Abstract
Rationale: Sarcoidosis is a systemic granulomatous and inflammatory disorder that most often involves the lungs but also affects many other organs. Data on sarcoidosis from large epidemiological studies remain scarce.
Objectives: To evaluate the baseline prevalence and 22-year incidence of sarcoidosis and their associations with demographic and geographic characteristics in a large cohort of U.S. women.
Methods: The Nurses’ Health Study II is a prospective cohort study of U.S. female nurses enrolled in 1989 (aged 25–44 yr, n = 116,430). Data on major illnesses were collected through biennial questionnaires (1989–2011). Cases were identified by the nurses’ self-report of physician-diagnosed sarcoidosis. Associations of demographic and geographic characteristics with sarcoidosis were evaluated by logistic regression and Cox models.
Measurements and Main Results: A total of 377 sarcoidosis cases were identified. The baseline prevalence was 100/100,000 women. The average annual incidence rate was 11/100,000 during 2,275,028 person-years of follow up. Incidence rate increased with age (P = 0.003), from 9 to 15/100,000 in women aged less than 35 to 55 or more years, respectively. Black women had a higher prevalence (odds ratio, 5.24; 95% confidence interval, 2.87–9.55) and incidence (hazard ratio, 3.80; 95% confidence interval, 2.31–6.24) than white women. Across U.S. regions, more than twofold differences were observed in sarcoidosis prevalence and incidence, with consistently higher rates in the Northeast.
Conclusions: We provide recent national data on the epidemiology of sarcoidosis among U.S. women. Important differences in prevalence and incidence were observed across U.S. regions. Large epidemiological studies are needed to better understand the causes of the observed demographic and geographic differences in sarcoidosis.
Keywords: epidemiology, prevalence, incidence, lung diseases
Sarcoidosis is a systemic granulomatous and inflammatory disorder that typically affects the lungs but can cause a variety of manifestations in many other organs (1–4). Skin, eye, cardiac, or nervous system involvements are common (2–4). The clinical course ranges from asymptomatic forms with spontaneous remission to chronic disease leading to significant organ impairment and death in 5% of patients (2, 5). The disease can occur at any age but is believed to be more common in young to middle-aged adults (1, 2, 4, 6). A slight female predominance has been described (3, 6–8). A higher risk of sarcoidosis in African-American men and women has been well documented (4, 6). Worldwide variations have also been observed in sarcoidosis occurrence, with higher incidence rates found in northern Europe and lower rates in Asian populations (1, 7, 9, 10). Although genetic, lifestyle, occupational, and environmental risk factors have been suggested (11–15), the exact etiology of sarcoidosis remains unknown (2).
Data on the prevalence and incidence of sarcoidosis from large epidemiological studies remain scarce. In the United States, currently available data on sarcoidosis are mostly based on studies restricted to small geographic areas (6, 16) or to specific, predominantly male occupational groups (17, 18). These studies do not cover recent time periods, with most data collected before 2000. More recently, the prevalence and incidence of sarcoidosis was investigated in the Black Women’s Health Study (4), providing nationwide data on sarcoidosis among black women, who are a higher-risk population.
The Nurses’ Health Study II (NHSII) is a large, ongoing, prospective study of U.S. female nurses. The aim of the current study was to evaluate the baseline prevalence and 22-year incidence of sarcoidosis and their associations with demographic and geographic characteristics in NHSII.
Methods
The NHSII began in 1989 when 116,430 female registered nurses from 14 U.S. states, aged 25 to 44 years, completed a mailed questionnaire on their medical history and lifestyle characteristics. Every 2 years, follow-up questionnaires have been sent to update information on potential risk factors and to identify newly diagnosed diseases (19, 20). The active follow-up rate (number of person-years in the cohort when participants are censored after their last questionnaire response) from 1989 to 2011 was 87% of the potential person-years. The NHSII and the current sarcoidosis study were approved by the Institutional Review Board at the Brigham and Women’s Hospital (Boston, MA).
At the baseline questionnaire (1989), participants were asked if they ever had any of a list of physician-diagnosed conditions. In all biennial questionnaires (1991–2011), participants were asked to report physician-diagnosed condition(s) they had since the last questionnaire cycle. Although no specific question on sarcoidosis was included, participants were asked to report “other major illness” in a final free-text field. We used this information to identify self-reported cases of physician-diagnosed sarcoidosis from 1989 onward.
