Among 43 830 lymphomas registered in the French network, 19 were ALK-negative anaplastic large cell lymphoma associated with breast implant (i-ALCL). We identified two distinct histological features (in situ versus infiltrative i-ALCL) correlated with clinical presentation and having different outcomes. Infiltrative i-ALCL subtype being more aggressive requires additional therapy to implant removal.
Keywords: implant-ALCL, seroma, mass, anaplastic/Hodgkin-like subtypes, cytotoxic T-cell
Abstract
Background
ALK-negative anaplastic large cell lymphoma associated with breast implant (i-ALCL) has been recently recognized as a distinct entity. Among 43 830 lymphomas registered in the French Lymphopath network since 2010, 300 breast lymphomas comprising 25 peripheral T-cell lymphomas (PTCL) were reviewed. Among PTCL, ALK-negative ALCL was the most frequent and all of them were associated with breast implants.
Patients and methods
Since 2010, all i-ALCL cases were collected from different institutions through Lymphopath. Immuno-morphologic features, molecular data and clinical outcome of 19 i-ALCLs have been retrospectively analyzed.
Results
The median age of the patients was 61 years and the median length between breast implant and i-ALCL was 9 years. Most implants were silicone-filled and textured. Implant removal was performed in 17 out of 19 patients with additional treatment based on mostly CHOP or CHOP-like chemotherapy regimens (n = 10/19) or irradiation (n = 1/19). CHOP alone or ABVD following radiation without implant removal have been given in two patients. The two clinical presentations, i.e. effusion and less frequently tumor mass correlated with distinct histopathologic features: in situ i-ALCL (anaplastic cell proliferation confined to the fibrous capsule) and infiltrative i-ALCL (pleomorphic cells massively infiltrating adjacent tissue with eosinophils and sometimes Reed–Sternberg-like cells mimicking Hodgkin lymphoma). Malignant cells were CD30-positive, showed a variable staining for EMA and were ALK negative. Most cases had a cytotoxic T-cell immunophenotype with variable T-cell antigen loss and pSTAT3 nuclear expression. T-cell receptor genes were clonally rearranged in 13 out of 13 tested cases. After 18 months of median follow-up, the 2-year overall survival for in situ and infiltrative i-ALCL was 100% and 52.5%, respectively.
Conclusions
In situ i-ALCLs have an indolent clinical course and generally remain free of disease after implant removal. However, infiltrative i-ALCLs could have a more aggressive clinical course that might require additional therapy to implant removal.
introduction
Non-Hodgkin lymphomas of the breast are rare and comprise ∼0.01–0.5% of all malignant breast tumors [1, 2]. The most common types of breast lymphomas are B-cell lymphomas such as diffuse large B-cell lymphomas and extranodal marginal zone lymphomas [3, 4]. Peripheral T-cell lymphomas (PTCL) of the breast are less frequently reported and represent only 10% of all breast lymphomas [5]. Breast implant-associated ALK- negative ALCL (i-ALCL) was described for the first time in 1997 by Keech and Creech [6]. Subsequently, anecdotal cases and series were reported and i-ALCL has recently emerged as a distinct clinicopathologic entity [7–20]. In 2011, the association between breast implant and ALCL was better documented since the US Food and Drug Administration was aware of a total of 60 potential cases. Recently, Brody et al. [21] have inventoried the largest literature review of clinical history of 173 i-ALCL cases. Neoplastic cells have common anaplastic features with a cytotoxic T-cell phenotype. They co-express CD30 and EMA but do not show ALK protein. As the literature has evolved, two distinct clinical subtypes of i-ALCL have emerged. Most patients present with accumulation of fluid around the breast implant referred to as seroma, and have a localized disease [8, 9, 12, 15, 17, 18, 21]. Less frequently, patients present with a palpable tumor mass limited to the breast with or without axillary lymphadenopathies (stage II to stage IV) [8, 15, 17, 18]. However, no specific pathologic features associated with each of these two groups have been yet reported.
