Abstract
The incidence of cancer will increase within the next 5 years up to 15 million patients per year worldwide with a further increasing tendency. Therefore, there is a requirement for more emphasis on cancer prevention, research and effective anticancer drugs including a rapid licensing and market availability for the patients. In the EU, the centralized procedure (CP) of the European Medicine Agency (EMA) is mandatory for marketing authorization for anticancer drugs. A CP will result in one marketing authorization for all Member States granted by the European Commission. At variance, numerous independent healthcare sys-tems are in operation across the EU, and each Health Technology Assessment (HTA) body follows its own methodologies and scientific value judgements in the assessment of the ad-ditional clinical benefit of a new anticancer drug. Payer organisations in the various member states consider these assessments to a varying degree as input in their pricing and reim-bursement negotiations. At the same time international reference pricing and parallel trade have an inherent tendency to establish rather uniform price levels across member states. Consequently, drug access for patients differs considerably within the EU. Initiatives to im-prove the interface between the different stakeholders are currently on the way, but are unlikely sufficient to overcome these fundamental problems.
This article intends to give an overview about developments in European Regulatory and Health Technology Assessment (HTA) of new cancer drugs. As background information, it will refer to an overview article by Bergmann et al. [1], which pointed out the status and the limitations of the current system. The authors discussed possible steps to improve the interface between regulators and HTA bodies but stated that this alone will not be sufficient to overcome heterogeneous HTA assessments between HTA agencies. Major issues and challenges for the foreseeable future will be to overcome the heterogeneity of patient access decisions of pharmaceutical payers across Europe which is due to (i) considerably different scientific approaches and methodology to the more or less formal evaluation of cost-effectiveness; (ii) differing health priorities across the countries that reflect historically developed cultural differences and values or different unmet medical needs and (iii) different economic strengths among nations, regions and locales that necessarily drive health care budgetary decisions. The authors consider that this needs a science-based common position on methodology, greater commitments by politicians and health care decision makers to ensure equal access for patients across the EU to anti-tumour medicines.
What is the present situation? For European patients, to benefit from a new medicine, three conditions must be fulfilled cumulatively: regulators need to approve the product, the Marketing Authorisation Holder (MAH) has to put it on the market in the Member State of the patient, and reimbursement has to be assured. Therefore, new oncology drugs do not only have to prove efficacy, safety and quality in order to gain a marketing authorisation by a regulatory agency, but also need to show cost-effectiveness or additional clinical benefit, according to early drug assessment in Germany, when compared with other available therapies. HTA with its direct impact on pricing and reimbursement and its indirect impact on MAHs' decision to market (or not) in a given Member State has become an important determinant for patients' access to innovative oncology medicines.
The field of cancer diseases is growing fast: the World Health Organization predicted 15 million new cases per year in 2020 [2], but as investigations could show, we already reached an incidence of 14.1 million cancer cases and 8.2 deaths from cancer worldwide in 2012 [3]. The World Cancer Report 2014, published by the International Agency for Research on Cancer, revealed that the cancer burden could rise to 22 million annually within the next two decades [4].
Having this in mind, more emphasis on cancer prevention, research, therapy and better-targeted anticancer drugs are required and, at the same time, there is a need for rapid licencing and market availability of innovative, more effective oncology drugs.
While the approval of new drugs belongs to the competences of regulatory agencies, most EU member states have delegated the assessments for relative effectiveness or additional clinical benefit to dedicated HTA bodies. The European Medicines Agency (EMA) is responsible for the centralised authorisation procedure, which is mandatory for human medicines for the treatment of cancer inter-alia and results in a single marketing authorisation that is valid in all EU countries, as well as in the European Economic Area. Although the marketing authorisation is granted on a European level by the European Commission (EC), the decision on the pricing, reimbursement and funding for those medicines is taken independently in every single European country due to different operating health care systems. Among the European HTA bodies, the Haute Autorité de Santé in France and the ‘Gemeinsamer Bundesausschuss (GBA)’ make their price negotiation and decision based on the determination of an added clinical benefit. The legal basis is the ‘Arzneimittelneuordnungsgesetz (AMNOG)’. The pharmaceutical companies can still set the initial list price for a new drug after marketing authorisation and after launch. Upon launch, however, they have to submit a detailed dossier based on the authorisation documents and all studies carried out on this pharmaceutical to the GBA. The GBA assesses recognition of any additional benefit claimed over the appropriate comparator and rates between ‘extensive benefit’ (level 1) to ‘less benefit’ (level 6) compared with the comparator, which is the basis for price negotiations. The benefit assessment can be delegated to the Institute for Quality and Efficiency in Health Care (IQWiG) or third parties. One year after market launch, this reimbursement price replaces the initial list price of the drug [5].