Participants provided demographic data at baseline. Race was categorized in three groups (white, black, other) and ethnicity in two groups (Hispanic versus non-Hispanic). U.S. region at baseline, with updating every 2 years thereafter, was categorized into four groups (West, Midwest, South, Northeast). Although the NHSII participants initially resided in 14 states, as of the mid-1990s they resided in all 50 states and the District of Columbia (21).
Statistical analyses evaluated the prevalence of sarcoidosis at baseline (1989) and the average annual incidence during follow up (1991–2011). Associations between demographics (race, ethnicity, and U.S. region) at baseline and sarcoidosis prevalence at baseline (diagnosis before 1989) were evaluated by multinomial logistic regressions. Associations between demographics and sarcoidosis incidence during follow up were evaluated by Cox proportional hazard models. We evaluated associations with U.S. region both at baseline and at the questionnaire cycle before time of diagnosis (referred to hereafter as “region at the time of diagnosis”), handling region as a time-varying covariate in the Cox proportional hazard model. All Cox models were stratified by age in months and calendar year. Results from age-adjusted and multivariable adjusted models are presented. A two-sided P less than 0.05 was considered statistically significant.
Results
Among the 116,430 participants in the NHSII, 116 reported sarcoidosis at baseline (1989), for a prevalence of 100/100,000 women or 0.1%. During 2,275,028 person-years of follow up from 1989 to 2011, 261 incident cases were reported, for an average annual incidence rate of 11/100,000.
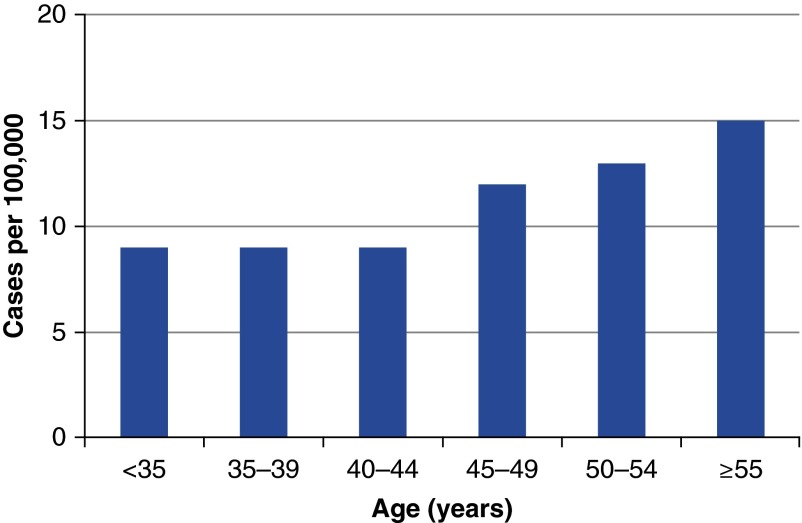
At baseline, women with sarcoidosis were slightly older (mean age [SD], 36.7 [4.1] yr) than women without sarcoidosis (34.4 [4.7] yr, P < 0.001). The median age at diagnosis among incident cases was 48 years (range, 28–63 yr). Age-specific incidence rates are presented in Figure 1. Incidence rate increased with age (P = 0.003) and ranged from 9/100,000 in women younger than 35 years to 15/100,000 in women 55 years and older. Similar results were observed when restricting analyses to white women only (96% of the study population); the number of nonwhite women was too low to study age-specific incident rates in this group specifically.
Figure 1.
Age-specific incidence rate of sarcoidosis in women, Nurses’ Health Study II (n = 261 incident cases; 2,275,028 person-years). Similar results were observed in sensitivity analyses where (1) cases were restricted to participants who specifically indicated that the diagnosis of sarcoidosis occurred within 2 years before the questionnaire cycle where they first reported sarcoidosis (n = 163 incident cases; 2,274,204 person-years), (2) women with less frequent medical examination (as measured by the number of times participants reported having had physical examination during follow up) were excluded (analysis conducted with n = 223 incident cases and 1,828,972 person-years), and (3) women who returned fewer than eight biennial follow-up questionnaires (out of 11) were excluded (analysis conducted with n = 236 incident cases and 2,076,332 person-years).