In this study, we reviewed the clinical, immunomorphologic, molecular and survival data of 19 cases collected from different institutions through Lymphopath over a 5-year period [22]. Lymphopath is a government-supported network which aims at reviewing all newly lymphoma diagnoses or suspected lymphoma diagnoses in France by an expert hematopathologist. This study emphasizes the distinctive features of each group and the importance of distinguishing between the two morphological subtypes to improve therapeutic management.
materials and methods
patients
We reviewed 19 i-ALCL diagnosed between 2010 and 2014 and collected through Lymphopath, a national network of 33 expert hematopathology reference centers, established by the French National Cancer Agency (INCa) in 2010 [22]. Patients underwent staging based on clinical examination and CT scan, complemented in some cases by positron emission tomography (PET) or bone marrow analysis. In each case, Ann Arbor staging could be determined (Table 1). Seventeen had immediate surgical removal of the implant and capsulectomy, while two were treated with chemotherapy without implant removal associated or not with radiation.
Table 1.
Clinical and implant characteristics, treatment and follow-up of 19 patients with an i-ALCL
| Case | Age (years) | Reason for implant | Implant characteristics |
Side | Time to i-ALCL diagnosis (years) | Clinical presentation | Ann Arbor staging | Therapy | Overall survival (outcome) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of implant | Texture of implant | Disrupted implant | |||||||||
| 1 | 67 | Breast cancer | Saline | Yes | Yes | Right | 10 | Seroma | IE | Implant removal + CT1 | 30 (NED) |
| 2 | 42 | Cosmetic | Silicone | Yes | Yes | Left | 20 | Seroma | IE | Implant removal + RT (36 Gy) | 18 (NED) |
| 3 | 56 | Breast cancer | Silicone | Yes | Yes | Left | 13 | Seroma | IE | Implant removal | 60 (NED) |
| 4 | 49 | Cosmetic | Silicone | Yes | No | Left | 10 | Seroma | IE | Implant removal + CT1 | 24 (NED) |
| 5 | 54 | Cosmetic | Silicone | Yes | No | Right | 9 | Seroma | IE | Implant removal | 24 (NED) |
| 6 | 62 | Breast cancer | Silicone | Yes | No | Bilateral | 17 | Seroma | IE | Implant removal | 18 (NED) |
| 7 | 77 | Breast cancer | Saline | Yes | No | Left | 14 | Seroma | IE | Implant removal | 12 (NED) |
| 8 | 44 | Breast cancer | Silicone | Yes | No | Right | 6 | Seroma | IE | Implant removal | 8 (NED) |
| 9 | 60 | Cosmetic | Silicone | Yes | No | Right | 10 | Mass + seroma | IE | Implant removal | 24 (NED) |
| 10 | 61 | Cosmetic | Silicone | Yes | No | Left | 17 | Mass + seroma | IE | Implant removal + CT1 | 36 (NED) |
| 11 | 54 | Cosmetic | Silicone | Yes | No | Left | 3 | Mass + seroma | II | Implant removal + CT1 | 7 (DOD) |
| 12 | 74 | Breast cancer | NA | NA | NA | Left | 9 | Mass | II | CT2 + RT (30Gy) | 36 (NED) |
| 13 | 83 | Breast cancer | Silicone | Yes | No | Right | 7 | Mass | II | Implant removal + CT1 | 13 (DOD) |
| 14 | 80 | Cosmetic | Silicone | Yes | Yes | Right | 8 | Mass + seroma | IE | Implant removal + CT1 | 3 (NED) |
| 15 | 50 | Cosmetic | Silicone | Yes | No | Left | 8 | Seroma | IE | Implant removal | NA |
| 16 | 77 | Breast cancer | Silicone | Yes | Yes | Right | 2 | Seroma | IE | Implant removal + CT3 | 18 (NED) |
| 17 | 74 | Breast cancer | Silicone | Yes | No | Left | 9 | Mass + seroma | IV | Implant removal + CT1 | 3 (NED) |
| 18 | 75 | Breast cancer | Silicone | Yes | Yes | Right | 1.5 | Seroma | IV | Implant removal + CT1 | 18 (NED) |
| 19 | 58 | Breast cancer | Silicone | Yes | Yes | Left | 7 | Mass + seroma | IV | CT1 | 3 (DOC) |
CT1, cyclophosphamide, adriamycin, vincristine and prednisone (6 cures) [CHOP] or [CHOP-like]; CT2, adriamycine, bleomycine, vinblastine and dacarbazine [ABVD]; CT3, dexamethasone, high-dose aracytine, oxaliplatin [DHAOX]; RT, radiotherapy; NA, not available; NED, no evidence of disease; DOD, dead of disease; DOC, dead from other cause (breast carcinoma).
