Abstract
Human ovary autotransplantation is a promising option for fertility preservation of young women and girls undergoing gonadotoxic treatments for cancer or some autoimmune diseases. Although experimental, it resulted in at least 42 healthy babies worldwide. According to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a systematic literature review was performed for all relevant full-text articles published in English from 1 January 2000 to 01 October 2015 in PubMed to explore the latest clinical and research advances of human ovary autotransplantation. Human ovary autotransplantation involves ovarian tissue extraction, freezing/thawing, and transplantation back into the same patient. Three major forms of human ovary autotransplantation exist including (a) transplantation of cortical ovarian tissue, (b) transplantation of whole ovary, and (c) transplantation of ovarian follicles (artificial ovary). According to the recent guidelines, human ovary autotransplantation is still considered experimental; however, it has unique advantages in comparison to other options of female fertility preservation. Human ovary autotransplantation (i) does not need prior ovarian stimulation, (ii) allows immediate initiation of cancer therapy, (iii) can restore both endocrine and reproductive ovarian functions, and (iv) may be the only fertility preservation option suitable for prepubertal girls or for young women with estrogen-sensitive malignancies. As any other fertility preservation option, human ovary autotransplantation has both advantages and disadvantages and may not be feasible for all cases. The major challenges facing this option are how to avoid the risk of reintroducing malignant cells and how to prolong the lifespan of ovarian transplant as well as how to improve artificial ovary results.
Keywords: Ovary autotransplantation, Female fertility preservation, Cancer, Cryopreservation, In vitro maturation, Oncofertility
1 Introduction
To preserve fertility of young women and girls especially those undergoing gonadotoxic treatments for cancer or some autoimmune diseases, several options are offered including embryo freezing, egg freezing, ovary autotransplantation, in vitro maturation (IVM), and ovarian protection [1-5].
According to the most recent female fertility preservation guidelines [6-21], human ovary autotransplantation is still considered experimental although it resulted in at least 42 healthy babies worldwide without any increased risk for miscarriage or congenital anomalies. Technically, human ovary autotransplantation involves ovarian tissue extraction, freezing/thawing, and transplantation back into the same patient. It is a promising option as it has several unique advantages in comparison to embryo freezing and egg freezing, the only two established options for female fertility preservation [22-27].
In this review, we explore the latest clinical and research advances of human ovary autotransplantation and investigate in detail its advantages and disadvantages as an option for female fertility preservation. We also suggest an updated model for integration of human ovary autotransplantation with the other options of female fertility preservation.
2 Methods
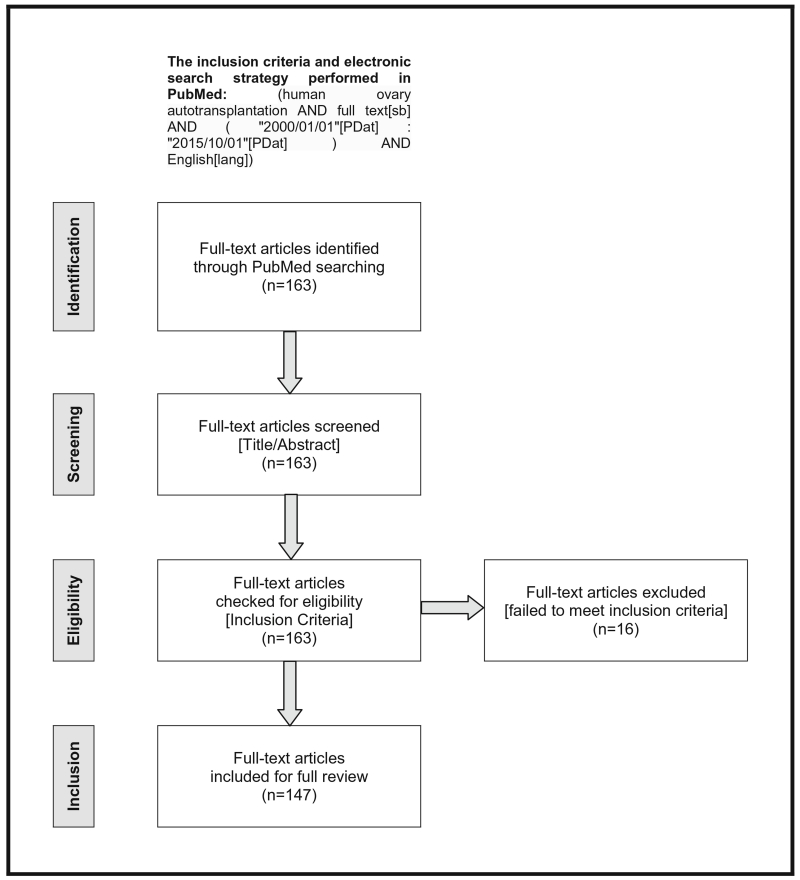
According to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a systematic review of the literature was performed for all relevant full-text articles published in English from 1 January 2000 to 1 October 2015 in PubMed to explore the latest clinical and research advances of human ovary autotransplantation. Based on these inclusion criteria, the following electronic search strategy was performed in PubMed: (human ovary autotransplantation AND full text[sb] AND (“2000/01/01”[PDat]: “2015/10/01”[PDat]) AND English[lang]).
The full-text articles identified from the initial search underwent screening for titles and abstracts, then were checked for eligibility according to the inclusion criteria. Only the full-text articles that focus primarily on human ovary autotransplantation were included and fully reviewed. Data was extracted from the text, tables, graphs, and references of the included articles.
3 Results
A total of 163 full-text articles were identified from the initial search. After screening titles and abstracts, all 163 full-text articles were checked for eligibility according to the inclusion criteria. Only 147 full-text articles that focus primarily on human ovary autotransplantation were included and fully reviewed. PRISMA flow diagram of the systematic review process is illustrated in Fig. 1. Some significant articles were not identified from the initial search, but we reviewed them as well. Therefore, the final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Fig. 1.
PRISMA four-phase flow diagram of identification, screening, eligibility, and inclusion steps
As an option for female fertility preservation, human ovary autotransplantation involves ovarian tissue extraction, freezing/thawing, and transplantation back into the same patient. Basically, there are three major types of human ovary autotransplantation including (a) transplantation of cortical ovarian tissue (orthotopic or heterotopic), (b) transplantation of a whole ovary, and (c) transplantation of ovarian follicles (artificial ovary) [22-27].
To date worldwide, the only forms of human ovary autotransplantation resulted in healthy live births are cortical ovarian tissue orthotopic transplantation (40 babies) and heterotopic transplantation (2 babies) although an accurate international registry is highly required (Tables 1, 2, and 3) [28-60].
Table 1.
Reported 42 live births after human ovary autotransplantation–updated and modified from [41] and [56]
| Group | Reference | Human ovary | Freezing | Autotransplantation | Live births (n) total=42 |
Country |
|---|---|---|---|---|---|---|
| Andersen et al. | [28-31] | Cortical tissue | Slow freezing | Orthotropic | 6 | Denmark |
| Demeestere et al. | [32-34] | Cortical tissue | Slow freezing | Orthotropic | 3 | Belgium |
| Donnez and Dolmans et al. | [35-41] | Cortical tissue | Slow freezing | Orthotropic | 6 | Belgium |
| Meirow and Dor et al. | [42, 43] | Cortical tissue | Slow freezing | Orthotropic | 3 | Israel |
| Revel et al. | [44] | Cortical tissue | Slow freezing | Orthotropic | 3 | Israel |
| Burmeister et al. | [45] | Cortical tissue | Slow freezing | Orthotropic | 1 | Australia |
| Sánchez-Serrano et al. | [46] | Cortical tissue | Slow freezing | Orthotropic | 2 | Spain |
| García Rada | [47] | Cortical tissue | Slow freezing | Orthotropic | 1 | Spain |
| Piver et al. | [48, 49] | Cortical tissue | Slow freezing | Orthotropic | 3 | France |
| Revelli et al. | [50] | Cortical tissue | Slow freezing | Orthotropic | 1 | Italy |
| Dittrich et al. | [51, 52] | Cortical tissue | Slow freezing | Orthotropic | 3 | Germany |
| Silber et al. | [53-56] | Cortical tissue | Slow freezing | Orthotropic | 4 | USA |
| Rodriguez-Wallberg et al. | [57] | Cortical tissue | Slow freezing | Orthotropic | 1 | Sweden |
| Kawamura et al. | [58] | Cortical tissue | Vitrification | Orthotropic | 1 | Japan |
| Suzuki et al. | [59] | Cortical tissue | Vitrification | Orthotropic | 2 | Japan |
| Stern et al. | [60] | Cortical tissue | Slow freezing | Heterotopic | 2 | Australia |
Table 2.