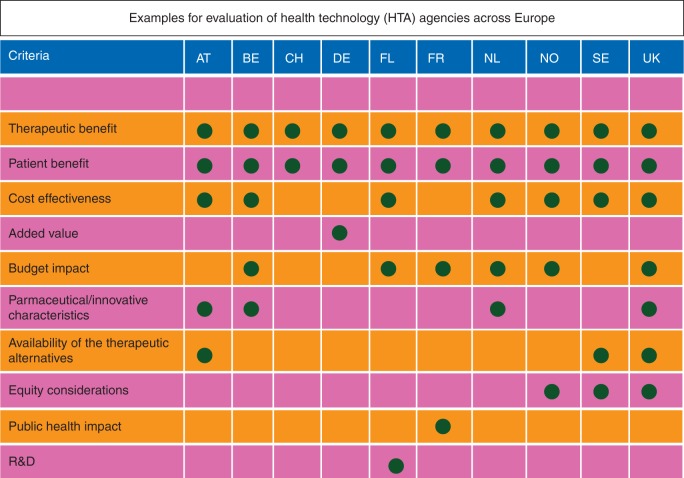
Other methods, as practiced by the National Institute for Health and Clinical Excellence in the UK and the Swedish Dental and Pharmaceutical Benefits Agency (TLV), make use of health economic analyses, which result in the comparison of costs per quality-adjusted life-year estimates to the threshold. Further European countries and their criteria taken into account in the evaluation of health technologies can be found in Figure 1. Those variations in HTA procedures affect the time needed by a new cancer drug to step to the market and lead to divergent prices in different countries. Prices proposed by HTA bodies and agreed by payer organisations may, in combination with other commercial considerations—e.g. market size and availability of competing products, prevent MAHs from marketing their products in some Member States. As a result, inequality of patients' access to medicines in the EU persists—in spite of the 20th anniversary of the EMA and the expansion of the mandatory scope of the centralised authorisation procedure.
Figure 1.
Heterogeneity of evaluation parameters of health technology agencies across Europe (AT = Austria, BE = Belgium, CH = Switzerland, DE = Germany, FI = Finland, NL = Netherlands, NO = Norway, SE = Sweden, UK = United Kingdom).
The development of a truly European HTA procedure, which links the knowledge of regulatory authorities and HTA bodies together, may be an important step towards a more equal access of European patients to innovative medicines. The regulatory assessment of the benefit–risk based on the evaluation of the pharmaceutical quality, safety and efficacy should be a plausible first building block for the subsequent work of HTA bodies. At the time of marketing authorisation, the efficacy and safety data gathered during drug development, which has been the basis of the preceding regulatory approval process, is all information available to determine the effectiveness of a drug.