histological and immunohistochemical analysis
All cases were reviewed by five pathologists (CL, AD, LL, PG and GD) without the knowledge of the clinical presentation. Institutional ethical approval was obtained in compliance with the Helsinki agreement. Samples fixed in 10% buffered formalin were processed for routine histopathological examination. For immunohistochemical examination, 3 µm thick sections were tested using a Ventana Benchmark XT (Ventana, Tucson, AZ) [23].
The antibodies used are detailed in supplementary Table S1, available at Annals of Oncology online.
in situ hybridization for Epstein–Barr virus and FISH study for DUSP22-IRF4 rearrangement
Epstein–Barr virus (EBV) detection was performed by in situ hybridization using EBV-encoded RNA (EBER) probes (Ventana Medical Systems). FISH was performed on whole-tissue sections as using homemade break-apart probes spanning the DUSP22-IRF4 breakpoints at 6p25.3.
polymerase chain reaction
Rearrangements of T-cell receptor (TCR) and of immunoglobulin chain genes (IG) genes were studied on DNA extracted from FFPE tissue by multiplex PCR targeting TCRβ, TCRγ, IGH and IGK genes. BIOMED-2 primers were used with standard PCR conditions [24]. Each reaction was performed in duplicate. TCRγ, TCRβ, IGH and IGK gene amplification products were analyzed by heteroduplex capillary electrophoresis (QIAxcel, QIAGEN, Hilden, Germany) and GeneScan analysis.
statistical analysis
Survival was determined from time of diagnosis until time of death or last follow-up. Survival curves were constructed by the Kaplan–Meier method. Survival distributions were compared with the log-rank test. Statistical significance was set at a P-value of 0.05. Analyses were performed using GraphPrism software.
results
demographics of i-ALCL in France
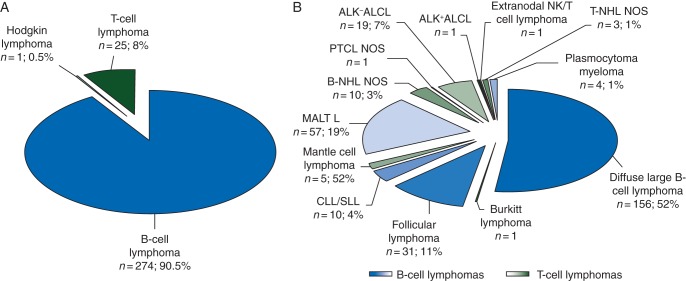
Since 2010, 43 830 lymphomas have been registered in the database of the Lymphopath network. Among them, 300 breast lymphomas were diagnosed in France. As expected, the most frequent were B-cell lymphomas (n = 274; 91.5%), whereas PTCL accounted for only 25 cases (8%) (Figure 1). Interestingly, the most frequent PTCLs were ALK-negative ALCL (n = 19) and were associated with breast implants in all cases, while such an association was not found for the other breast lymphomas.
Figure 1.
Distribution of lymphoma subtypes in 300 breast lymphomas registered in Lymphopath network from 2010 to 2014. Repartition of main lymphoma categories of all breast lymphomas in total number (n) and in percentage (%) (A). Relative frequencies of B- and T-cell lymphoma subtypes in total number (n) and in percentage (%) (B) (MALT L, mucosae-associated lymphoid tissue lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; NHL NOS, non-Hodgkin lymphoma not otherwise specified; PTCL NOS, peripheral T-cell lymphoma not otherwise specified, ALK+ or ALK− ALCL, anaplastic lymphoma kinase positive or negative anaplastic large cell lymphoma).