Reported 42 live births after human ovary autotransplantation (simplified)
Table 3.
Human ovary allotransplantation (related references are cited within the original text)
| Human ovary autotransplantation | (A) Transplantation of cortical ovarian tissue |
(B) Transplantation of whole ovary | (C) Transplantation of ovarian follicles (artificial ovary) |
|
|---|---|---|---|---|
| Othrotopic | Heterotopic | |||
| 1. Definition | Transplantation of frozen-thawed cortical ovarian tissue back to the same patient into the remaining ovary, ovarian fossa, or broad ligament |
Transplantation of frozen-thawed cortical ovarian tissue back to the same patient into the subcutaneous space of abdominal wall or forearm |
Transplantation of frozen-thawed whole ovary with its vascular pedicle back to the same patient |
Reimplantation of isolated preantral ovarian follicles into a biodegradable artificial ovary made of three- dimensional alginate matrigel matrix |
| 2. Aim | To resume endocrine and reproductive ovarian functions |
To resume endocrine and reproductive ovarian functions |
|
|
| 3. Ovarian Extraction | At least half of one ovary via laparoscopy or mini-laparotomy |
At least half of one ovary via laparoscopy or mini-laparotomy |
At least one whole ovary with its vascular pedicle via laparoscopy or mini-laparotomy |
At least half of one ovary via laparoscopy or mini-laparotomy. Isolation of preantral ovarian follicles can be performed before or after ovarian tissue freezing |
| 4. Freezing/thawing | Slow freezing is the standard. Vitrification is promising. |
Slow freezing is the standard. Vitrification is promising. |
Slow freezing is the standard. Vitrification is promising. |
Slow freezing is the standard. Vitrification is promising. |
| 5. Transplantation | Avascular grafting of frozen-thawed cortical ovarian tissue pieces into the remaining ovary, ovarian fossa, or broad ligament of the same patient |
Avascular grafting of frozen- thawed cortical ovarian tissue pieces into the subcutaneous space of abdominal wall or forearm of the same patient |
Vascular grafting of frozen- thawed whole ovary back to the same patient |
Reimplantation of isolated preantral ovarian follicles into a biodegradable artificial ovary made of three- dimensional alginate matrigel matrix. The biodegradable artificial ovary containing follicles may be either cultured in vitro or in vivo (xenotransplantation or autotransplantation) to allow follicular development. |
| 6. Advantages |
|
|
|
|
| 7. Disadvantages |
|
|
|
|
| 8. Outcome |
|
|
|
|
| 9. Indications | Fertility preservation for young women (<40 years old) and girls scheduled to receive gonadotoxic treatments for cancer or some autoimmune diseases. |
As an alternative to orthotopic autotransplantation in case of severe pelvic adhesions or poor pelvic vasculature due to previous intensive pelvic irradiation |
Still in research settings as a fertility preservation option for young women and girls scheduled to receive gonadotoxic treatments for cancer or some autoimmune diseases |
|
| 10. Contraindications |
|
|
|
Xenotransplantation to mature human ovarian follicles is not allowed in clinical practice due to safety and ethical reasons. |
| 11. Animal research (large mammals) |
Resulted in healthy offsprings in sheep |
Resulted in healthy offsprings in monkeys |
Resulted in healthy offsprings in sheep |
Resulted in mature oocytes in monkeys |
In this section, we discuss in detail ovarian tissue extraction, ovarian tissue freezing/thawing as well as the three major types of human ovary autotransplantation.
3.1 Ovarian tissue extraction
Ovarian tissue is extracted surgically via laparoscopy, mini-laparotomy, or laparotomy. It should be indicated before initiation of gonadotoxic treatments for cancer or some autoimmune diseases. If feasible, it may be performed in the same setting with surgical ovarian transposition (oophoropexy) or surgical excision of primary pelvic and abdominal malignancies [22, 23, 61]. Ovarian tissue extraction is also indicated in some nonmalignant conditions such as benign ovarian tumors, severe or recurrent endometriosis, BRCA1 or BRCA2 mutation carriers, risk of premature ovarian failure, transgender operations, and bone marrow transplantation [22-26].
Three surgical possibilities of ovarian tissue extraction exist including partial, unilateral, or bilateral oophorectomy. If clinically reasonable, it is usually recommended to excise at least half of one ovary (partial oophorectomy), while the other ovary is left intact in situ to act as an ideal site for future orthotopic autotransplantation. The remaining ovary may also keep the potential for spontaneous resumption of endocrine and reproductive ovarian functions after gonadotoxic treatments. However, if severe ovarian damage is highly expected due to scheduled aggressive chemotherapy and radiotherapy, unilateral or bilateral oophorectomy can be performed as prophylaxis. In case future autotransplantation of a whole ovary is planned, excision of an ovary with a large part of its vascular pedicle is then a must [22-25]. Surgical excision of less than half of one ovary may put the whole procedure of ovarian tissue freezing and autotransplantation at risk for suboptimal histological and functional investigations of ovarian tissue before and after freezing and thereafter at risk for reintroducing malignant cells, transplanting nonviable ovarian tissue, or transplanting ovarian tissue with poor endocrine and reproductive functions [6-21].
Immediately after ovarian tissue extraction, the excised tissue is transported on ice to the local laboratory for further processing. If the local laboratory team is less experienced, the excised ovarian tissue could be shipped within 24 h under special transport conditions to centralized cryobanks for further processing by experts. After histological examination of a portion to exclude malignancy, most of the excised ovarian tissue is frozen for future autotransplantation. When possible, another portion (~20 %) of the excised ovarian tissue may be processed as fresh for future transplantation of ovarian follicles (artificial ovary), IVM of oocytes or other research purposes [62, 63].
3.2 Ovarian tissue freezing
Ovarian follicles are the functional units of human ovary. Histologically, most of ovarian follicles are located within the ovarian cortex, and about 90 % of them are in the primor-dial stage. By freezing ovarian cortex, most of primordial follicles are preserved with favorable survival rate and uncompromised developmental potential due to their small size and low metabolic rate that make them resistant to cryoinjury [64-66]. Ovarian tissue freezing involves cryopreservation of cortical ovarian tissue or cryopreservation of a whole ovary either by slow freezing or by vitrification. To date, the standard method for ovarian tissue freezing is slow freezing; however, vitrification is also encouraged due to its promising results [67-71]. As a preparation for freezing, ovarian cortex should be dissected from medulla and further cut into ultra-thin strips (~10×5×1 mm each), slices (~4×2×1 mm each), or cubes (~2 mm3 each). In case of cryopreservation of a whole ovary, a large part of its vascular pedicle must be present in situ. The ultra-thinness of ovarian cortical pieces or the presence of the vascular pedicle of a whole ovary is essential not only for proper perfusion of cryoprotectants but also for efficient revascularization after autotransplantation [22-25]. Some oocytes may expel spontaneously in the medium during dissection of the extracted ovarian tissue and then can be used for IVM [72-74] and subsequent in vitro fertilization (IVF), resulting recently in a reported live birth [75].
