Abstract
Chronic obstructive pulmonary disease (COPD) is a complex chronic disease. Chronic inflammation is the hallmark of COPD, involving the interplay of a wide variety of cells in the lung microenvironment. Cigarette smoke (CS) induces chronic lung inflammation and is considered a key etiological factor in the development and pathogenesis of COPD. Structural and inflammatory cells in the lung respond to CS exposure by releasing proinflammatory mediators that recruit additional inflammatory immune cells, which collectively contribute to the establishment of a chronic inflammatory microenvironment. Chronic inflammation contributes to lung damage, compromises innate and adaptive immune responses, and facilitates the recurrent episodes of respiratory infection that punctuate and further contribute to the pathological manifestations of the stable disease. A number of studies support the conclusion that immune dysfunction leads to exacerbations and disease severity in COPD. Our group has clearly demonstrated that CS exacerbates lung inflammation and compromises immunity to respiratory pathogens in a mouse model of COPD. We have also investigated the phenotype of immune cells in patients with COPD compared with healthy control subjects and found extensive immune dysfunction due to the presence and functional activity of T regulatory cells, CD4+PD-1+ exhausted effector T cells and myeloid-derived suppressor cells. Manipulation of these immunosuppressive networks in COPD could provide a rational strategy to restore functional immune responses, reduce exacerbations, and improve lung function. In this review, we discuss the role of immune dysfunction in COPD that may contribute to recurrent respiratory infections and disease severity.
Keywords: adaptive immunity, COPD, immune dysfunction, innate immunity
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease of the airways with progressive and irreversible decline in lung function caused by airway obstruction and destruction of parenchyma (1–3). Three pathological disorders, including chronic bronchitis, small airway disease, and emphysema, existing either separately or in combination culminate in COPD. COPD affects around 200 million people worldwide; it is the third most common cause of death in the United States; and it is predicted to be the third leading cause of death worldwide by 2020 (2, 4, 5). Approximately 12.7 million U.S. adults were estimated to have COPD in 2011; however, nearly 24 million U.S. adults have proof of impaired lung function, suggesting a potential underdiagnosis of COPD (6–8). The overall associated cost to the health care system is substantial, which in the United States in 2010 was estimated to be about $49.9 billion, including $29.5 billion in direct health care expenditures, $8.0 billion in indirect morbidity costs, and $12.4 billion in indirect mortality costs (9).
Although cigarette smoke (CS) is regarded as the principal causative factor in the development and pathogenesis of COPD (10), in developing countries indoor pollution from the burning of biomass fuel is associated with an increased risk of COPD (11, 12). Genetic makeup and environmental factors likely also play a role in disease development because not all smokers develop COPD (1, 13). Other risk factors include exposure to air pollution, occupational dust and chemicals, a history of childhood respiratory infections, and socioeconomic status (12).
Chronic exposure to inhaled irritants activates structural and inflammatory cells within the respiratory tract (14, 15). For example, CS activates airway epithelial cells; reduces cilia, thus impacting mucus removal; and constitutively activates alveolar macrophages. These activated cells release potent inflammatory cytokines and chemoattractants in the lung microenvironment that collectively induce a state of chronic inflammation, recruiting additional inflammatory cells, including monocytes and neutrophils. These physiological alterations in the lung ultimately cause structural changes and obstruction in the airways, leading to emphysema, tissue destruction, and mucus hypersecretion (16–18). Overall, chronic inflammation and compromised immunity to respiratory pathogens are strongly associated with the induction of immune-suppressive networks that likely contribute to COPD pathogenesis. Our group has demonstrated that CS exposure exacerbates lung inflammation and compromises immunity to a respiratory pathogen non-typeable Haemophilus influenzae (NTHI) in a mouse model of COPD (19). Additionally, our studies of patients with COPD have clearly demonstrated that immune dysfunction is associated with increased numbers and function of immunosuppressive cells, including regulatory T cells (Tregs), PD-1+ T cells, and myeloid-derived suppressor cells (MDSCs), that contribute to poor immune responses to NTHI (20, 21).