As expected, both prevalence (Table 1) and incidence (Table 2) of sarcoidosis were significantly higher in black women than in white women. Lower incidence rates were observed among women of other races (6/100,000) and among Hispanic women (5/100,000), although formal statistical testing of the difference could not be performed due to the low number of cases in these groups.
Table 1.
Prevalent sarcoidosis (before 1989) in women according to demographic and geographic characteristics at baseline, Nurses’ Health Study II
| Total | No. of Cases | Prevalence* | Age-adjusted OR |
Mutually Adjusted OR |
|||
|---|---|---|---|---|---|---|---|
| OR | CI | OR | CI | ||||
| Race | |||||||
| White | 111,230 | 102 | 92 | 1 | — | 1 | — |
| Black | 2,308 | 12 | 519 | 5.24 | 2.87–9.55 | 5.34 | 2.92–9.77 |
| Other | 2,892 | 2 | 69 | — | — | — | — |
| Ethnicity | |||||||
| Non-Hispanic | 114,270 | 116 | 102 | — | — | — | — |
| Hispanic | 2,160 | 0 | — | — | — | — | — |
| U.S. geographic region† | |||||||
| West | 17,126 | 13 | 75 | 1 | — | 1 | — |
| Midwest | 38,478 | 30 | 78 | 1.16 | 0.60–2.22 | 1.18 | 0.61–2.31 |
| South | 20,404 | 19 | 93 | 1.38 | 0.68–2.81 | 1.32 | 0.64–2.73 |
| Northeast | 40,225 | 54 | 134 | 1.90 | 1.04–3.48 | 1.92 | 1.02–3.59 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Results in bold are statistically significant.
Cases per 100,000.
At baseline, Nurses’ Health Study II participants resided in 14 states (California, Connecticut, Indiana, Iowa, Kentucky, Massachusetts, Michigan, Missouri, New York, North Carolina, Ohio, Pennsylvania, South Carolina, and Texas).
Table 2.
Incident sarcoidosis (1991–2011) in women according to demographic and geographic characteristics, Nurses’ Health Study II
| Person-Years | No. of Cases | Incidence Rate* | Age-adjusted IRR |
Mutually Adjusted IRR† |
Mutually Adjusted IRR‡ |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | HR | CI | HR | CI | ||||
| Race | |||||||||
| White | 2,182,697 | 241 | 11 | 1 | — | 1 | — | 1 | — |
| Black | 39,543 | 17 | 43 | 3.80 | 2.31–6.24 | 3.96 | 2.40–6.52 | 4.06 | 2.46–6.68 |
| Other | 52,787 | 3 | 6 | — | — | — | — | — | — |
| Ethnicity | |||||||||
| Non-Hispanic | 2,234,513 | 259 | 12 | — | — | — | — | — | — |
| Hispanic | 40,514 | 2 | 5 | — | — | — | — | — | — |
| U.S. geographic region at baseline§ | |||||||||
| West | 334,591 | 20 | 6 | 1 | — | 1 | — | — | — |
| Midwest | 762,654 | 106 | 14 | 2.52 | 1.56–4.06 | 2.49 | 1.54–4.04 | — | — |
| South | 388,490 | 41 | 11 | 1.90 | 1.11–3.24 | 1.81 | 1.05–3.09 | — | — |
| Northeast | 785,558 | 94 | 12 | 2.14 | 1.31–3.47 | 2.13 | 1.32–3.47 | — | — |
| U.S. geographic region at the time of diagnosis|| | |||||||||
| West | 357,490 | 27 | 8 | 1 | — | — | — | 1 | — |
| Midwest | 720,983 | 101 | 14 | 2.00 | 1.31–3.07 | — | — | 1.97 | 1.28–3.01 |
| South | 476,070 | 42 | 9 | 1.25 | 0.77–2.03 | — | — | 1.18 | 0.72–1.91 |
| Northeast | 716,189 | 90 | 13 | 1.78 | 1.15–2.74 | — | — | 1.76 | 1.14–2.71 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; IRR = incidence rate ratio; NHSII = Nurses’ Health Study II.
Results in bold are statistically significant.