Slow freezing is the standard method for cryopreservation of cortical ovarian tissue or whole ovary. It involves exposure of cortical ovarian tissue or whole ovary to lower concentrations of cryoprotectants followed by slow cooling to −140 °C at low rates (~1 °C/min) via an automated freezing machine. It carries a very low risk for intracellular crystallization. However, it is a time-consuming method (within several hours) and usually needs a computerized freezing machine [64-71]. To date, several protocols for slow freezing/thawing have been described and at least 39 live births were reported worldwide after slow freezing/thawing of human cortical ovarian tissue (Tables 1 and 2) [28-57, 60].
Vitrification is a promising cryopreservation method as it has comparable outcomes to slow freezing. Vitrification involves exposure of cortical ovarian tissue or whole ovary to higher concentrations of cryoprotectants followed by ultra-rapid cooling to −196 °C at very fast rates (~20,000 °C/min) by direct plunging into liquid nitrogen. Vitrification is a cost-effective method as it is simple, time-saving (within few minutes), and it does not need an expensive freezing machine. Vitrification carries a very low risk for intracellular crystallization. However, it carries a risk for cellular toxicity and osmotic trauma due to higher concentrations of cryoprotectants as well as a risk for contamination due to direct exposure to liquid nitrogen [64-71]. To date, several protocols for vitrification/warming have been described, but only three live births were reported worldwide after vitrification/warming of human cortical ovarian tissue (Tables 1 and 2) [58, 59].
A group of histological and functional investigations of ovarian tissue should be performed before and after freezing to exclude the risk of reintroducing malignant cells, transplanting nonviable ovarian tissue, or transplanting ovarian tissue with poor endocrine and reproductive functions. Examples of these investigations include histological examination, transmission electron microscopy, immunohistochemistry, terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphate nick end-labeling (TUNEL) assay, polymerase chain reaction (PCR), estradiol and progesterone production in vitro, and xenografting [76-83]. After freezing, the ovarian tissue is stored in liquid nitrogen at −196 °C for up to 10 years. When the patient becomes cancer-free, her frozen ovarian tissue can be thawed and transplanted back to her (autotransplantation) in order to resume the endocrine and reproductive ovarian functions [6-21, 84].
3.3 Orthotopic autotransplantation of ovarian tissue
Orthotopic autotransplantation of ovarian tissue refers to transplantation of frozen-thawed cortical ovarian tissue back to the same patient into pelvic sites such as the remaining ovary, ovarian fossa, or broad ligament. It aims to resume both endocrine and reproductive ovarian functions. Usually, at least half of one ovary is excised via laparoscopy or mini-laparotomy. After dissection from medulla, ovarian cortex is further cut into ultra-thin pieces for cryopreservation via slow freezing as standard or via vitrification as a promising alternative. When autotransplantation is decided, avascular grafting of the frozen-thawed cortical ovarian tissue pieces is performed into the remaining ovary, ovarian fossa, or broad ligament via laparoscopy or mini-laparotomy [85-90].
Orthotopic autotransplantation of frozen-thawed cortical ovarian tissue has several advantages including the following: (1) It requires no prior ovarian stimulation nor delay in cancer treatment; (2) it provides normal environment for follicle and oocyte development; (3) it allows spontaneous pregnancy, otherwise further ovarian stimulation, ovum pickup, and IVF can be performed; and (4) it may reduce the risk of reintroducing malignant cells due to avascular grafting [22-27, 85-90]. However, orthotopic autotransplantation of frozen-thawed cortical ovarian tissue has also some disadvantages including the following: (1) It carries the risk of reintroducing malignant cells especially in case of ovarian malignancies or in malignancies that may metastasize in ovaries; (2) it may increase post-grafting ischemia and follicle atresia due to avascular grafting; and (3) although it allows spontaneous pregnancy, spontaneous pregnancy may occur due to ovulation from the remaining ovary and not from the orthotopically grafted cortical ovarian tissue [22-27, 85-91]. To reduce the risk of reintroducing malignant cells, histological examination, immunohistochemistry, polymerase chain reaction, and long-term xenografting of ovarian tissue portion can be performed [77-83]. To reduce the window of post-grafting ischemia that may be responsible for atresia of ~70 % of follicles, angiogenic and antiapoptotic factors, gonadotropins, antioxidants, and mesenchymal stem cells (MSCs) can be used to enhance neovascularization and to prolong the lifespan of ovarian transplant [92-97].
With a great variability observed, ovarian function after orthotopic autotransplantation of frozen-thawed cortical ovarian tissue may resume 2–9 months post-operatively and it may last for up to 7 years. To date, this technique has resulted in at least 40 healthy babies worldwide; most of them were due to spontaneous pregnancies without the need for IVF (Tables 1 and 2) [28-59]. Although an accurate international registry is highly required, it is roughly estimated that the live birth rate per cortical ovarian tissue transplant is ~30 % [56]. In contrast, orthotopic transplantation of fresh and frozen-thawed cortical ovarian tissue was successful between monozygotic twin sisters and resulted in healthy babies [53-56].
Orthotopic autotransplantation of frozen-thawed cortical ovarian tissue is indicated as a fertility preservation option for young women (<40 years old), and girls scheduled to receive gonadotoxic treatments for cancer or some autoimmune diseases. It may be the only fertility preservation option suitable for prepubertal girls or for young women with estrogen-sensitive malignancies where ovarian stimulation and embryo or egg freezing are contraindicated [22]. Recently, the first live birth after orthotopic autotransplantation of cortical ovarian tissue cryopreserved during childhood has been reported [34].
According to several studies, systematic reviews and meta-analyses assessing the risk of reintroducing malignant cells, orthotopic autotransplantation of frozen-thawed cortical ovarian tissue should be contraindicated in ovarian carcinomas and malignancies that may metastasize in ovaries such as leukemia (high risk), gastrointestinal cancer (moderate risk), breast cancer, lymphoma, gynecological malignancies, and sarcoma of the bone and connective tissue (low risk). The risk of metastasizing into the ovaries is related not only to the type but also to the stage of the primary cancer at the time of ovarian tissue extraction [98-100]. In such cases, IVM or ovarian follicle transplantation (artificial ovary) may be offered as alternatives [101-103]. It is also not recommended to perform orthotopic autotransplantation of frozen-thawed cortical ovarian tissue in relatively old patients (>40 years old) or in patients with poor ovarian reserve [22].
For decades in animal research, orthotopic autotransplantation of frozen-thawed cortical ovarian tissue was successful and resulted in healthy offsprings in large mammals as sheep [104, 105].
3.4 Heterotopic autotransplantation of ovarian tissue
Heterotopic autotransplantation of ovarian tissue refers to transplantation of frozen-thawed cortical ovarian tissue back to the same patient into extrapelvic sites such as the subcutaneous space of abdominal wall or forearm. It aims to resume both endocrine and reproductive ovarian functions. Usually, at least half of one ovary is excised via laparoscopy or mini-laparotomy. After dissection from medulla, ovarian cortex is further cut into ultra-thin pieces for cryopreservation via slow freezing as standard or via vitrification as a promising alternative. When autotransplantation is decided, avascular grafting of the frozen-thawed cortical ovarian tissue pieces is performed into the subcutaneous space of abdominal wall or forearm [85-90].