Immune Modulation in COPD
Innate immunity
Innate immune defenses in the lung include epithelial barrier, mucociliary clearance, antimicrobial peptides, complement components, and surfactants. Immune cells, including macrophages, dendritic cells (DCs), neutrophils, monocytes, mast cells, and natural killer cells also contribute to immunity in the lung (Figure 1). Immune responses induced during normal inflammatory processes augment tissue immunity that protects against infections. However, in patients with COPD, chronic pulmonary inflammation is accompanied by the induction of defective immune responses that contribute to intermittent respiratory infections, worsening the inflammatory lung microenvironment and disease severity (15, 22, 23).
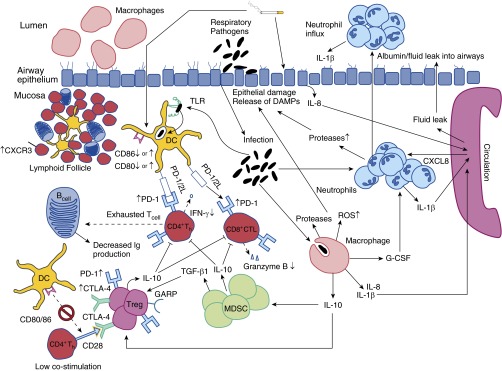
Figure 1.
Immune dysfunction in patients with chronic obstructive pulmonary disease (COPD). The figure depicts modulation of various critical parameters in the lung microenvironment of patients with COPD that result in chronic inflammation and immune dysfunction and facilitate recurrent respiratory infections. Chronic pulmonary inflammation occurs with the formation of prominent tertiary lymphoid structures overexpressing homing chemokines, such as chemokine (C-X-C motif) receptor 3 (CXCR3) (57, 72). Additionally, normal immune cell activation is modulated, increasing reactive oxygen species (ROS) production and neutrophil survival while decreasing macrophage phagocytosis, culminating in tissue damage (37, 38, 73, 74). To counteract an overexuberant chronic inflammatory response, immunosuppressive pathways are augmented, ultimately compromising the response necessary to prevent recurrent infections such as those seen in patients with COPD (21, 75). Additional details are provided in the text. CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; CXCL8 = chemokine (C-X-C motif) ligand 8; DAMP = damage-associated molecular pattern molecule; DC = dendritic cell; GARP = glycoprotein A repetitions predominant; G-CSF = granulocyte colony-stimulating factor; MDSC = myeloid-derived suppressor cell; TGF-β1 = transforming growth factor β1; Th = helper T cell; TLR = Toll-like receptor; Treg = regulatory T cell.
Tissue destruction and respiratory infections in the lungs of patients with COPD are sensed by the innate immune cells via pathogen-associated molecular pattern–pattern recognition receptor (PRR) and/or damage-associated molecular pattern (DAMP)–PRR pathways (22, 23) and are critical in mounting adequate host immune responses. We have demonstrated that a host Toll-like receptor 2 (TLR2)–bacterial lipoprotein P6 signaling axis is essential to mediate enhanced airway inflammation and elicit robust adaptive immune responses against NTHI (24). We used wild-type and TLR2-deficient mice and instilled either wild-type or P6 lipoprotein-null NTHI strains and established that both inflammation and immune responses were diminished when either P6 or TLR2 was absent. Also, reduced immune cell infiltration into the lungs, along with decreased levels of inflammatory cytokines tumor necrosis factor (TNF)-α, IL-6, IL-17, and IFN-γ, was noted. Our compelling evidence suggests that this signaling axis could be a potential therapeutic target in managing NTHI respiratory infections in patients with COPD. Recently, DAMPs such as extracellular ATP have been shown to activate the inflammasome, and they have been implicated in mediating inflammation in COPD (23, 25–30). Furthermore, CS also activates PRRs, either directly by binding of its components to arylhydrocarbon receptor or indirectly by causing epithelial injury and leading to DAMP production (31).
Phagocytes control infection and accelerate the resolution of infection-associated inflammation. During an inflammatory response, alveolar macrophages phagocytose infiltrated neutrophils to control infection and regulate the extent of inflammation (32, 33). However, this functional cooperation is impaired in patients with COPD. Although the number of alveolar macrophages are increased in patients with COPD, their phagocytic ability, compared with that in smokers without COPD, is diminished (34–36). This impairment in macrophage activity, along with the induction of neutrophil survival, increases the neutrophilic load in airways (37, 38).