Cases per 100,000 person-years.
Race and geographic region at baseline.
Race and geographic region at the time of diagnosis.
At baseline, NHSII participants resided in 14 states (California, Connecticut, Indiana, Iowa, Kentucky, Massachusetts, Michigan, Missouri, New York, North Carolina, Ohio, Pennsylvania, South Carolina, and Texas).
At the questionnaire cycle before time of diagnosis; during follow up, NHSII participants had resided in all 50 states and the District of Columbia (participants residing overseas were excluded from the analyses).
Variation in sarcoidosis prevalence and incidence were observed across U.S. regions, with consistently higher prevalence and incidence rates for women living in the Northeast than those in other regions, especially the West. Significantly higher incidence rates were also observed for women living in the Midwest (either at baseline or at the time of diagnosis) and in the South (at baseline only) than in the West. Although race distribution varied by region (proportion of white women: 90% in the West, 95% in the South, 97% in the Midwest and Northeast), geographical variations in sarcoidosis prevalence and incidence remained similar after adjustment for race (Tables 1 and 2, mutually adjusted odds ratios and incidence rate ratios).
Discussion
In a large prospective cohort study of predominantly white women in the United States, we observed an annual incidence rate of sarcoidosis of 11/100,000 over 22 years of follow up. The incidence rate increased with age and varied across U.S. regions, with more than twofold differences observed. Moreover, the risk of developing sarcoidosis was almost four times higher in black women than in white women.
Identification of sarcoidosis cases was based on self-report of a physician diagnosis of the disease on biennial questionnaires. Although cases have not been validated by medical records, the validity of these registered nurses’ reports for many other health outcomes is generally high (>90%) (19–23) and provides reassurance about the accuracy of these reports. Moreover, in the Black Women’s Health Study, investigators observed a very high agreement (96%) between self-report of sarcoidosis diagnosis and physician’s report (4); an even higher agreement might be expected in NHSII, which is a study of registered nurses. Case misclassification may occur in our study but is likely to have a limited impact on the results. Nevertheless, sarcoidosis is generally underdiagnosed, especially among patients with no or minimal symptoms (24). Thus, we acknowledge that the prevalence and incidence rates in the current study may be underestimates.
Despite this limitation, our findings are very similar to previous estimates in white U.S. women: 12/100,000 reported by Rybicki and colleagues (6) and 10 to 16/100,000 reported by Henke and colleagues (16), among similar age groups. We also found, as expected, an increased risk of sarcoidosis in black women compared with white women, with very similar estimates to those previously reported (6).
Sarcoidosis has often been described as a disease occurring most commonly in younger adults, although there is some recent evidence of an increasing age at diagnosis in various populations (25, 26) as well as differences in age at onset by race (27). In a U.S. population, the peak in annual incidence in white women was observed at age 40 to 49 years and a decade earlier in black women (6). In the U.S. Black Women’s Health Study, the highest incidence rate was observed at age 40 to 49 years among black women (4). In the current study of predominantly white women, sarcoidosis incidence increased with age, and the highest incidence rates were observed in the oldest age group (50–55 yr).
Several reasons may explain this different pattern. First, the diagnosis of sarcoidosis is often delayed (24), possibly even more so among patients over the age of 50 years (28). African Americans are known to be at higher risk of sarcoidosis, and a further delayed diagnosis is possible among white patients. However, race was not associated with time to diagnosis in a study of U.S. patients with sarcoidosis (24). Alternatively, the different age patterns may be explained by race-related differences in clinical presentation (3, 5) and possibly etiology of sarcoidosis.
Studies in northern Europe (8, 9) and in Japan (7) have described a bimodal pattern in the age-specific incidence rate among women, with a first peak in young adulthood (20–35 yr) and a second peak over the age of 50 years. A recent study in Japan found that in women, only the second peak was still observed in the last decade (25). In the current study, women were already aged 25 to 44 years at baseline, and incidence in young adulthood could not be assessed; however, our results are consistent with an increase in sarcoidosis incidence after the age of 50 years for women. Interestingly, in the previous studies the bimodal distribution was observed in women only, suggesting a potential role of endogenous hormones. In the Black Women’s Health Study, a later age at menopause was associated with a reduced risk of sarcoidosis incidence (29). Further studies are needed to better understand these sex and race differences in age-specific incidence pattern in sarcoidosis and the potential role of hormonal factors.