Heterotopic autotransplantation of frozen-thawed cortical ovarian tissue has several advantages including the following: (1) It requires no prior ovarian stimulation nor delay in cancer treatment, (2) it is surgically easier and considered as a good alternative to orthotopic autotransplantation in case of severe pelvic adhesions or poor pelvic vasculature, (3) it allows easy monitoring of the grafted ovarian tissue, and (4) it may reduce the risk of reintroducing malignant cells due to avascular grafting [22-27, 85-90]. However, heterotopic autotransplantation of frozen-thawed cortical ovarian tissue has also some disadvantages including the following: (1) It carries the risk of reintroducing malignant cells especially in the case of ovarian malignancies or in malignancies that may metastasize in ovaries; (2) it may increase post-grafting ischemia and follicle atresia due to avascular grafting; (3) it provides abnormal environment for follicle and oocyte development; (4) it does not allow spontaneous pregnancy; therefore, subsequent ovarian stimulation, ovum pickup, and IVF must be performed [22-27, 85-90].
To reduce the risk of reintroducing malignant cells and the window of post-grafting ischemia, same measures as described in orthotopic autotransplantation can be applied [77-83, 92-97].
With a great variability observed, ovarian function after heterotopic autotransplantation of frozen-thawed cortical ovarian tissue may resume 2–9 months post-operatively and it may last for up to 7 years. To date, this technique has resulted in the first two babies worldwide (Tables 1 and 2) [60]; in addition, it resulted in a four-cell embryo [106], a biochemical pregnancy [107], and a clinical pregnancy [108].
Heterotopic autotransplantation of frozen-thawed cortical ovarian tissue is indicated as an alternative to orthotopic autotransplantation in case of severe pelvic adhesions or poor pelvic vasculature due to previous intensive pelvic irradiation. It has also the same contraindications as described in orthotopic autotransplantation [22, 98-100].
In animal research, heterotopic autotransplantation of frozen-thawed cortical ovarian tissue was successful and resulted in healthy offsprings in large mammals as monkey [109].
3.5 Autotransplantation of whole ovary
Autotransplantation of whole ovary refers to transplantation of frozen-thawed whole ovary with its vascular pedicle back to the same patient. It aims to resume both endocrine and reproductive ovarian functions as well as to overcome post-grafting ischemia and follicle atresia. One whole ovary is excised with a large part of its vascular pedicle (≥5 cm of the infundibulopelvic ligament) via laparoscopy or mini-laparotomy. The whole ovary must then undergo immediate cryoperfusion and cryopreservation via slow freezing as standard or via vitrification as a promising alternative. When autotransplantation is decided, vascular grafting and anastomosis of frozen-thawed whole ovary back to the same patient is performed via mini-laparotomy or laparotomy [110-118].
Autotransplantation of frozen-thawed whole ovary has several advantages including the following: (1) It requires no prior ovarian stimulation nor delay in cancer treatment; (2) it provides normal environment for follicle and oocyte development; (3) it allows spontaneous pregnancy, otherwise further ovarian stimulation, ovum pickup, and IVF can be performed; (4) it should reduce post-grafting ischemia and follicle atresia due to vascular grafting [110-118]. However, autotransplantation of frozen-thawed whole ovary has also some disadvantages including the following: (1) It has a higher risk of cryoinjury during freezing due to inadequate diffusion of cryoprotectants throughout the entire ovary, nonhomogenous cooling rate between the core and the periphery of the ovary, or cryoinjury of ovarian vasculature; (2) it has a higher risk of reintroducing malignant cells especially in case of ovarian malignancies or in malignancies that may metastasize in ovaries; (3) there is a surgical difficulty of vascular anastomosis due to the small size of ovarian artery (~0.5 mm in diameter), short ovarian vascular pedicle (~5 cm in length), discrepancy between the diameters of graft and recipient vessels, and possible failure of microvascular anastomosis; (4) it has a higher risk of post-operative vascular complications including anastomotic bleeding, pseudoaneurysm, stenosis, or microvascular thrombosis; (5) its vascular complications can severely affect the survival of the entire ovary leaving no other attempt for transplantation [110-118].
To reduce the risk of reintroducing malignant cells, same measures as described in orthotopic autotransplantation can be applied [77-83]. To reduce vascular complications, microvascular anastomosis should be performed by an expert to attempt different types of anastomosis when needed such as end-toend, end-to-side, or fishmouth modifications [113, 119]. In the case of damaged remaining ovary by previous gonadotoxic treatments, its vascular pedicle may be used for vascular anastomosis of the transplanted frozen-thawed ovary [120].
After transplantation of frozen-thawed whole ovary, resumption of ovarian function is questionable. To date, this technique has not resulted yet in any reported live births. However, it is important to mention that fresh whole ovary transplantation between a donor and a recipient was successful with one reported live birth [121].
Autotransplantation of frozen-thawed whole ovary is still in research settings as an option for female fertility preservation. It has also the same contraindications as described in orthotopic autotransplantation [22].
In animal research, autotransplantation of frozen-thawed whole or hemi ovary was successful in large mammals as sheep and resulted in healthy offsprings [122-125].
3.6 Transplantation of ovarian follicles (artificial ovary)
Transplantation of human ovarian follicles refers to reimplantation of isolated preantral ovarian follicles into a biodegradable artificial ovary made of a three-dimensional alginate matrigel matrix. It aims to resume reproductive ovarian function as well as to avoid the risk of reintroducing malignant cells. At least half of one ovary is excised via laparoscopy or mini-laparotomy. Isolation of preantral ovarian follicles from ovarian cortex can be performed mechanically and/or enzymatically before or after cortical ovarian tissue freezing via slow freezing or vitrification. However, isolation of preantral ovarian follicles from fresh ovarian cortex improves the results. After isolation, preantral ovarian follicles are reimplanted into a biodegradable artificial ovary made of a three-dimensional alginate matrigel matrix. To allow follicular development, the biodegradable artificial ovary containing preantral follicles may be either cultured in vitro or in vivo via xenotransplantation into nude mice or theoretically via autotransplantation into the same patient. After the preantral ovarian follicles reach antral stage, the oocytes can be isolated for further IVM and IVF [23, 126-131].
Transplantation of human ovarian follicles has several advantages including the following: (1) It requires no prior ovarian stimulation nor delay in cancer treatment and (2) it has no risk of reintroducing malignant cells. However, transplantation of ovarian follicles has also some disadvantages including the following: (1) It allows limited follicle and oocyte development; (2) it requires further oocyte isolation, IVM, and IVF; (3) it may require xenotransplantation to mature human ovarian follicles and that is not allowed in clinical practice due to several safety and ethical reasons; (4) it does not resume the endocrine ovarian function of the patient [132-135].
To date, transplantation of human ovarian follicles has not resulted yet in any reported live births. However, development of isolated small preantral follicles into large preantral stage or early antral stage after in vitro culture or xenotransplantation was achieved [136-139].
Transplantation of human ovarian follicles is still in research settings as an option for female fertility preservation. It is a promising alternative to autotransplantation to avoid the risk of reintroducing malignant cells. Nevertheless, xenotransplantation to mature human ovarian follicles is not allowed in clinical practice due to safety and ethical reasons [22, 23].
In animal research, transplantation of ovarian follicles into three-dimensional in vitro culture was successful in large mammals as monkeys and resulted in antrum formation, steroid hormone production [140, 141], and mature oocytes [142, 143].
4 Discussion
Female fertility loss refers to the permanent inability of a woman to conceive due to complete depletion of her oocytes. The most common causes of fertility loss in women are aging and gonadotoxic treatments for cancer and some autoimmune diseases. Other less common causes of female fertility loss include bilateral oophorectomy, familial premature ovarian failure, gonadal agenesis, and some genetic disorders [1-5].
Each year worldwide, several million women are diagnosed with different types of cancer. According to GLOBOCAN study in 2012, the number of new cases of cancer in women was about 0.7 million in the USA, 1.7 million in Europe, and 6.6 million worldwide [144]. Approximately 10 % of women with cancer are diagnosed during their reproductive years, and nearly 80–90 % of them can survive due to advances in cancer diagnosis and treatment [145].