CS also causes differentiation of alveolar monocyte precursors into the M2 macrophage phenotype. In patients with COPD, increased numbers of M2 macrophages correlate with decreased FEV, disease progression, and disease severity, owing to their secretion of matrix metalloproteinases (39, 40). In the mouse model we have developed, we observed that increased numbers of macrophages and neutrophils in the airways were accompanied by increased levels of inflammatory cytokines, including IL-1β, IL-6, TNF-α, and IL-17 in the bronchoalveolar lavage (BAL) fluid, and heightened airway inflammation (19).
There is a lack of consensus on the role of DCs in patients with COPD. Some studies indicate that, in patients with COPD, there is an increase in DC number and function (41–43), whereas others have found decreased numbers of DCs and impairment in their maturation status (41, 43–46). Similarly, in some studies in mice, researchers have reported that CS exposure increases the number of lung DCs, whereas others have shown a decrease in their numbers and/or function (47–50). Exposure to nicotine, one of the major components of CS, adversely affects DC functionality, leading to compromised immunity (51).
Adaptive Immunity
Patients with COPD are prone to recurrent respiratory infections leading to disease severity. In COPD, not only the initial response to pathogens but also the strength with which the adaptive immune system responds to such challenges is impaired (15, 52). Such weakened immune responses can lead to recurrent infections. NTHI, a bacterial cause of exacerbations in patients with COPD, is associated with excessive lung inflammation and disease-related pathology (53, 54). Moghaddam and colleagues reported that chronic exposure of mice to a lysate of NTHI elicits an inflammatory rather than a protective response (54). Mouse models have been used to evaluate the effects of chronic irritants such as CS and nicotine on adaptive immune responses. Chronic exposure of mice to nicotine leads to immune suppression by induction of T cell anergy (55). Mice exposed to chronic CS and a high dose of influenza virus elicited lower protective but higher inflammatory immune responses, which ultimately resulted in reduced survival rates (56).
We developed a mouse model to evaluate the cumulative impact of prior CS exposure followed by chronic infection with NTHI on inflammation and antigen-specific immune responses (19). Mice were first exposed to CS with subsequent chronic intratracheal instillation with NTHI. We found that prior smoke exposure induced increased lung inflammation and compromised adaptive immunity against NTHI compared with air-exposed controls. Immune cell infiltration surrounding airways and bronchovasculature was greatly increased in smoke-exposed mice. Specifically in our model, lymphocyte infiltration led to the formation of bronchus-associated lymphoid tissue (BALT), which is considered one of the characteristic features in the lungs of patients with COPD (57). Furthermore, in the BAL, CS exposure elevated the frequency and number of macrophages and neutrophils, but lymphocyte numbers were reduced. Levels of proinflammatory cytokines IL-1β, IL-6, TNF-α, and IL-17 were elevated in these mice, and IFN-γ was decreased. The NTHI-specific lung and splenic T lymphocytes were decreased in number as well as in frequency and exhibited lower secretion of IFN-γ and IL-4, with an increase in IL-17 production. Additionally, we found that NTHI-specific B cell responses were impaired, resulting in poor antibody responses in smoke-exposed mice compared with air-exposed controls. The extent of immune dysfunction was found to be systemic, with a reduction in immune responses noted in the lung, spleen, serum, BAL, and bone marrow in smoke- plus NTHI-exposed mice (19).
Because we observed impaired immune responses to NTHI in mice exposed to CS, we evaluated if vaccination could have protective effects against subsequent infections. We found that vaccination with P6, an outer membrane protein of NTHI, was less efficient in mice exposed to CS than in air-exposed controls. We further observed that smoking decreased the antibody response and diminished bacterial and neutrophil clearance in response to NTHI challenge following immunization. However, in CS-exposed mice, immunization significantly reduced the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α and minimized the lung damage compared with sham-immunized mice. These results indicate that P6 immunization does afford some protection against subsequent exposure to NTHI in CS-exposed animals, although the effect was not comparable to that in air-exposed mice. These findings, to our knowledge, are the first reported on the systemic effect of CS exposure attenuating both the adaptive immune responses to infections and the efficacy of vaccination (19).