Geographical differences in the occurrence of sarcoidosis have been described worldwide (1, 7, 9, 10, 30–32). Higher rates have been observed in northern countries than in the rest of Europe (9, 10, 32), and lower rates have been observed in Asia (7). However, few studies have investigated regional differences at a local level (10, 33). In the United States, regional distribution of sarcoidosis has not been described recently (34). In the current study, we observed up to twofold differences in prevalence and incidence of sarcoidosis across U.S. regions. Prevalence and incidence were consistently higher in the Northeast and lower in the West. Increased incidence rates were also observed in the Midwest and the South compared with the West.
Etiological agents in sarcoidosis remain unknown, and many reasons might explain the observed geographic differences. Although an increased risk of sarcoidosis in rural areas (10) and associations with rural exposures (14, 34) have been described, they are unlikely to be the sole explanation for regional disparities; in particular, it would not explain our current observation of a higher risk in the Northeast than in the West, because the Northeast is highly urbanized. Other hypotheses, such as the role of residing in a colder environment and viral infections during the immune-forming period, have been formulated (32) but remain speculative. In the current study, one association (residence in the South) was restricted to the region of residence at baseline but was not observed for the region at the time of diagnosis, suggesting a potential role of exposures occurring earlier in life. However, this result should be interpreted with caution, because at baseline nurses resided in only 14 states, whereas all 50 states were represented during follow up. Overall, the factors explaining geographic disparities in sarcoidosis remain largely unknown and provide an important clue for future research into the etiology of this poorly understood disease.
In summary, the current study provides recent national data on the epidemiology of sarcoidosis among U.S. women. Our finding of a continuously increasing incidence rate in 45- to 65-year-old women adds evidence to the emerging recognition that sarcoidosis often presents in older women (3, 35). This result is of clinical importance, as recent data suggest differences in older patients with sarcoidosis that may require specific management (5). We also describe, for the first time since the 1970s, regional difference across the United States in sarcoidosis occurrence and in particular a higher risk in the Northeast. Our findings call for more large epidemiological studies on sarcoidosis, with better phenotypic characterization, to better understand the causes behind the observed demographic and geographic differences in disease occurrence.
Acknowledgments
Acknowledgment
The authors thank the participants and staff of the Nurses’ Health Study II for their valuable contributions.
Footnotes
Supported by National Institutes of Health grant UM1 CA176726. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: O.D. contributed to study conception and hypothesis delineation, statistical programming, data analysis, data interpretation, and primary manuscript preparation. L.A. contributed to data management, statistical programming, data analysis, data interpretation, and critical revision of the manuscript. A.S.W. contributed to data management, data interpretation, and critical revision of the manuscript. Y.C.C. contributed to data interpretation and critical revision of the manuscript. C.A.C. contributed to study conception and hypothesis delineation, data interpretation, and critical revision of the manuscript. All authors approved the final version of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011;305:391–399. doi: 10.1001/jama.2011.10. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 4.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139:144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472–478. doi: 10.1097/MCP.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, Oritsu M, Nukiwa T. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–379. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 8.Byg K-E, Milman N, Hansen S. Sarcoidosis in Denmark 1980-1994: a registry-based incidence study comprising 5536 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:46–52. [PubMed] [Google Scholar]
- 9.Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson TT, Plant BJ, Henry MT, Bredin CP. Sarcoidosis in Ireland: regional differences in prevalence and mortality from 1996-2005. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:111–120. [PubMed] [Google Scholar]