The most common cancers in young women (age <40) are breast (29 %), lung (13 %), colorectal (8 %), uterine and cervical (6 %) cancer, thyroid carcinoma (6 %), lymphoma (4 %), melanoma (4 %), leukemia (3 %), kidney (3 %), and pancreatic (3 %) cancer [145]. The most common cancers in female adolescents (age 15–19) are lymphoma (23 %), leukemia (12 %), thyroid (11 %), central nervous system malignancies (10 %), bone tumors (7 %), melanoma (6 %), and ovarian germ cell tumors (2 %) [146]. The most common cancers in prepubertal girls (age 0–14) are leukemia (31 %), central nervous system malignancies (21 %), lymphoma (10 %), neuroblastoma (7 %), Wilms tumor (5 %), bone tumors (4 %), rhabdomyosarcoma (3 %), and retinoblastoma (3 %) [146].
As a common side effect of cytotoxic cancer treatments, the probability of young female cancer survivors to conceive after chemotherapy and radiotherapy is markedly reduced by ~50 % with an increased risk of miscarriage, preterm labor, and low birth weight babies [147-149]. However, when ovaries are exposed to aggressive chemotherapy and radiotherapy, gonadotoxicity occurs leading to irreversible damage of ovarian follicles and oocytes, premature ovarian failure, and fertility loss in more than 80 % of cases [150, 151]. Alkylating chemotherapy such as cyclophosphamide, ifosfamide, and busulfan, in addition to ionizing radiotherapy to the pelvis and abdomen, and cranial or total body irradiation are the most aggressive cancer treatments to the ovaries and can lead to gonadotoxicity and subsequent fertility loss in almost all cases [152-154]. Several studies have assessed the risk of chemotherapy- and radiotherapy-induced gonadotoxicity and subsequent fertility loss for common cancers in females. The risk of gonadotoxicity and subsequent female fertility loss is significantly related to the type, dose, site, and fractionation of the chemotherapy and radiotherapy, the type and stage of cancer as well as to the age of the young women and girls at the beginning of treatment [147-154].
To preserve fertility of young women and girls undergoing gonadotoxic cancer treatments, several options can be offered including embryo freezing, egg freezing, ovary autotransplantation, IVM, and ovarian protection. However, each of those fertility preservation options has advantages and disadvantages and may not be feasible for all cases [1-5]. That is why many guidelines have been published concerning the possible options and strategies for female fertility preservation. Most of these guidelines were published by the American Society of Clinical Oncology (ASCO) [6, 7], American Society for Reproductive Medicine (ASRM) [8-10], European Society for Medical Oncology (ESMO) [11, 12], US Oncofertility Consortium [13, 14], International Society for Fertility Preservation (ISFP) [15-18], Fertility Preservation Network FertiPROTEKT [19], American Academy of Pediatrics (AAP) [20], and Association of Pediatric Hematology/Oncology Nurses (APHON) [21].
According to the most recent guidelines [6-21], human ovary autotransplantation is still considered an experimental option for female fertility preservation. Although experimental, ovarian autotransplantation is promising due to its unique advantages in comparison to embryo freezing and egg freezing, the only two established options for female fertility preservation. Distinctively, human ovary autotransplantation (i) does not need prior ovarian stimulation, (ii) allows immediate initiation of cancer therapy, (iii) can restore both endocrine and reproductive ovarian functions, and (iv) may be the only fertility preservation option suitable for prepubertal girls or for young women with estrogen-sensitive malignancies where ovarian stimulation and embryo or egg freezing are contraindicated (Table 3).
The major and serious challenges facing ovarian autotransplantation are how to avoid the risk of reintroducing malignant cells and how to prolong the lifespan of ovarian transplant as well as how to improve artificial ovary results [6-21]. That is why several medical and ethical concerns should be taken into account before offering this technique to cancer patients. Recently, the Edinburgh selection criteria have recommended that ovarian autotransplantation can be offered to young women, female adolescents, and prepubertal girls undergoing gonadotoxic cancer treatments only when there are a realistic chance of survival, a risk of fertility loss >50 %, no or low risk for reintroducing malignant cells, negative serology for blood transmitted diseases, and informed consent by parents or legal guardians in case of a child patient [61]. Overall, ovarian autotransplantation should be contraindicated in ovarian carcinomas and malignancies that may metastasize in ovaries such as leukemia (high risk), gastrointestinal cancer (moderate risk), breast cancer, lymphoma, gynecological malignancies, and sarcoma of the bone and connective tissue (low risk) [98-100].
Technically, ovarian autotransplantation is a very sophisticated procedure that requires a highly skilled oncofertility team of oncologists, gynecologists, reproductive biologists, and transplantation surgeons. That is why it should be performed only at highly specialized centers, and it should be offered only to preserve fertility of young women (<40 years old) and girls undergoing gonadotoxic treatments for cancer or some autoimmune diseases and not to preserve fertility of patients with benign conditions nor the women who want to postpone their childbearing for nonmedical reasons [22, 155].
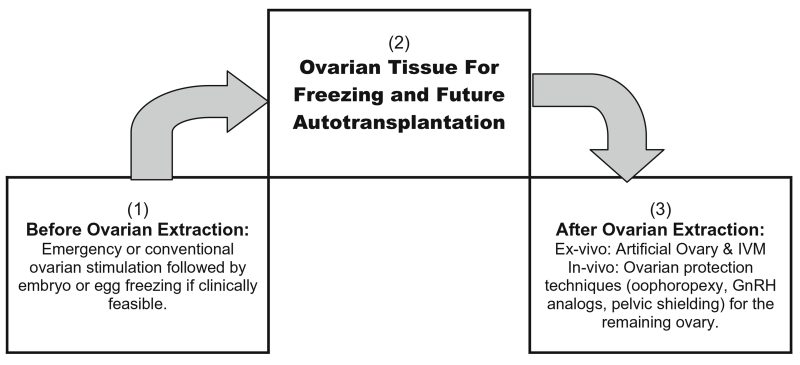
We suggest, by integrating ovarian autotransplantation with other options of female fertility preservation, the chance of preserving fertility can be comprehensively amplified (Fig. 2). If clinically feasible, ovarian autotransplantation can be proceeded by emergency or conventional ovarian stimulation and further embryo or egg freezing. After ovarian tissue extraction, ex vivo isolation of follicles and oocytes for artificial ovary and IVM can be attempted. In case one ovary is left in situ, ovarian protection techniques such as oophoropexy, GnRH analogs, and pelvic shielding should be considered [22-27, 156-160].
Fig. 2.
Integrating ovarian autotransplantation with other options of female fertility preservation
5 Conclusion
Although experimental, human ovary autotransplantation is a promising option for female fertility preservation. The major challenges facing this option are how to avoid the risk of reintroducing malignant cells and how to prolong the lifespan of ovarian transplant as well as how to improve artificial ovary results. Moreover, an international registry is absolutely required for better evaluation, improvement, and establishment of this procedure.
Key message (implications for practice).
Human ovary autotransplantation involves ovarian tissue extraction, freezing/thawing, and transplantation back into the same patient. Although experimental, it is a promising option for fertility preservation of young women and girls undergoing gonadotoxic treatments for cancer or some autoimmune diseases. To date, it resulted in at least 42 healthy babies worldwide. The major challenges facing this option are how to (i) avoid the risk of reintroducing malignant cells, (ii) prolong the lifespan of ovarian transplants, (iii) improve artificial ovary results, and (iv) establish an accurate international registry.
Acknowledgments
Funding Not applicable
Footnotes
Compliance with ethical standards
Conflict of interest The authors have declared no conflicts of interest.