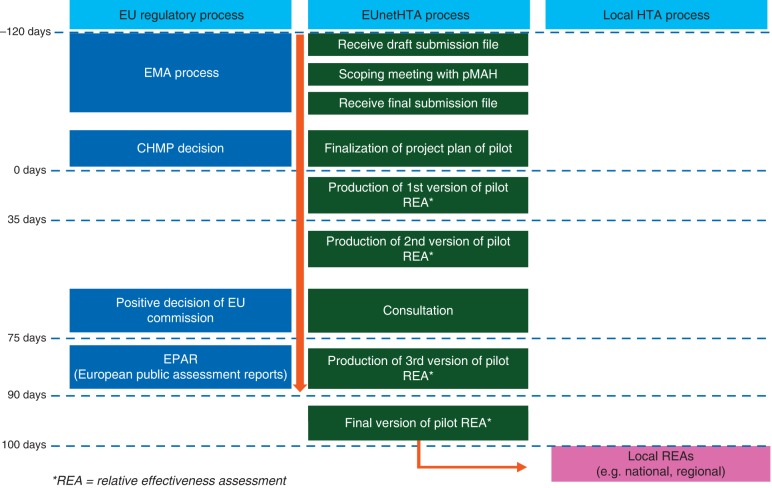
A consensual and aligned, or at least not contradictory, scientific interpretation of the available data by regulators and HTA bodies is needed for a more consistent basis of local price negotiations in Europe. The founding elements of the regulatory process remain exclusively efficacy, safety and quality (risk-benefit assessment). The relative effectiveness assessment (REA) is carried out in parallel with but separate from the regulatory approval process and information might be shared with EU REA Committee before publication of European Public Assessment report (EPAR), providing confidentiality. Two important initiatives support the development towards a European-wide facilitation of patients' access to medicines: EUnetHTA is a joint action on HTA founded by the EC and Member States and charged with the development of standards in the field of relative (clinical) effectiveness of pharmaceuticals. ‘STAMP’ is the acronym for the Expert Group on ‘Safe and Timely Access to Medicines for Patients’ initiated by the Directorate General for Health and Consumers of the European Commission with the intention to improve regulatory procedures, HTA and the interface. Currently, after methodological guidelines have been developed, EUnetHTA performs a second phase of joint action with the aim of strengthening the practical application of tools and approaches to cross-border HTA collaboration [5]. This project to be in force until the end of 2015, consists of eight work packages of which work package 5 is of special interest, because it deals with the application of the so-called HTA Core Model for Rapid Assessment for national adaptation and reporting. The HTA Core Model defines the content elements to be considered in HTA and facilitates standardised reporting by sharing information and avoiding duplication of work. An objective is to test the capacity of national HTA bodies to produce structured core HTA information together and apply it in national context, including collection of data on costs and overall efficiency of the production in the network. The parallel progress and timeline of the EU regulatory process, including the decisions of Committee for Human Medicinal Products (CHMP) and EC and publication of the EPAR, and the HTA process ending with the final REA (Figure 2) is an important result achieved, so far. Presently this procedure is done on a voluntary initiation by the manufacturer until the process is proven to be validated by all parties.
Figure 2.
EUnetHTA project of parallel assessment of regulatory bodies and HTA agencies (adapted from http://www.eunethta.eu). The aim of this project is to have early parallel assessments on new drugs between the regulatory body (EMA) and HTA agencies including early consultations. The participation of the HTA agencies is voluntary and the HTA bodies are selected by the sponsor.
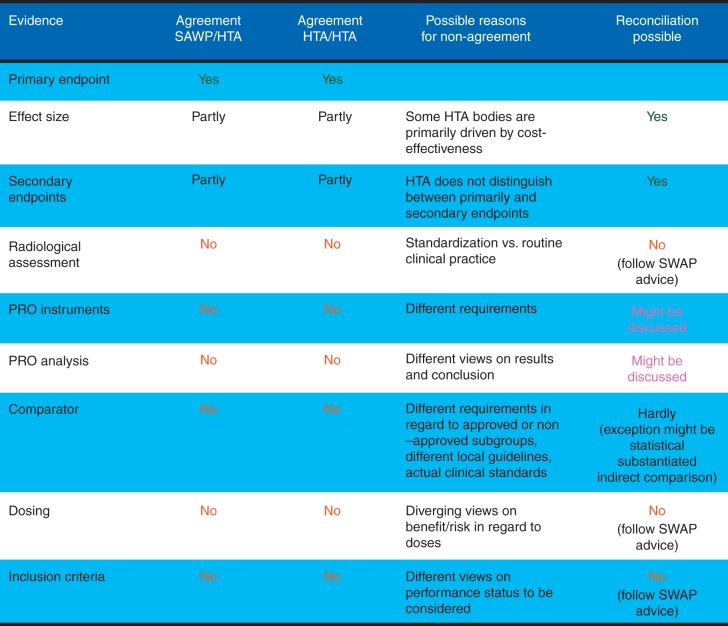
The experiences collected in about 25 pilot procedures for parallel advice and protocol assistance of EMA and HTA bodies were discussed in a workshop held in November 2013 [6]. Afterwards, a draft best practice guidance for EMA-HTA parallel scientific advice [7] was developed and published in May 2014 for 2 months of public consultation, but the final version has not been published yet. An example for a parallel EMA-HTA scientific advice dealing with evidentiary requirements in an oncology case study is shown in Figure 3. The parallel procedure, established so far, includes four stages [8]: (i) Pre-notification, where applicants have early informal meetings with EMA and HTAs announcing their intentions for the procedure, product and timescale, and specifying which HTAs will participate. (ii) Pre-validation, which includes a teleconference with HTAs to discuss the scope, wording and clarity of the questions, and whether the material provided is sufficient to answer the questions posed. (iii) Meeting face-to-face between all stakeholders, for which the applicant prepares the agenda according to priorities, and (iv) outcome, when meeting minutes are circulated by the company for individual written HTA agreement and the regulatory scientific advice is provided in a CHMP advice letter.
