Abstract
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are thought to be associated with—and perhaps to mediate—accelerated loss of lung function in COPD. Although the application of culture-independent methods for detection of bacteria have shown COPD to be associated with marked differences in the burden, diversity, and composition of the bronchial bacterial microbiome, few studies have examined the changes associated with community-acquired exacerbations of the disease. In a longitudinal cohort study of COPD, the availability of sputum samples from subjects obtained at the onset of an exacerbation and during periods of clinical stability before and after the event enabled us to recently address this gap in knowledge, using culture-independent, 16S rRNA–based analysis methods combined with in silico inference of metagenomic functions. We observed sputum bacterial composition to be generally stable over the preexacerbation period of clinical stability, but to change at the time of exacerbation, with specific enrichment in not only typical COPD-associated bacterial species (e.g., Haemophilus influenzae) but also other phylogenetically related species with pathogenic potential. Concurrently, we observed depleted abundance of other bacteria whose predicted metagenomes suggest functional capacities to produce a variety of antiinflammatory compounds. Most strikingly, we found that resolution of these exacerbation-related changes in sputum microbiota composition differed significantly, depending on the exacerbation treatments prescribed. Treatment with corticosteroids resulted in microbiome enrichment for a number of bacterial communities, mostly members of the Proteobacteria phylum, whereas prolonged suppression of microbiota was seen in those treated with antibiotics alone. Taken together, our findings suggest that exacerbations of COPD are associated with heterogeneous changes in the bronchial microbiome, with increases in the abundance of species related to typical COPD pathogens and decreases in microbiota members that contribute to compositional and functional homeostasis. The findings further suggest that exacerbation treatments may have very different impacts on the bronchial microbiome’s rate of return toward baseline composition.
Keywords: bacterial microbiome, COPD, exacerbations
The pathogenesis of chronic obstructive pulmonary disease (COPD) has been conceived of as a vicious cycle of airway injury, inflammation, and infection (1). Cigarette smoking, of course, is regarded as the most common initiating factor, but chronic exposure to occupational fumes or dusts, as well as some childhood respiratory diseases, may also impair innate mechanisms of defense, permitting microbial colonization of the bronchial mucosa. Local immune responses to microbial antigens are thought to worsen bronchial epithelial injury, leading to further impairment of host defense (2). This vicious cycle is greatly amplified by acute exacerbations of COPD, typically associated with the overgrowth of pathogenic bacteria, especially Haemophilus influenzae, Moraxella catarrhalis, Pseudomonas aeruginosa, or Streptococcus pneumoniae. The acute increase in proteolytic activity stimulated by these microbial pathogens is thought to upset the balance between protease and antiprotease activity, accelerating progression of the fibrotic remodeling of the bronchial wall and the development of the irreversible airflow obstruction characteristic of the condition.
In several recent studies, with use of culture-independent methods for identifying bacteria based on characteristic 16S rRNA gene sequences that classify species, researchers have reported that the airways of patients with stable COPD are colonized by rich, complex bacterial communities (3). However, very few studies have examined changes in the airway microbiome associated with acute exacerbations of COPD. In an early study, Huang and coworkers (4) examined the microbiota identified in tracheal aspirates from patients intubated for treatment of severe exacerbations of COPD and reported marked variability in bacterial richness across subjects. The subjects were clustered into two groups distinguished by significant differences in bacterial richness and illness duration, with richness decreasing as illness duration increased, presumably reflecting the effects of prolonged antibiotic treatment. Millares and colleagues examined exacerbation-related changes in sputum bacterial communities in patients with severe COPD who either were or were not colonized with P. aeruginosa (5). No significant changes in measures of microbial diversity were observed, and the patterns of bacterial compositional changes at exacerbation were heterogeneous. However, irrespective of Pseudomonas colonization state, increases in the relative abundance of several bacterial genera were observed, including Streptococcus, Pseudomonas, Moraxella, Haemophilus, Neisseria, Achromobacter, and Corynebacterium. Molyneaux and colleagues subsequently reported that viral respiratory infection, recognized as a trigger of exacerbations of COPD (6), can be associated with alterations in bronchial bacterial microbiota (7). In subjects with mild COPD, they observed increases in sputum bacterial burden at 15 days following inoculation with human rhinovirus 16. Although changes in community composition were uniform across subjects, increases in the relative abundance of specific taxa, especially members of the Proteobacteria phylum, were seen.