clinical features
The mean age at diagnosis was 61 years (ranged 42–83 years) (Table 1). In 11 cases (58%), the implant followed mastectomy for breast carcinoma; in 8 cases (42%), the reason for implant was cosmetic. Breast implants were filled with saline solution (n = 2) or silicone gel (n = 16). All patients but one received textured breast implant. In the majority of cases (61%), implants were not disrupted at the time of i-ALCL diagnosis. Five patients presented with erythematous skin eruption before i-ALCL diagnosis (n°3, n°11, n°12, n°17 and n°19). The median interval time between implant surgery and i-ALCL diagnosis was 9 years (range 1.5–20 years). Pretreatment PET scan has been positive in eight out of eight patients. The majority of patients (84%) were Ann Arbor stage IE (n = 13) or II (n = 3), the three remaining patients (16%) considered as stage IV with bone (n°18 and n°19) or muscle lesions (n°17).
Two distinct clinical presentations were observed: (i) patients presenting with seroma (n = 11) and (ii) patients with palpable breast tumor mass (n = 8). Of the 13 patients with stage IE disease, 10 patients presented with an effusion without mass and 3 patients had a tumor mass. Of the six patients with more advanced stage, only one presented as a seroma without palpable tumor. Capsulectomy with implant removal was performed in 17 out of 19 (89%) patients. Additional treatments in 10 out of 17 patients comprised polychemotherapy (n = 9) as an anthracyclin-based regimen CHOP (cyclophosphamide, adriamycin, vincristine and prednisone) or CHOP-like (n = 8), or as a platinum-based regimen DHAOx (dexamethasone, high-dose aracytine and oxaliplatin) (n = 1) and one patient received radiotherapy alone after implant removal (n = 1). One patient, in whom implant was not removed, received CHOP chemotherapy alone. Finally, one patient, who had been first misdiagnosed as classical Hodgkin lymphoma, has been given ABVD chemotherapy (adriamycine, bleomycine, vinblastine and dacarbazine) followed by radiation without implant removal.
Clinical outcome was available in 18 patients. The median follow-up was 18 months (range 2–60 months). The 2-year overall survival was 80% [95% confidence interval (CI) = 51.1–99.2]. At the time of analysis, 15 patients were alive and in complete remission at the last follow-up, three patients died, two from lymphoma progression at 7 and 13 months after the diagnosis of lymphoma and one from relapsed breast carcinoma at 3 months.
relationship between histopathologic features and clinical presentation
We identified two distinct pathological patterns with respect to both cytological features of tumor cells and their environment. They correlated with clinical presentation.
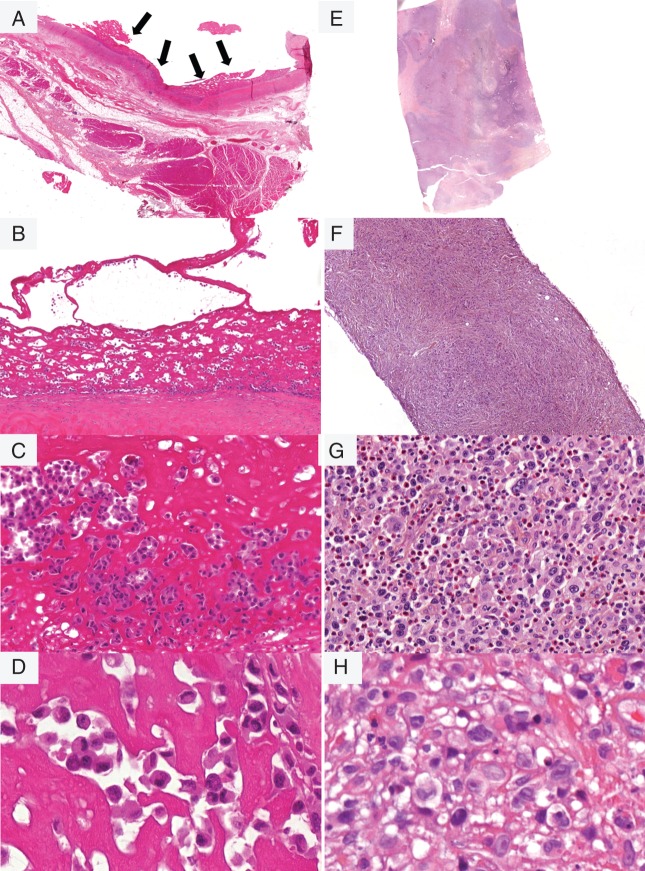
Indeed, the 11 i-ALCL patients with presentation as seroma were characterized by a lymphoid neoplastic proliferation confined to the implant capsule and therefore referred to as ‘in situ i-ALCL’. Non-cohesive malignant cells lined the capsule border (Figure 2A) and/or were suspended in a serous material. Non-cohesive malignant cells lined the capsule border (Figure 2A) and/or were embedded within serous or fibrinoid material (Figure 2B). There was only a sparse inflammatory infiltrate (Figure 2C). Aspiration of seroma (n = 3) showed non-cohesive tumor cells suspended in serous/fibrinoid background. Tumor cells were large and pleomorphic surrounded by a clear halo, with irregular nuclei dispersed chromatin and sometimes prominent nucleoli, on histological and cytological preparation. Their cytoplasm was variably abundant and eosinophilic. In addition, anaplastic ‘hallmark’ cells with eccentric kidney-shaped nuclei were observed, although in variable number from case to case (Figure 2D).