Figure 3.
EMA - HTA parallel scientific advice. Eventiary requirements in an oncologic case study (adapted from Britta Paschen, EMA/HTA workshop 2013; http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2013/11/WC500155673.pdf) (PRO = patient reported outcome; SAWP = Scientific advice working party; HTA = Health technology assessment).
However, even if we could have a common EU HTA agency to establish one European Assessment procedure and subsequently a common price, it remains uncertain whether this would enhance access to cancer medicines. The key bottleneck is that Public Health is a national sovereignty matter at this time. All EU countries therefore initiate their own bureaucracy to manage health care costs. At present, this uses a form of HTA assessment to localise the value debate. If an EU price would be determined, then the focus will move to reimbursement and this would shift the bureaucracy and the debate. Even once national reimbursements are given the debate in many states moves to the regions since they have devolved budgets to the country regions. Thus, it is a far too sweeping statement to say that, by encouraging a pan EU price, it would improve access as it may just shift the debate.
In lack of a common EU health care system, of course, some aspects of the evaluation have still to be taken at the country level due to economical differences and different health care systems, but the initiatives may help to overcome some inequities in access to innovative oncological drugs due to different assessment criteria. In general, there is a need for a better science-based common position on methodology, greater commitments by politicians and health care decision makers to ensure a widely equal access as feasible for patients across the EU to innovative anti-tumour medicines.
disclosure
The authors have declared no conflicts of interest.
references
- 1.Bergmann L, Enzmann H, Broich K et al. Actual developments in European regulatory and health technology assessment of new cancer drugs: what does this mean for oncology in Europe? Ann Oncol 2013; 25: 303–306. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Levin B. WHO World Cancer Report. 2008.
- 3.WHO GLOBOCAN PROJECT 2012 - Estimated Cancer Incidence, Mortality and Prevalence worldwide in 2012. n.d.
- 4.Stewart BW, Wild CP. WHO World Cancer Report. 2014.
- 5.Fischer KE, Stargardt T. Early benefit assessment of pharmaceuticals in Germany: manufacturers’ expectations versus the Federal Joint Committee's decisions. Med Decis Making 2014; 34(8): 1030–1047. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency (EMA). EMA-HTA Workshop Report: Bringing together stakeholders for early dialogue in medicines development. London, 2013. [Google Scholar]
- 7.European Medicines Agency (EMA). Best Practice Guidance for Pilot EMA HTA Parallel Scientific Advice Procedures. vol. 44 2014.
- 8.European Medicines Agency (EMA). Scientific advice and protocol assistance, Parallel scientific advice with health-technology-assessment bodies n.d. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000049.jsp&mid=WC0b01ac05800229b9 (14 June 2015, date last accessed).