References
- 1.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salama M, Winkler K, Murach KF, Seeber B, Ziehr SC, Wildt L. Female fertility loss and preservation: threats and opportunities. Annals of Oncology. 2013;24(3):598–608. doi: 10.1093/annonc/mds514. [DOI] [PubMed] [Google Scholar]
- 3.Hirshfeld-Cytron J, Gracia C, Woodruff TK. Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation. Journal of Women’s Health. 2011;20(10):1467–1477. doi: 10.1089/jwh.2010.2625. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Wallberg KA, Oktay K. Fertility preservation during cancer treatment: clinical guidelines. Cancer Management and Research. 2014;6:105–117. doi: 10.2147/CMAR.S32380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinology. 2015;3(7):556–567. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K, American Society of Clinical Oncology American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 7.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K, American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ethics Committee of the American Society for Reproductive Medicine et al. Fertility preservation and reproduction in cancer patients. Fertility and Sterility. 2005;83(6):1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Ethics Committee of American Society for Reproductive Medicine Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertility and Sterility. 2013;100(5):1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertility and Sterility. 2013;100(5):1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N, ESMO Guidelines Working Group Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21(Suppl 5):v266–v273. doi: 10.1093/annonc/mdq198. [DOI] [PubMed] [Google Scholar]
- 12.Peccatori FA, Azim HA, Jr., Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, Pentheroudakis G, ESMO Guidelines Working Group Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24(Suppl 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 13.Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nature Reviews. Clinical Oncology. 2010;7(8):466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waimey KE, Duncan FE, Su HI, Smith K, Wallach H, Jona K, Coutifaris C, Gracia CR, Shea LD, Brannigan RE, Chang RJ, Zelinski MB, Stouffer RL, Taylor RL, Woodruff TK. Future directions in oncofertility and fertility preservation: a report from the 2011 Oncofertility Consortium Conference. J Adolesc Young Adult Oncol. 2013;2(1):25–30. doi: 10.1089/jayao.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Practice Committee, I. S. F. P. Kim SS, Donnez J, Barri P, Pellicer A, Patrizio P, Rosenwaks Z, Nagy P, Falcone T, Andersen C, Hovatta O, Wallace H, Meirow D, Gook D, Kim SH, Tzeng CR, Suzuki S, Ishizuka B, Dolmans MM. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. Journal of Assisted Reproduction and Genetics. 2012;29(6):465–468. doi: 10.1007/s10815-012-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemp JR, Kim SS, ISFP Practice Committee Fertility preservation in young women with breast cancer. Journal of Assisted Reproduction and Genetics. 2012;29(6):469–472. doi: 10.1007/s10815-012-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadoul P, Kim SS, ISFP Practice Committee Fertility considerations in young women with hematological malignancies. Journal of Assisted Reproduction and Genetics. 2012;29(6):479–487. doi: 10.1007/s10815-012-9792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt KT, Andersen CY, ISFP Practice Committee Recommendations for fertility preservation in patients with lymphomas. Journal of Assisted Reproduction and Genetics. 2012;29(6):473–477. doi: 10.1007/s10815-012-9787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women—a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Archives of Gynecology and Obstetrics. 2011;284(2):427–435. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallat ME, Hutter J, American Academy of Pediatrics Committee on Bioethics. American Academy of Pediatrics Section on Hematology/Oncology. American Academy of Pediatrics Section on Surgery Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121(5):e1461–e1469. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 21.Fernbach A, Lockart B, Armus CL, Bashore LM, Levine J, Kroon L, Sylvain G, Rodgers C. Evidence-based recommendations for fertility preservation options for inclusion in treatment protocols for pediatric and adolescent patients diagnosed with cancer. Journal of Pediatric Oncology Nursing. 2014;31(4):211–222. doi: 10.1177/1043454214532025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Practice Committee of American Society for Reproductive Medicine Ovarian tissue cryopreservation: a committee opinion. Fertility and Sterility. 2014;101(5):1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 23.Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertility and Sterility. 2012;97(6):1260–1268. doi: 10.1016/j.fertnstert.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. New England Journal of Medicine. 2009;360(9):902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clinical Obstetrics and Gynecology. 2010;53(4):787–796. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 26.Donnez J, Dolmans MM. Fertility preservation in women. Nature Reviews Endocrinology. 2013;9(12):735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 27.Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, Dechene J, Ferster A, Veys I, Fastrez M, Englert Y, Demeestere I. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Human Reproduction. 2014;29(9):1931–1940. doi: 10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- 28.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Human Reproduction. 2008;23(10):2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 29.Ernst E, Bergholdt S, Jørgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Human Reproduction. 2010;25(5):1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 30.Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, Andersen AN, Andersen CY. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reproductive Biomedicine Online. 2011;22(2):162–171. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. Journal of Assisted Reproduction and Genetics. 2014;31(11):1557–1564. doi: 10.1007/s10815-014-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryo-preserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. The Oncologist. 2007;12(12):1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 33.Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Human Reproduction. 2010;25(6):1590–1591. doi: 10.1093/humrep/deq096. [DOI] [PubMed] [Google Scholar]
- 34.Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Human Reproduction. 2015 doi: 10.1093/humrep/dev128. doi:10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 35.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 36.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Human Reproduction Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 37.Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Annals of Medicine. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 39.Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, Dolmans MM. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertility and Sterility. 2011;95(5):1787.e1–4. doi: 10.1016/j.fertnstert.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 40.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, Smitz J, Dolmans MM. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertility and Sterility. 2012;98(3):720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovariantissue: a review of 60 cases of reimplantation. Fertility and Sterility. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. New England Journal of Medicine. 2005;353(3):318–821. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 43.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, Dor J. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrinestudies, in vitro fertilization cycles, and live birth. Fertility and Sterility. 2007;87(2):418.e7–418.e15. doi: 10.1016/j.fertnstert.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 44.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Human Reproduction. 2011;26:1097–1105. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]
- 45.Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Medical Journal of Australia. 2013;198(3):158–159. doi: 10.5694/mja12.11768. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-Serrano M, Crespom J, Mirabet V, Cobo AC, Escribá MJ, Simón C, Pellicer A. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertility and Sterility. 2010;93(1):268.e11–13. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 47.García Rada A. Spanish woman becomes pregnant through ovarian tissue transplantation. BMJ. 2012;344:d8350. doi: 10.1136/bmj.d8350. doi:10.1136/bmj.d8350. [DOI] [PubMed] [Google Scholar]
- 48.Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue; 25th Annual Meeting of ESHRE; Amsterdam, the Netherlands: Oxford University Press, Hum Reprod. 28 June - 1 July, 2009.2009. p. i15. [Google Scholar]
- 49.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertility and Sterility. 2010;93:2413.e15–19. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, Benedetto C. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertility and Sterility. 2013;99:227–230. doi: 10.1016/j.fertnstert.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, van der Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertility and Sterility. 2012;97:387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 52.Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertility and Sterility. 2015;103(2):462–468. doi: 10.1016/j.fertnstert.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 53.Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Human Reproduction. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 54.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertility and Sterility. 2010;94:2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 55.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Molecular Human Reproduction. 2012;18(2):59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 56.Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384(9950):1311–1319. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, Sheikhi M, Ouvrier B, Bozóky B, Olofsson JI, Lundqvist M, Hovatta O. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(3):324–328. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Human Reproduction. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 60.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, Jobling T. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Human Reproduction. 2014;29(8):1828. doi: 10.1093/humrep/deu119. [DOI] [PubMed] [Google Scholar]