To investigate in greater detail airway microbiome dynamics in “natural” COPD exacerbations, we examined temporal changes in sputum microbiota composition associated with spontaneous, community-acquired exacerbations of COPD (8). For bacterial taxa found to significantly change between time points, we examined their predicted functional capacities by using a predictive metagenomic analysis pipeline (9).
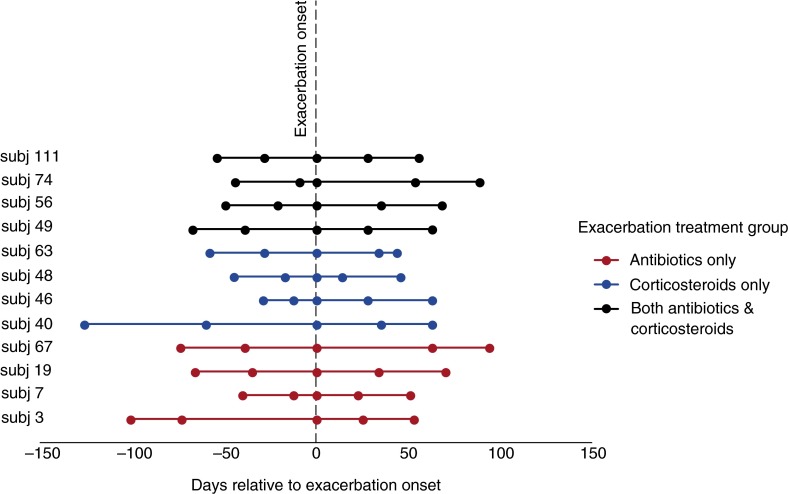
For these detailed studies, we selected 12 subjects from among a cohort of adults with COPD participating in a longitudinal study that involved repeated sputum sampling (8). The great majority of these subjects had moderately severe COPD (Global Initiative for Chronic Obstructive Lung Disease Stage 2 on the basis the criteria current at the time of their participation [10]). All had chronic sputum production (enabling analysis of expectorated as opposed to induced sputum samples). All but one had an FEV1 less than 65% of predicted value, and all but one was older than 60 years of age. Spontaneously expectorated sputum was collected at the time of the exacerbation and on at least two occasions before and at least two occasions after the exacerbation (Figure 1). Symptoms of an exacerbation led to clinical evaluation by expert physicians, who prescribed treatment. On the basis of clinical signs and symptoms, we analyzed four subjects who received treatment with an antibiotic, four who received a course of systemic corticosteroids, and four who were treated with both an antibiotic and a systemic corticosteroid. Importantly, the “exacerbation sample” of sputum was obtained before the institution of these therapies.
Figure 1.
Time points of samples collected before onset of and after exacerbation. Actual sample collection dates (i.e., “days relative to exacerbation”) for each time point did not significantly differ between exacerbation treatment groups. Reprinted by permission from Reference 8.
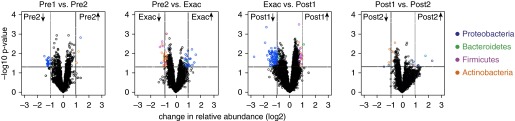
Analysis of a total of 60 samples demonstrated that significant differences in sputum bacterial community composition existed among the groups of subjects with COPD treated with different therapeutic approaches. Within-subject analysis of samples obtained during weeks of clinical stability preceding the exacerbation demonstrated no significant differences, indicative of general stability of the bacterial microbiome during this period (Figure 2). In contrast, several bacterial taxa were found to change in abundance at the time of exacerbation. Communities enriched at the time of exacerbations included both typical and nontypical COPD-associated bacteria, many in the Proteobacteria phylum. Not surprisingly, an increase in H. influenzae was noted in several samples, and increases in this member of the Pasteurellaceae family were strongly correlated with increased abundance of other phylogenetically related species, such as other Gammaproteobacteria (e.g., Enterobacteriaceae, Pseudomonadaceae, Moraxellaceae) as well as Delta- and Betaproteobacteria. These correlative relationships across Proteobacteria members suggest that functions of bacterial species in addition to H. influenzae contribute additively or synergistically to the pathogenesis of respiratory exacerbations. In contrast, communities relatively depleted at the time of exacerbation included members of the Firmicutes and Actinobacteria phyla. Predictive metagenomic analyses revealed that functional pathways encoded by these depleted communities included their capacity to produce antiinflammatory compounds (e.g., betalain, flavonoid, macrolide, and indole alkaloid biosynthesis).