Figure 2.
Pathological features of i-ALCL subtypes. The in situ i-ALCL subtype shows a lymphoproliferation confined to the surface of the breast implant (black arrows), H&E ×400 (A). Tumor cells line the fibrous capsule, H&E ×200 (B), and consist in large pleomorphic tumor cells with irregular nuclei surrounded by a clear halo, H&E ×400 (C), with variable number anaplastic ‘hallmark’-like cells, H&E ×600 (D). The infiltrative i-ALCL subtype shows a lymphoproliferation invading capsule and surrounding tissue H&E ×400 (E). This subtype sometimes associated with fibrosis H&E ×100 (F) consist in sheets of large tumor cells with variable number of Hodgkin or Reed–Sternberg-like cells with an abundant inflammatory background H&E ×400 (G) and ×600 (H).
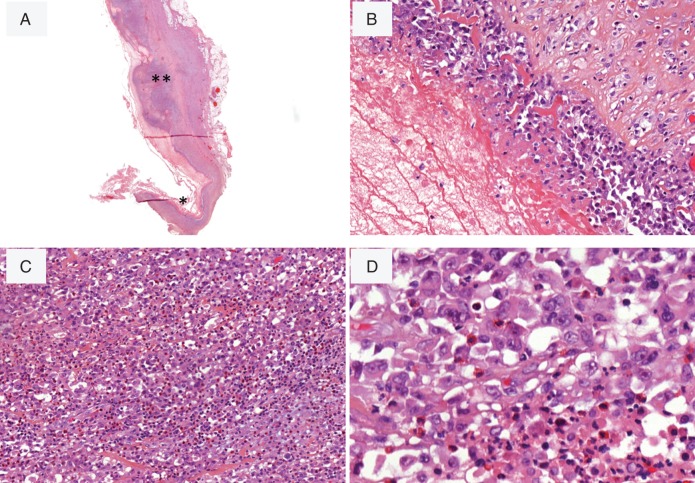
In contrast, the eight patients who presented with a breast tumor mass showed a diffuse infiltration of the capsule and the adjacent tissues and were designed ‘infiltrative i-ALCL’. Sheets of large malignant cells extended beyond the capsule (Figure 2F). Necrosis was frequent and sclerosis was sometimes observed (Figure 2E). Malignant cells were associated with a prominent inflammatory background with a large number of eosinophils (Figure 2G). Neoplastic cells contained large mono or polylobated nuclei with prominent eosinophilic nucleoli, resembling Hodgkin or Reed–Sternberg cells (Figure 2H). In two cases with tumor mass presentation, the two morphologic patterns co-existed and showed besides a proliferation confined to the fibrous capsule, a focally (n°14) or massive (n°10) proliferation invading the capsule (Figure 3).
Figure 3.
i-ALCL with the two morphologic patterns. The case N°10 shows, beside a proliferation confined to the fibrous capsule (*), a focally bulging proliferation that invades capsule tissue (**) H&E ×40 (A), ×200 (B), ×200 (C), ×600 (D) and ×600 (E).
According to the two histological subtypes of i-ALCL, the 2-year overall survival was significantly shorter for patients with infiltrative i-ALCL subtype compared with those with in situ i-ALCL subtype (52.5% versus 100% respectively, P = 0.023) (Figure 4).