- 61.Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncology. 2014;15(10):1129–1136. doi: 10.1016/S1470-2045(14)70334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss JM. Effect of long-term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertility and Sterility. 2009;91(4 Suppl):1556–1559. doi: 10.1016/j.fertnstert.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 63.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Andersen CY. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy - a technique in its infancy but already successful in fertility preservation. European Journal of Cancer. 2009;45(9):1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 64.Hovatta O. Methods for cryopreservation of human ovarian tissue. Reproductive Biomedicine Online. 2005;10(6):729–734. doi: 10.1016/s1472-6483(10)61116-9. [DOI] [PubMed] [Google Scholar]
- 65.Jeong K, Aslan E, Ozkaya E, Sonmezer M, Oktay K. Ovarian cryopreservation. Minerva Medica. 2012;103(1):37–46. [PubMed] [Google Scholar]
- 66.Sarnacki S. Ovarian tissue cryopreservation in children with cancer. Lancet Oncology. 2014;15(10):1049–1050. doi: 10.1016/S1470-2045(14)70378-X. [DOI] [PubMed] [Google Scholar]
- 67.Isachenko V, Isachenko E, Weiss JM. Human ovarian tissue: vitrification versus conventional freezing. Human Reproduction. 2009;24(7):1767–1768. doi: 10.1093/humrep/dep094. author reply 1768-1769. [DOI] [PubMed] [Google Scholar]
- 68.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138(2):319–327. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 69.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Human Reproduction. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 70.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reproductive Biomedicine Online. 2011;23(2):160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Klocke S, Bündgen N, Köster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Archives of Gynecology and Obstetrics. 2015;291(2):419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 72.Fatemi HM, Kyrou D, Al-Azemi M, Stoop D, De Sutter P, Bourgain C, Devroey P. Ex-vivo oocyte retrieval for fertility preservation. Fertility and Sterility. 2011;95(5):1787.e15–17. doi: 10.1016/j.fertnstert.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Bocca S, Dedmond D, Jones E, Stadtmauer L, Oehninger S. Successful extracorporeal mature oocyte harvesting after laparoscopic oophorectomy following controlled ovarian hyperstimulation for the purpose of fertility preservation in a patient with borderline ovarian tumor. Journal of Assisted Reproduction and Genetics. 2011;28(9):771–772. doi: 10.1007/s10815-011-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escribá MJ, Grau N, Escrich L, Novella-Maestre E, Sánchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. Journal of Assisted Reproduction and Genetics. 2012;29(11):1261–1265. doi: 10.1007/s10815-012-9860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, Loh SF, Chia YN. First pregnancy and live birth resulting from cryopre-served embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Human Reproduction. 2014;29(2):276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 76.Fabbri R, Pasquinelli G, Keane D, Mozzanega B, Magnani V, Tamburini F, Venturoli S. Culture of cryopreserved ovarian tissue: state of the art in 2008. Fertility and Sterility. 2009;91(5):1619–1629. doi: 10.1016/j.fertnstert.2009.03.109. [DOI] [PubMed] [Google Scholar]
- 77.Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, Nagler A, Yehuda BD. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Human Reproduction. 2008;23(5):1007–1013. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 78.Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertility and Sterility. 2010;94(6):2186–2190. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 79.Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, Anderson M, Schmidt KT, Andersen CY. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertility and Sterility. 2011;95(6):2158–2161. doi: 10.1016/j.fertnstert.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 80.Bastings L, Liebenthron J, Westphal JR, Beerendonk CC, van der Ven H, Meinecke B, Montag M, Braat DD, Peek R. Efficacy of ovarian tissue cryopreservation in a major European center. Journal of Assisted Reproduction and Genetics. 2014;31(8):1003–1012. doi: 10.1007/s10815-014-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Human Reproduction. 2005;20(1):72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 82.Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertility and Sterility. 2008;89(6):1787–1794. doi: 10.1016/j.fertnstert.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 83.Dittrich R, Lotz L, Fehm T, Krüssel J, von Wolff M, Toth B, van der Ven H, Schüring AN, Würfel W, Hoffmann I, Beckmann MW. Xenotransplantation of cryopreserved human ovarian tissue-a systematic review of MII oocyte maturation and discussion of it as a realistic option for restoring fertility after cancer treatment. Fertility and Sterility. 2015;103(6):1557–1565. doi: 10.1016/j.fertnstert.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Macklon KT, Ernst E, Andersen AN, Andersen CY. Cryobanking of human ovarian tissue: do women still want their tissue stored beyond 5 years? Reproductive Biomedicine Online. 2014;29(4):452–456. doi: 10.1016/j.rbmo.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 85.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Human Reproduction Update. 2009;15(6):649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonmezer M, Oktay K. Orthotopic and heterotopic ovarian tissue transplantation. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2010;24(1):113–126. doi: 10.1016/j.bpobgyn.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 87.Gamzatova Z, Komlichenko E, Kostareva A, Galagudza M, Ulrikh E, Zubareva T, Sheveleva T, Nezhentseva E, Kalinina E. Autotransplantation of cryopreserved ovarian tissue—effective method of fertility preservation in cancer patients. Gynecological Endocrinology. 2014;30(Suppl 1):43–47. doi: 10.3109/09513590.2014.945789. [DOI] [PubMed] [Google Scholar]
- 88.Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2014;28(8):1188–1197. doi: 10.1016/j.bpobgyn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Meirow D, Ra’anani H, Biderman H. Ovarian tissue cryopreservation and transplantation: a realistic, effective technology for fertility preservation. Methods in Molecular Biology. 2014;1154:455–473. doi: 10.1007/978-1-4939-0659-8_21. [DOI] [PubMed] [Google Scholar]
- 90.Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reproductive Biomedicine Online. 2015;30(6):643–650. doi: 10.1016/j.rbmo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 91.Oktay K, Türkçüoğlu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertility and Sterility. 2011;95(2):804.e7–10. doi: 10.1016/j.fertnstert.2010.07.1072. [DOI] [PubMed] [Google Scholar]
- 92.Yang H, Heun Lee H, Chang Lee H, Sung Ko D, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after transplantation? Fertility and Sterility. 2008;90(4 Suppl):1550–1558. doi: 10.1016/j.fertnstert.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 93.Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, Mallmann P, Nawroth F, Isachenko E. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2010;149(1):63–67. doi: 10.1016/j.ejogrb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 94.Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, Dolmans MM. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertility and Sterility. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 95.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, Abir R. Possible improvements in human ovarian grafting by various host and graft treatments. Human Reproduction. 2012;27(2):474–482. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 97.Xia X, Yin T, Yan J, Yan L, Jin C, Lu C, Wang T, Zhu X, Zhi X, Wang J, Tian L, Liu J,, Li R, Qiao J. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplantation. 2015;24(10):1999–2010. doi: 10.3727/096368914X685267. [DOI] [PubMed] [Google Scholar]
- 98.Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. Journal of Assisted Reproduction and Genetics. 2013;30(1):11–24. doi: 10.1007/s10815-012-9912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Human Reproduction Update. 2013;19(5):483–506. doi: 10.1093/humupd/dmt020. [DOI] [PubMed] [Google Scholar]
- 100.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertility and Sterility. 2013;99(6):1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 101.Silber SJ, Woodruff TK, Shea LD. To transplant or not to transplant—that is the question. Cancer Treatment and Research. 2010;156:41–54. doi: 10.1007/978-1-4419-6518-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berwanger AL, Finet A, El Hachem H, le Parco S, Hesters L, Grynberg M. New trends in female fertility preservation: in vitro maturation of oocytes. Future Oncology. 2012;8(12):1567–1573. doi: 10.2217/fon.12.144. [DOI] [PubMed] [Google Scholar]
- 103.Chian RC, Uzelac PS, Nargund G. In vitro maturation of human immature oocytes for fertility preservation. Fertility and Sterility. 2013;99(5):1173–1181. doi: 10.1016/j.fertnstert.2013.01.141. [DOI] [PubMed] [Google Scholar]
- 104.Baird DT, Campbell B, de Souza C, Telfer E. Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2004;113(Suppl 1):S55–S59. doi: 10.1016/j.ejogrb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 105.Shaw JM, Trounson AO. Experimental models for ovarian tissue and immature follicles. Seminars in Reproductive Medicine. 2002;20(1):51–62. doi: 10.1055/s-2002-24950. [DOI] [PubMed] [Google Scholar]