Figure 2.
Serial analysis of changes in specific taxon abundance between paired time points. “Pre1” and “Pre2” refer to samples collected before the exacerbation. “Exac” refers to samples collected at the exacerbation (before any treatment was instituted). “Post1” and “Post2” refer to samples collected after treatment was instituted. Each symbol represents an identified operational taxonomic unit, with the change in relative abundance in the samples collected at the two different time points plotted on the x-axis and the P value for the significance of the change plotted on the y-axis (false discovery rate set at 0.05). So, for example, in the comparison of Pre2 with Exac (second from left), the leftmost upper field shows taxa significantly more abundant in the second (most recent) preexacerbation sample than in the exacerbation sample (i.e., decreased with exacerbation), and the rightmost upper field shows taxa significantly more abundant in the exacerbation sample than in the second preexacerbation sample (i.e., increased with exacerbation). Reprinted by permission from Reference 8.
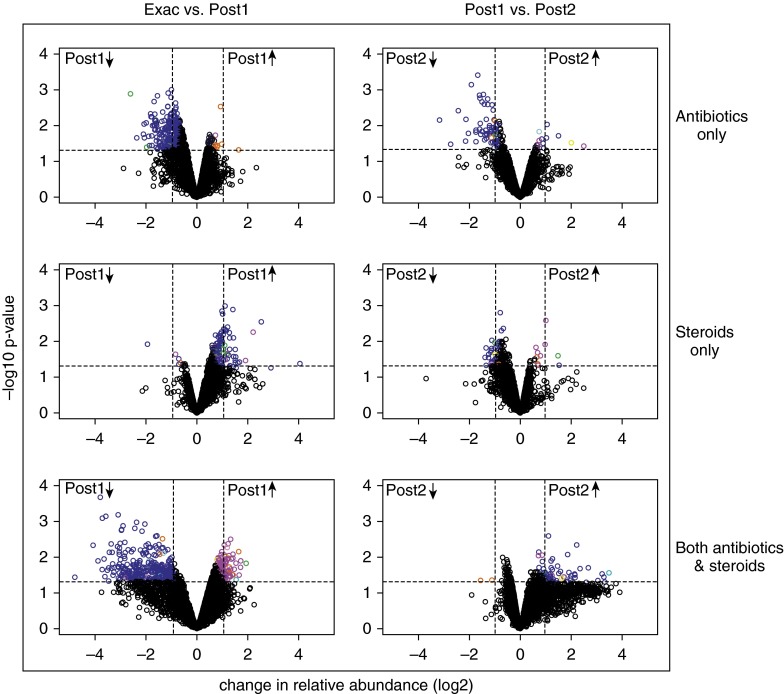
We further observed that, after exacerbation changes in sputum, microbiota composition varied by the type of treatment received (Figure 3). In subjects treated with antibiotics alone, a sharp decrease in Proteobacteria was observed, whereas an increase in members of this phylum was noted among subjects treated with oral corticosteroids only. In subjects treated with both an antibiotic and a systemic corticosteroid, slightly mixed effects on community composition were seen, but the predominant effect appeared to be antibiotic-mediated depletion of Proteobacteria. Further decreases in the abundance of bacterial communities from the first to the second postexacerbation sample were seen only in sputum samples from the subjects treated with antibiotics alone, in which further decreases in Proteobacteria were noted. In the other two groups, the abundance of particular taxa had either stabilized (oral corticosteroid treatment only) or trended back toward baseline values (both antibiotic and corticosteroid treatments).
Figure 3.
Differing impacts of exacerbation treatments on the airway bacterial microbiome. See Figure 2 legend for explanation of graphs and symbols. Reprinted by permission from Reference 8.