Figure 4.
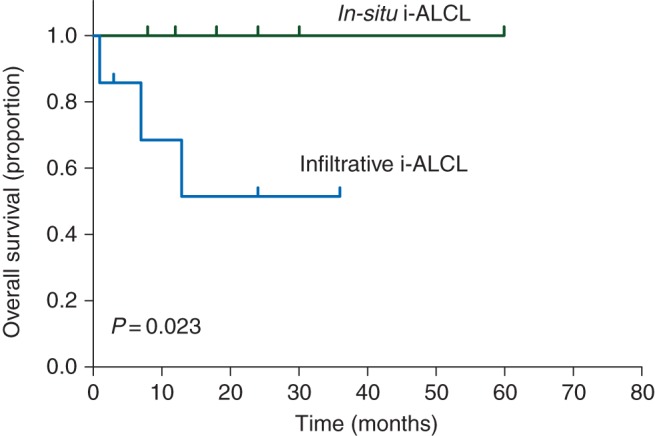
Kaplan–Meier curve showing overall survival estimates for the in situ and infiltrative i-ALCL subtypes.
immunophenotypic, EBV and FISH results
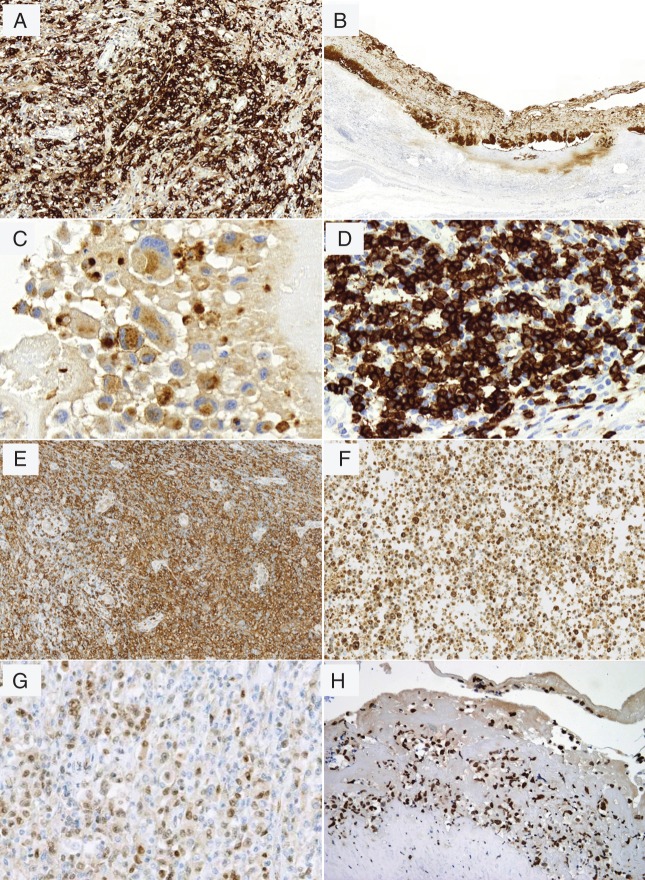
All cases were strongly and uniformly positive for CD30 (Figure 5A and B) (supplementary Table S2, available at Annals of Oncology online). Neoplastic cells were often positive for EMA (90%) (Figure 5C). ALK staining was consistently absent. All cases showed incomplete T-cell phenotype expressing one or several T-cell markers (Figure 5D–F) with extensive antigen loss such as CD3, CD5 and CD7. All cases tested for TCRβF1 (n = 12) and TCRγ (n = 12) were negative for both, and all had an activated cytotoxic profile [TIA-1+/−, granzyme B+/− (Figure 5F) and/or perforin+/−]. The majority of cases were positive for CD4 (84%) and CD43 (95%). Interestingly, co-expression of CD4 and CD8 was seen in two cases (n°7 and n°10). CD56 was only expressed in two cases (n°13 and n°19). CD45/LCA and BCL2 were positive in the majority of cases. CD15 was weakly expressed in 53% of cases. CD20 and CD79a were negative in the tumor cells in all cases, but two cases (10.5%), one in situ (n°3) and one of infiltrative subtype (n°9), were weakly positive for PAX5 (Figure 5G), as already reported in a small proportion of systemic ALCL [25]. IRF4/MUM1 was positive in 89% of cases. All cases tested for phospho-STAT3 (p-STAT3) were positive in both i-ALCL subtypes (n = 12/12) (Figure 5H). ALK-negative i-ALCLs were consistently negative for EBV. In addition, FISH testing with the DUSP22 break-apart probe was negative in all contributive cases tested (n = 9/9).
