asthma Index Score during virus-induced exacerbations. Clin Exp Allergy. 2014;44:813–821. doi: 10.1111/cea.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, Serra ME, Bhat N, Batalle JP, Mohamed Y, et al. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy. 2008;38:781–788. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Upham JW, Zhang G, Rate A, Yerkovich ST, Kusel M, Sly PD, Holt PG. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. J Allergy Clin Immunol. 2009;124:707–713.e2. doi: 10.1016/j.jaci.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF, Jr, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, Upham JW. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol. 2012;188:5898–5905. doi: 10.4049/jimmunol.1103507. [DOI] [PubMed] [Google Scholar]
- 73.Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, Kieninger E, Gupta A, Shoemark A, Bossley C, et al. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons J Allergy Clin Immunol[online ahead of print] 25 Jan 2015; DOI: 10.1016/j.jaci.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, Hegde RS, Divanovic S, Karp CL, Whitsett JA. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA. 2012;109:16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 77.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244, quiz 1245–1246. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 78.Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013;13:1–9. doi: 10.1007/s11882-012-0317-9. [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L373–L381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 80.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, et al. INTERCIA Study Group. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]