Diminished adaptive immune responses to respiratory pathogens colonizing the airways of patients with COPD contribute to disease exacerbation and intermittent lung infections (23, 58, 59). NTHI, Moraxella catarrhalis, and Streptococcus pneumonia are the three most common bacteria responsible for exacerbations in patients with COPD (15, 58). Researchers in our laboratory were interested in evaluating whether the immune dysfunction observed was a general phenomenon or was pathogen-specific. To accomplish our goals, lymphocytes isolated from patients with COPD were stimulated with P6 antigen of NTHI, tetanus toxoid antigen, and phytohemagglutinin, and their ability to proliferate in response to these stimuli was evaluated. We observed that lymphocytes from a subset of patients with COPD displayed an impaired response to P6 antigen of NTHI while eliciting a normal response to unrelated control antigens. These results suggested that impairment of adaptive immune responses in COPD is pathogen-specific (20). Importantly, this subset of patients had more frequent exacerbations caused by NTHI within the prior 12 months. Following this initial observation, we hypothesized that underlying immune suppression could be one of the mechanisms responsible for defective antibacterial immunity in these patients. To fully address this question, we evaluated the presence and functionality of Tregs, exhausted T effector cells (CD4+ PD-1+), and CD14−HLA-DR−CD11b+CD33+ MDSCs in patients with COPD, as these cells are known to play a pivotal role in suppressing immune responses (Figure 1).
On the basis of our studies, we have demonstrated that the decline in adaptive immune response to bacterial antigens in patients with COPD could be attributed to the net effect of augmented Treg function and decreased effector T cell function (21). A limited number of studies have investigated the presence of Tregs in patients with COPD, and researchers have reported different findings in lung tissue, BAL fluid, and peripheral blood. Increased numbers of Foxp3+ Tregs in BALT and CD25bright Tregs in BAL fluid (60, 61) or peripheral blood of patients with COPD have been found (62). In one study decreased numbers of CD25+Tregs in the BAL fluid of patients with COPD and nonsmokers compared with healthy smokers were observed; however, no differences in the numbers of circulating Tregs were detected between study groups (63).
A key finding at our laboratory was that the addition of autologous Tregs to purified effector T cells stimulated with P6 lipoprotein resulted in greater suppression of COPD effector T cell proliferation compared with that observed in healthy control subjects. Our data are the first demonstration that Foxp3+ Tregs from patients with COPD are effective at suppressing NTHI-specific effector T cells. Lee and colleagues conducted a functional analysis of Tregs in patients with emphysema and control subjects and reported that Tregs from both groups markedly inhibited the proliferation of autologous T cells in response to anti-CD3/anti-CD28 stimulation (64). However, our study significantly differs from their study in that we evaluated Treg function by measuring the proliferation of autologous antigen-specific T cells in response to stimulation by purified outer membrane bacterial antigen P6 from NTHI.
In our studies, even though the levels of CD4+CD127+CD25− effector T cells were similar between patients with COPD and healthy subjects, effector T cells from patients with COPD displayed an overall diminished response to P6, which was further decreased by the potent suppressive capacity of their own Tregs. Thus, antibacterial immunity in patients with COPD is limited by two important factors: the inability of effector T cells to robustly respond to bacterial antigens and the increased accumulation of functionally suppressive Tregs. Factors that may account for these suppressive aspects are the elevated expression of PD-1 on effector T cells and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) on Tregs. Our observation of high levels of PD-1+ exhausted phenotype in patients with COPD could provide an explanation for the decreased proliferation of patient-derived effector T cells stimulated with P6 antigen as compared with effector cells from healthy control subjects. In a recent study, researchers reported downregulation of T cell receptor signaling in CD8+ T cells isolated from the BAL fluid of patients with COPD compared with smokers and healthy control subjects, which may also contribute to T cell dysfunction in COPD (65).
Importantly, our studies have also established that blocking of immune checkpoint receptors CTLA-4 and PD-1 resulted in increased T cell proliferation and IFN-γ production, which strengthens the functional relevance of overexpression of these molecules on COPD T cells. Therefore, CTLA-4+ Tregs and PD-1+ T cells in patients with COPD may represent a targetable source for alleviating immunosuppression of antibacterial immunity. Our study represents the first demonstration of the differential effect of CTLA-4 and PD-1 blockade on T cell responses in COPD.