Figure 5.
Immunophenotype of i-ALCL. Tumor cells are positive for CD30, ×200 (A) and ×100 (B); EMA, ×600 (C); CD3, ×400 (D); CD4, ×200 (E); express cytotoxic granules of granzyme B, ×200 (F); PAX5, (×400) (G) and pSTAT3 (×200) (H).
TCR gene rearrangements
TCRG genes were clonally rearranged in the 13 cases tested. TCRB genes were clonally rearranged in 46% of cases (6/13). Polyclonal rearrangements in IGH and IGK genes were detected in the two cases tested (n°3 and n°9).
discussion
Our study confirms in a cohort of 19 patients the main clinicopathological characteristics of i-ALCL. In agreement with previous reports [6–21], they typically present with a persistent peri-implant seroma, although a significant proportion disclose a palpable tumor. In addition, we describe here that the two distinct clinical forms of i-ALCL correlate with specific pathological features: (i) in situ i-ALCL subtype, consisting in an anaplastic proliferation with ‘hallmark’ cells confined to the capsule is associated with seroma; (ii) infiltrative i-ALCL, with Hodgkin-like cells massively infiltrating the capsule and/or adjacent tissues is associated with a tumor mass. Interestingly, the two morphologic patterns coexisted in two patients, showing not only a histogenetic relationship between in situ and infiltrative i-ALCL, but also suggesting that a lesion confined to the capsule may evolve to an infiltrative proliferation. Consistent with previous reports [6–21], i-ALCL tumor cells express CD30, frequently EMA and IRF4/MUM1, but are ALK negative. Most cases have an activated cytotoxic phenotype with often extensive T-cell antigen loss, including absence of TCR expression. It should be stressed that some tumor cells had an ‘NK/T’ cell phenotype with CD56 expression and cytotoxic molecules alone. However, all cases tested were EBV-negative and disclosed clonal rearrangement of the TCRγ genes, allowing distinction from extranodal NK/T-cell lymphoma nasal-type.
In addition to the Hodgkin-like morphologic features of i-ALCL subtype, phenotypic features can be misleading. Indeed, besides the common T-cell antigen loss, i-ALCL cells variably expressed CD15 including two cases showing both positivity for PAX5 and CD15. This peculiar aberrant phenotype had resulted in an initial misdiagnosis of classical Hodgkin lymphoma in one patient. These two cases showed a clonal rearrangement of TCR genes but no B-cell clone. This highlights that the diagnosis of i-ALCL requires integration of the clinical context together with immunohistochemical and molecular investigations.
As suggested in the literature, i-ALCL is generally an indolent localized disease and could be efficiently treated with capsulectomy alone [9, 10, 12, 14]. Previously reported in a minority of cases (10%–35% of i-ALCL patients) [8, 15, 17, 18], we show here that 42% of patients have a palpable tumor mass generally limited to the breast, more rarely associated with lymphadenopathies or bone marrow involvement. As recently described [21], we show here that i-ALCL has a variable outcome ranging from indolent to aggressive and fatal disease. Miranda et al. [17] and others [7, 11, 13, 16, 19] reported that only i-ALCL patients with seroma have a good prognosis. In our series, we show that this clinical subtype is associated with in situ i-ALCL morphological features (n = 11) and has an excellent prognosis, all the patients being alive without active disease with a median follow-up of 18 months. This is in agreement with the proposal that in situ i-ALCL is similar to other indolent CD30-positive lymphoproliferative disorders such as primary cutaneous ALCL [11, 14, 21, 26].