Implications
Because of the relatively small number of subjects examined in this study, extrapolations to a larger COPD population must be considered carefully, although the collection of sputum at multiple time points enabled some insightful within-subject comparisons. It seems permissible, therefore, to draw some conclusions. First, changes in the COPD airway bacterial microbiome at exacerbation show marked heterogeneity. This is consistent with the heterogeneity of COPD exacerbations (as well as with COPD itself) and concurs also with the observations of Molyneaux and colleagues in their human model of virus-induced exacerbations in subjects with mild COPD (7). Differences in etiologies and/or the airway microenvironment may affect microbiota composition and behavior, leading to the development of exacerbations. Another possible modulating factor suggested by our findings is chronic use of inhaled corticosteroids, which may be associated with differences in airway microbiota composition.
Second, it seems likely that certain microbiota members, especially those found to be relatively depleted at the time of exacerbation, contribute to compositional and functional homeostasis in the COPD bronchial microbiome. This is supported by predictive functional analyses which indicated that microbial pathways for biosynthesis of antiinflammatory compounds exist among those bacterial groups whose abundance diminished at exacerbation. These observations suggest that a loss in production of counterinflammatory mediators may in effect allow inflammation stimulated by specific pathogens to elaborate unchecked.
Third, inflammation at exacerbation is likely augmented by cumulative proinflammatory effects resulting from increased abundance of many other bacterial communities related to those pathogenic species typically associated with COPD. For example, the abundance of many bacterial groups phylogenetically related to H. influenzae increased in concert with this species. Many of these, being members of the Gammaproteobacteria class, have similar pathogenic potential. From a microbial community ecological perspective, it thus seems highly likely that bacteria–bacteria interactions, such as via quorum-sensing mechanisms, would foster the expression of virulent traits across many members of the community, leading to augmented inflammatory responses.
Finally, it seems clear that the pattern of and time to restoration of a stable bronchial microbiome are quite different, depending on the treatment administered, with restoration appearing to take longer after treatment with an antibiotic than after treatment with a systemic corticosteroid alone. Indeed, we noted that treatment with antibiotics alone was associated with prolonged suppression of the bacterial population, whereas combination treatment with steroids and antibiotics appeared to allow reassemblage or reconstitution of the community during the same time frame following completion of treatment (Figure 3, Post1 versus Post2).
Comparison of the findings of this study with those of studies of the role of the airway microbiome in asthma suggests some commonality of the two conditions. In both, viral respiratory infections are commonly associated with exacerbations, and members of the Proteobacteria phylum also seem important, especially H. influenzae and other members of the Gammaproteobacteria class (11–13). A recent study of asthmatic children also suggests that airway colonization by certain bacteria—Haemophilus, Moraxella, or Streptococcus—is associated with a greater likelihood of exacerbation caused by viral respiratory infection (14). However, among the many important questions still to be addressed are whether this is also true in COPD and whether alteration of airway microbial community composition by selective antibiotic or probiotic treatment strategies could alter susceptibility to virally induced exacerbations. In the pursuit of these and related studies, it will be important to keep in mind the heterogeneity of phenotypes observed in both COPD and asthma. Relevant phenotypic characteristics should be carefully considered in designing such studies to maximize the likelihood of identifying clinically informative predictive features derived from knowledge of the airway microbiome.
Footnotes
Supported by National Institutes of Health Grants K23 HL105572 (Y.J.H.) and HL098107 (H.A.B.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S. Chronic obstructive pulmonary disease and infection: disruption of the microbiome? Ann Am Thorac Soc. 2014;11(Suppl 1):S43–S47. doi: 10.1513/AnnalsATS.201307-212MG. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, Curtis JL, Erb-Downward J, Lynch SV, Sethi S, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67:456–463. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, Lynch SV. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14:9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millares L, Ferrari R, Gallego M, Garcia-Nuñez M, Pérez-Brocal V, Espasa M, Pomares X, Monton C, Moya A, Monsó E. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2014;33:1101–1111. doi: 10.1007/s10096-013-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 7.Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SA, Homola D, Trujillo-Torralbo MB, Elkin S, Kon OM, Cookson WO, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52:2813–2823. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauwels RA, Buist SA, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 11.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–352.e3. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, Gangnon RE, Bochkov YA, Jackson DJ, Lemanske RF, Jr, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307.e3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]