In our studies, immunosuppression in patients with COPD was also reflected by significant increases in the peripheral blood levels of two Treg-generated immunosuppressive cytokines: IL-10 and transforming growth factor (TGF)-β1. The high frequency of Foxp3+ Tregs demonstrated significant correlation with levels of TGF-β1 in patients with COPD. In addition to this immunosuppressive milieu, we found that the levels of proinflammatory helper T cell type 1–associated cytokines IFN-γ and IL-12 were significantly increased in patients with COPD. Elevated levels of these proinflammatory cytokines exhibited an inverse relationship with lung function (FEV1), suggesting that the proinflammatory immune response worsens the lung function of patients with COPD. Therefore, our results demonstrate that the plasma cytokine milieu in patients with COPD is shifted toward an immunosuppressive and proinflammatory phenotype.
Although studies in our laboratory have been focused largely on CD4 T cell subsets, it is known that CD8+ T cells may also contribute to progression of the disease in patients with COPD (59). Increased numbers of CD8+ T cells expressing IL-17 have been found in the lungs of patients with COPD, which highlights the important role played by these cells in the pathogenesis of COPD (66). Nonetheless, the T cell receptor signaling molecule CD247 (ζ-chain) has been shown to be downregulated in lung CD8+ T cells of patients with COPD, indicating T cell dysfunction and the potential role of dysfunctional lung CD8+ T cells in COPD pathogenesis (65). Increased expression of IL-18, CD69, T-bet, perforin, and granzyme B on CD8+ T cells has been shown to be positively correlated with decline in FEV1 in patients with COPD (67). Freeman and colleagues have also shown that an increased percentage of CD8+ T cells isolated from lung tissue expressed cell surface TLRs and that this correlated with the emphysema score. Freeman and colleagues speculate that the modulation of TLRs during bacterial infections may contribute to lung destruction (68). In our studies, we found a positive correlation between the frequency of glycoprotein A repetitions predominant–positive (GARP+)Foxp3+ Tregs cells and lung function in patients with COPD, and it is tempting to speculate that these cells may counteract the activity of the tissue-damaging CD8+ T cells.
In addition to our studies on T cells, we provide the first evidence of MDSC accumulation in patients with COPD. We observed that elevated levels of MDSCs correlated with high levels of Tregs, which is in agreement with studies that suggest reciprocal control of these two cell types. Inflammation promotes the accumulation of MDSCs, which induce Tregs, producing TGF-β1 and IL-10, which contribute to effector T cell suppression (69). In support of these findings, we have demonstrated that the frequency of circulating Tregs in patients with COPD shows an excellent correlation with the percentage of MDSCs and level of plasma TGF-β1. The direct relationship between GARP+Foxp3+ Tregs and lung function in patients with COPD can be considered a beneficial effect of Tregs. Potentially suppressive Tregs may attenuate effector T cell–mediated destruction of lung epithelium, which, if unregulated, may eventually lead to an emphysematous condition. Our studies highlight the notion that, in COPD, a fine balance must be achieved between (1) proinflammatory responses that clear pathogens but damage lung tissue and (2) immunosuppressive cells and cytokines that attenuate proinflammatory responses but hinder antibacterial immunity.
Summary and Future Perspectives
Chronic lung inflammation plays a critical role in COPD, leading to extensive lung damage and impaired immunity to respiratory infections. COPD is associated with lung-specific and systemic immune dysfunction that facilitate disease exacerbations (21, 23, 70, 71). Host immunosuppressive networks are induced in an effort to reduce the inflammation and minimize tissue destruction. On the basis of the findings at our laboratory, as well as those derived from studies from other investigators, therapeutic targeting of dysfunctional immune cells could be beneficial in COPD management. Restoration of immune function could potentially prevent pathogen-mediated disease exacerbations and also lessen infection-induced inflammation and tissue destruction. However, a fine balance between the induction of immunosuppressive networks and adaptive immunity is needed to minimize lung inflammation without compromising immunity against respiratory infections. Thus, immunosuppressive feedback loops could be important targets in COPD management; however, additional studies are needed to evaluate the detailed mechanisms of such immune suppression and their role in COPD. Although it is more challenging, the evaluation of Tregs and exhausted effector T cells isolated from the lungs of patients would provide additional important insights.