- 106.Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 107.Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, Ottosen C, Andersen CY. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Human Reproduction. 2006;21(8):2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- 108.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, Jobling T. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Human Reproduction. 2013;28(11):2996–2999. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 109.Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP. Live birth after ovarian tissue transplant. Nature. 2004;428(6979):137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 110.Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Human Reproduction. 2006;21(12):3258–3269. doi: 10.1093/humrep/del227. [DOI] [PubMed] [Google Scholar]
- 111.Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultra-structural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertility and Sterility. 2007;87(5):1153–1165. doi: 10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 112.Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertility and Sterility. 2007;87(4):971–975. doi: 10.1016/j.fertnstert.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Bedaiwy MA, Shahin AY, Falcone T. Reproductive organ transplantation: advances and controversies. Fertility and Sterility. 2008;90(6):2031–2055. doi: 10.1016/j.fertnstert.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 114.Silber SJ. Fresh ovarian tissue and whole ovary transplantation. Seminars in Reproductive Medicine. 2009;27(6):479–485. doi: 10.1055/s-0029-1241058. [DOI] [PubMed] [Google Scholar]
- 115.Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Seminars in Reproductive Medicine. 2009;27(6):465–471. doi: 10.1055/s-0029-1241056. [DOI] [PubMed] [Google Scholar]
- 116.Kim SS. Time to re-think: ovarian tissue transplantation versus whole ovary transplantation. Reproductive Biomedicine Online. 2010;20(2):171–174. doi: 10.1016/j.rbmo.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 117.Bedaiwy MA, Falcone T. Whole ovary transplantation. Clinical Obstetrics and Gynecology. 2010;53(4):797–803. doi: 10.1097/GRF.0b013e3181f97c94. [DOI] [PubMed] [Google Scholar]
- 118.Zhang JM, Sheng Y, Cao YZ, Wang HY, Chen ZJ. Cryopreservation of whole ovaries with vascular pedicles: vitrification or conventional freezing? Journal of Assisted Reproduction and Genetics. 2011;28(5):445–452. doi: 10.1007/s10815-011-9539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bedaiwy MA, Falcone T. Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques. Reproductive Biomedicine Online. 2007;14(3):360–371. doi: 10.1016/s1472-6483(10)60880-2. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, Chen H, Yin H, Kim SS, Lin Tan S, Gosden RG. Fertility after intact ovary transplantation. Nature. 2002;415(6870):385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]
- 121.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. New England Journal of Medicine. 2008;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 122.Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after auto-graft of frozen-thawed hemiovaries into ewes. Fertility and Sterility. 2002;77(2):403–408. doi: 10.1016/s0015-0282(01)02960-0. [DOI] [PubMed] [Google Scholar]
- 123.Imhof M, Hofstetter G, Bergmeister H, Rudas M, Kain R, Lipovac M, Huber J. Cryopreservation of a whole ovary as a strategy for restoring ovarian function. Journal of Assisted Reproduction and Genetics. 2004;21(12):459–965. doi: 10.1007/s10815-004-8763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertility and Sterility. 2006;85(Suppl 1):1208–1215. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 125.Courbiere B, Caquant L, Mazoyer C, Franck M, Lornage J, Salle B. Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertility and Sterility. 2009;91(6):2697–2706. doi: 10.1016/j.fertnstert.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 126.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation inovarian follicle culture. Biomaterials. 2011;32(10):2524–2531. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, Donnez J, Amorim CA. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–6085. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 128.Luyckx V, Dolmans MM, Vanacker J, Scalercio SR, Donnez J, Amorim CA. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J Ovarian Res. 2013;6(1):83. doi: 10.1186/1757-2215-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luyckx V, Dolmans MM, Vanacker J, Legat C, Fortuño Moya C, Donnez J, Amorim CA. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertility and Sterility. 2014;101(4):1149–1156. doi: 10.1016/j.fertnstert.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 130.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annual Review of Biomedical Engineering. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soares M, Sahrari K, Chiti MC, Amorim CA, Ambroise J, Donnez J, Dolmans MM. The best source of isolated stromal cells for the artificial ovary: medulla or cortex, cryopre-served or fresh? Human Reproduction. 2015;30(7):1589–1598. doi: 10.1093/humrep/dev101. [DOI] [PubMed] [Google Scholar]
- 132.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reproductive Sciences. 2007;14(8 Suppl):6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Seminars in Reproductive Medicine. 2007;25(4):287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith RM, Woodruff TK, Shea LD. Designing follicle-environment interactions with biomaterials. Cancer Treatment and Research. 2010;156:11–24. doi: 10.1007/978-1-4419-6518-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Human Reproduction Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reproduction. 2009;24(10):2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amorim CA, van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Human Reproduction. 2009;24(1):92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 138.Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, Camboni A, Van Langendonckt A, Donnez J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134(2):253–262. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- 139.Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, Donnez J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reproductive Biomedicine Online. 2008;16(5):705–711. doi: 10.1016/s1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- 140.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles fromcryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Human Reproduction. 2011;26(9):2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Human Reproduction. 2013;28(5):1267–1279. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Human Reproduction. 2011;26(5):1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biology of Reproduction. 2011;84(4):689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2014;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 145.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 146.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 147.Magelssen H, Melve KK, Skjaerven R, Fosså SD. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Human Reproduction. 2008;23:178–186. doi: 10.1093/humrep/dem362. [DOI] [PubMed] [Google Scholar]
- 148.Mueller BA, Chow EJ, Kamineni A, Daling JR, Fraser A, Wiggins CL, Mineau GP, Hamre MR, Severson RK, Drews-Botsch C. Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Archives of Pediatrics & Adolescent Medicine. 2009;163(10):879–886. doi: 10.1001/archpediatrics.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stensheim H, Cvancarova M, Møller B, Fosså SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. International Journal of Cancer. 2011;129:1225–1236. doi: 10.1002/ijc.26045. [DOI] [PubMed] [Google Scholar]
- 150.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Human Reproduction Update. 2001;7(6):534–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 151.Green DM, Sklar CA, Boice JD, Jr., Mulvihill JJ, Whitton JA, Stovall M, Yasui Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2374–2381. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shelling AN. Premature ovarian failure. Reproduction. 2010;140(5):633–641. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 153.Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertility and Sterility. 2009;92(2):417–427. doi: 10.1016/j.fertnstert.2008.07.1714. [DOI] [PubMed] [Google Scholar]
- 154.Meirow D, Biederman H, Anderson RA, Wallace W. Toxicity of chemotherapy and radiation on female reproduction. Clinical Obstetrics and Gynecology. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 155.von Wolff M, Germeyer A, Nawroth F. Fertility preservation for non-medical reasons: controversial, but increasingly common. Deutsches Ärzteblatt International. 2015;112(3):27–32. doi: 10.3238/arztebl.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, Zelinski MB, Stouffer RL. Primate follicular development and oocyte maturation in vitro. Advances in Experimental Medicine & Biology. 2013;761:43–67. doi: 10.1007/978-1-4614-8214-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertility and Sterility. 2013;99(6):1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chung K, Donnez J, Ginsburg E, Meirow D. Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertility and Sterility. 2013;99(6):1534–1542. doi: 10.1016/j.fertnstert.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 159.Dittrich R, Lotz L, Mueller A, Hoffmann I, Wachter DL, Amann KU, Beckmann MW, Hildebrandt T. Oncofertility: combination of ovarian stimulation with subsequent ovarian tissue extractionon the day of oocyte retrieval. Reproductive Biology and Endocrinology. 2013;11:19. doi: 10.1186/1477-7827-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Salama M, Mallmann P. Emergency fertility preservation for female patients with cancer: clinical perspectives. Anticancer Research. 2015;35(6):3117–3127. [PubMed] [Google Scholar]