- 11.Cozier YC, Ruiz-Narvaez EA, McKinnon CJ, Berman JS, Rosenberg L, Palmer JR. Fine-mapping in African-American women confirms the importance of the 10p12 locus to sarcoidosis. Genes Immun. 2012;13:573–578. doi: 10.1038/gene.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cozier Y, Ruiz-Narvaez E, McKinnon C, Berman J, Rosenberg L, Palmer J. Replication of genetic loci for sarcoidosis in US black women: data from the Black Women’s Health Study. Hum Genet. 2013;132:803–810. doi: 10.1007/s00439-013-1292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147:1086–1093. doi: 10.1378/chest.14-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, Terrin ML, Weinberger SE, Moller DR, McLennan G, et al. ACCESS Research Group. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 15.Kucera GP, Rybicki BA, Kirkey KL, Coon SW, Major ML, Maliarik MJ, Iannuzzi MC. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527–1535. doi: 10.1378/chest.123.5.1527. [DOI] [PubMed] [Google Scholar]
- 16.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123:840–845. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 17.Prezant DJ, Dhala A, Goldstein A, Janus D, Ortiz F, Aldrich TK, Kelly KJ. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183–1193. doi: 10.1378/chest.116.5.1183. [DOI] [PubMed] [Google Scholar]
- 18.Gorham ED, Garland CF, Garland FC, Kaiser K, Travis WD, Centeno JA. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431–1438. doi: 10.1378/chest.126.5.1431. [DOI] [PubMed] [Google Scholar]
- 19.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Spiegelman D, Colditz GA. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst. 1998;90:1155–1160. doi: 10.1093/jnci/90.15.1155. [DOI] [PubMed] [Google Scholar]
- 20.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingaiah S, Hart JE, Laden F, Aschengrau A, Missmer SA. Air pollution exposures during adulthood and risk of endometriosis in the Nurses’ Health Study II. Environ Health Perspect. 2014;122:58–64. doi: 10.1289/ehp.1306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernán MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53:1711–1718. doi: 10.1212/wnl.53.8.1711. [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 24.Judson MA, Thompson BW, Rabin DL, Steimel J, Knattereud GL, Lackland DT, Rose C, Rand CS, Baughman RP, Teirstein AS ACCESS Research Group. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 25.Sawahata M, Sugiyama Y, Nakamura Y, Nakayama M, Mato N, Yamasawa H, Bando M. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med. 2015;109:272–278. doi: 10.1016/j.rmed.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Martusewicz-Boros MM, Boros PW, Wiatr E, Roszkowski-Śliż K. What comorbidities accompany sarcoidosis? A large cohort (n=1779) patients analysis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:115–120. [PubMed] [Google Scholar]
- 27.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–127. [PubMed] [Google Scholar]
- 28.Lenner R, Schilero GJ, Padilla ML, Teirstein AS. Sarcoidosis presenting in patients older than 50 years. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:143–147. [PubMed] [Google Scholar]
- 29.Cozier YC, Berman JS, Palmer JR, Boggs DA, Wise LA, Rosenberg L. Reproductive and hormonal factors in relation to incidence of sarcoidosis in US Black women: the Black Women’s Health Study. Am J Epidemiol. 2012;176:635–641. doi: 10.1093/aje/kws145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coquart N, Cadelis G, Tressières B, Cordel N. Epidemiology of sarcoidosis in Afro-Caribbean people: a 7-year retrospective study in Guadeloupe. Int J Dermatol. 2015;54:188–192. doi: 10.1111/ijd.12633. [DOI] [PubMed] [Google Scholar]
- 31.Gribbin J, Hubbard RB, Le Jeune I, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurata A. Hygiene hypothesis: why south/north geographical differences in prevalence of asthma and sarcoidosis? Med Hypotheses. 2012;79:363–364. doi: 10.1016/j.mehy.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Kajdasz DK, Judson MA, Mohr LC, Jr, Lackland DT. Geographic variation in sarcoidosis in South Carolina: its relation to socioeconomic status and health care indicators. Am J Epidemiol. 1999;150:271–278. doi: 10.1093/oxfordjournals.aje.a009998. [DOI] [PubMed] [Google Scholar]
- 34.Kajdasz DK, Lackland DT, Mohr LC, Judson MA. A current assessment of rurally linked exposures as potential risk factors for sarcoidosis. Ann Epidemiol. 2001;11:111–117. doi: 10.1016/s1047-2797(00)00179-4. [DOI] [PubMed] [Google Scholar]
- 35.Jamilloux Y, Bonnefoy M, Valeyre D, Varron L, Broussolle C, Sève P. Elderly-onset sarcoidosis: prevalence, clinical course, and treatment. Drugs Aging. 2013;30:969–978. doi: 10.1007/s40266-013-0125-5. [DOI] [PubMed] [Google Scholar]