Footnotes
Supported by Grant AI069379 from the National Institute of Allergy and Infectious Diseases (Y.T.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2007;28:479–513. doi: 10.1016/j.ccm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 5.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention National Center for Health StatisticsNational Health Interview Survey Raw Data, 2011. Analysis performed by the American Lung Association Research and Health Education Division using SPSS and SUDAAN software
- 8.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117. [PubMed] [Google Scholar]
- 9.Confronting COPD in America, 2000. Schulman, Ronca and Bucuvalas, Inc. (SRBI). Funded by Glaxo Smith Kline
- 10.Office of the Surgeon General, Public Health Service, US Department of Health and Human Services The health consequences of smoking—50 years of progress: a report of the Surgeon General, 2014 Washington, DC:US Department of Health and Human Services; 2014[accessed 2015 Jun 29]. Available from: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/#fullreport [Google Scholar]
- 11.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: revised 2011 [accessed 2015 Jun 29]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf
- 13.Postma DS, Kerkhof M, Boezen HM, Koppelman GH. Asthma and chronic obstructive pulmonary disease: common genes, common environments? Am J Respir Crit Care Med. 2011;183:1588–1594. doi: 10.1164/rccm.201011-1796PP. [DOI] [PubMed] [Google Scholar]
- 14.Thorley AJ, Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:409–428. [PMC free article] [PubMed] [Google Scholar]
- 15.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:524–526. doi: 10.1513/pats.200904-016DS. [DOI] [PubMed] [Google Scholar]
- 19.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192:5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe Y, Murphy TF, Sethi S, Faden HS, Dmochowski J, Harabuchi Y, Thanavala YM. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:967–971. doi: 10.1164/ajrccm.165.7.2109009. [DOI] [PubMed] [Google Scholar]
- 21.Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:40–50. doi: 10.1164/rccm.201312-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013;2013:413735. doi: 10.1155/2013/413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugade AA, Bogner PN, Murphy TF, Thanavala Y. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Front Immunol. 2011;2:10. doi: 10.3389/fimmu.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polosa R, Blackburn MR. Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2009;30:528–535. doi: 10.1016/j.tips.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12:54–59. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 27.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 29.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 30.Eltom S, Dale N, Raemdonck KR, Stevenson CS, Snelgrove RJ, Sacitharan PK, Recchi C, Wavre-Shapton S, McAuley DF, O’Kane C, et al. Respiratory infections cause the release of extracellular vesicles: implications in exacerbation of asthma/COPD. PLoS One. 2014;9:e101087. doi: 10.1371/journal.pone.0101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiba T, Chihara J, Furue M. Role of the arylhydrocarbon receptor (AhR) in the pathology of asthma and COPD. J Allergy (Cairo) 2012;2012:372384. doi: 10.1155/2012/372384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010;87:805–813. doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- 33.Silva MT. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. J Leukoc Biol. 2011;89:675–683. doi: 10.1189/jlb.0910536. [DOI] [PubMed] [Google Scholar]
- 34.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 35.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 36.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, Stewart CC, Sethi S. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun. 2004;318:32–37. doi: 10.1016/j.bbrc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, et al. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc Natl Acad Sci USA. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435. doi: 10.3389/fimmu.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaku Y, Imaoka H, Morimatsu Y, Komohara Y, Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M, et al. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One. 2014;9:e87400. doi: 10.1371/journal.pone.0087400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 42.Lommatzsch M, Bratke K, Knappe T, Bier A, Dreschler K, Kuepper M, Stoll P, Julius P, Virchow JC. Acute effects of tobacco smoke on human airway dendritic cells in vivo. Eur Respir J. 2010;35:1130–1136. doi: 10.1183/09031936.00090109. [DOI] [PubMed] [Google Scholar]
- 43.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers AV, Adelroth E, Hattotuwa K, Dewar A, Jeffery PK. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax. 2008;63:108–114. doi: 10.1136/thx.2007.078253. [DOI] [PubMed] [Google Scholar]
- 45.Tsoumakidou M, Bouloukaki I, Koutala H, Kouvidi K, Mitrouska I, Zakynthinos S, Tzanakis N, Jeffery PK, Siafakas NM. Decreased sputum mature dendritic cells in healthy smokers and patients with chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2009;150:389–397. [Google Scholar]
- 46.Liao SX, Ding T, Rao XM, Sun DS, Sun PP, Wang YJ, Fu DD, Liu XL, Ou-Yang Y. Cigarette smoke affects dendritic cell maturation in the small airways of patients with chronic obstructive pulmonary disease. Mol Med Rep. 2015;11:219–225. doi: 10.3892/mmr.2014.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeid NA, Muller HK. Tobacco smoke induced lung granulomas and tumors: association with pulmonary Langerhans cells. Pathology. 1995;27:247–254. doi: 10.1080/00313029500169063. [DOI] [PubMed] [Google Scholar]
- 48.D’hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005;26:204–213. doi: 10.1183/09031936.05.00095204. [DOI] [PubMed] [Google Scholar]
- 49.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Cox G, Stampfli MR. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol. 2004;30:202–211. doi: 10.1165/rcmb.2003-0259OC. [DOI] [PubMed] [Google Scholar]
- 50.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol. 2008;180:6623–6628. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nouri-Shirazi M, Guinet E. Exposure to nicotine adversely affects the dendritic cell system and compromises host response to vaccination. J Immunol. 2012;188:2359–2370. doi: 10.4049/jimmunol.1102552. [DOI] [PubMed] [Google Scholar]
- 52.Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc. 2007;4:512–521. doi: 10.1513/pats.200701-002FM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis. 2011;6:113–123. doi: 10.2147/COPD.S15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moghaddam SJ, Clement CG, De la Garza MM, Zou X, Travis EL, Young HW, Evans CM, Tuvim MJ, Dickey BF. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 56.Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, Stampfli MR. Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am J Respir Crit Care Med. 2006;174:1342–1351. doi: 10.1164/rccm.200604-561OC. [DOI] [PubMed] [Google Scholar]
- 57.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 58.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol. 2009;182:3270–3277. doi: 10.4049/jimmunol.0802622. [DOI] [PubMed] [Google Scholar]
- 60.Plumb J, Smyth LJ, Adams HR, Vestbo J, Bentley A, Singh SD. Increased T-regulatory cells within lymphocyte follicles in moderate COPD. Eur Respir J. 2009;34:89–94. doi: 10.1183/09031936.00100708. [DOI] [PubMed] [Google Scholar]
- 61.Smyth LJ, Starkey C, Vestbo J, Singh D. CD4-regulatory cells in patients with COPD. Chest. 2007;132:156–163. doi: 10.1378/chest.07-0083. [DOI] [PubMed] [Google Scholar]
- 62.Brandsma CA, Hylkema MN, Geerlings M, van Geffen WH, Postma DS, Timens W, Kerstjens HA. Increased levels of (class switched) memory B cells in peripheral blood of current smokers. Respir Res. 2009;10:108. doi: 10.1186/1465-9921-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barceló B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agusti AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31:555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 64.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 65.Grundy S, Plumb J, Lea S, Kaur M, Ray D, Singh D. Down regulation of T cell receptor expression in COPD pulmonary CD8 cells. PLoS One. 2013;8:e71629. doi: 10.1371/journal.pone.0071629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, Hamid Q. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:43. doi: 10.1186/1465-9921-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, Polak TJ, Sonstein J, Todt JC, Ames TM, et al. Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freeman CM, Martinez FJ, Han MK, Washko GR, Jr, McCubbrey AL, Chensue SW, Arenberg DA, Meldrum CA, McCloskey L, Curtis JL. Lung CD8+ T cells in COPD have increased expression of bacterial TLRs. Respir Res. 2013;14:13. doi: 10.1186/1465-9921-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 71.Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelsen SG, Aksoy MO, Georgy M, Hershman R, Ji R, Li X, Hurford M, Solomides C, Chatila W, Kim V. Lymphoid follicle cells in chronic obstructive pulmonary disease overexpress the chemokine receptor CXCR3. Am J Respir Crit Care Med. 2009;179:799–805. doi: 10.1164/rccm.200807-1089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bowler RP, Barnes PJ, Crapo JD. The role of oxidative stress in chronic obstructive pulmonary disease. COPD. 2004;1:255–277. doi: 10.1081/copd-200027031. [DOI] [PubMed] [Google Scholar]
- 74.Hackett TL, Scarci M, Zheng L, Tan W, Treasure T, Warner JA. Oxidative modification of albumin in the parenchymal lung tissue of current smokers with chronic obstructive pulmonary disease. Respir Res. 2010;11:180. doi: 10.1186/1465-9921-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKendry R, Staples K, Spalluto CM, Wilkinson T. Modulation of PD-1 and PD-L1 in response to acute viral infection of human lung tissue. Eur Respir J. 2014;44:3855. [Google Scholar]