In contrast, as already suggested [10, 11, 13, 15, 17, 18, 27], the presentation as a tumor mass associated with an infiltrative i-ALCL morphology (n = 8) appeared much more aggressive with a 52.5% overall survival at 2 years, close to that of systemic ALK-negative ALCL [28]. According to recently published clinical guidances, surgical removal of the implant and complete capsulectomy should be performed when i-ALCL is suspected. This treatment appears successful and no other therapy is generally needed. However, i-ALCLs presenting as a mass require additional chemotherapy [29]. Although the mechanisms of lymphomagenesis remain elusive in systemic ALK negative ALCL, recurrent translocations involving DUSP22 locus and oncogenic STAT3 mutations have been recently described [30–32]. The detection of nuclear pSTAT3 expression in all our cases might suggest that STAT3 is also mutated in such tumors, although alternative mechanisms for the constitutive activation of STAT3 could not be precluded. Nevertheless, JAK/STAT3-targeted tyrosine kinase inhibitor (sunitinib) could be envisaged as an interesting strategy in infiltrative i-ALCL. Despite the indolent course of i-ALCL, our results showed that none of tested cases had a DUSP22 rearrangement, in contrast with primary cutaneous ALCL and a subset of systemic ALK-negative ALCL with good prognosis. However, given the small number of cases in our series, further studies are needed to determine whether i-ALCL represents a new genetic subset of ALK-negative ALCL or is a part of the still heterogeneous systemic ALK-negative ALCL entity.
Despite the very low risk to develop ALCL in women with breast implant (0.1–0.3 per 100 000 women with prostheses) [8, 17], several key observations in line with our results lead to support an association between ALCL and implants [8]. Actually, among 300 breast lymphomas registered in the Lymphopath network, only 8% were PTCLs, but the vast majority (80%) of the latter was i-ALCL. In our series, all implants were textured suggesting a site and material-specific chronic inflammatory etiology, as already suggested [8, 12, 13, 17, 18, 21, 29]. Although some authors found no significant association between i-ALCL and fluid fill (saline or silicone) [29], all cases reported here but two were in the context of silicone implants. However, we cannot eliminate a possible selection bias, given that silicone-breast implant is most commonly used in France. Silicone and its degradation products have been shown to be directly toxic in living tissues by causing fibrosis and inflammation [33, 34]. However, in half of our cases with silicone gel, implants were not disrupted. This could suggest that other factors, such as immunologic response to chronic irritation by the prosthesis, in particular having a textured surface, could trigger ALCL proliferation [12, 35]. In i-ALCL, a direct link with viral/or bacterial infection has not been demonstrated, but a recent study found a biofilm Gram-negative bacteria in some implants, associated with an increased T-cell response [36]. In this respect, we have reported that insect bite-associated antigens could result in an influx of T lymphocytes, some bearing the t(2;5). The subsequent release of cytokines could act as a ‘second hit’, eliciting activation of the latter cells, which would then express the oncogenic NPM-ALK protein and undergo ALK+ALCL cell proliferation [37].
In conclusion, i-ALCL is a distinct clinicopathological entity comprising two histological subtypes, correlating to clinical presentation. Clinical presentation as an effusion around the implant corresponds histologically to an ‘in situ’ i-ALCL confined to the capsule. Conversely, patients with a palpable mass have an ‘infiltrative’ i-ALCL massively invading the capsule and adjacent tissues and show histological features resembling classical Hodgkin lymphoma. Although i-ALCL is considered as an indolent T-cell lymphoma, ‘infiltrative’ i-ALCL subtype requires more intensive therapeutic approaches.
funding
This work was supported in part by institutional grants from the Institut National du Cancer (INCA), the Laboratoire d'Excellence Toulouse Cancer (TOUCAN) (contract ANR11-LABX), the Programme Hospitalo-Universitaire en Cancérologie CAPTOR (contract ANR11-PHUC0001) and the Institut Carnot Lymphome (CALYM).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank Dr E. Jaffe (NIH, Bethesda, USA) for her critical review of the manuscript. We thank L. Jalabert and G. Perez for immunohistochemistry staining (IUCT, Toulouse, France). We thank S. Boussetta for statistical analysis (LYSA). We thank F.X. Frenois for whole slide imaging (IUCT Toulouse, France). We thank N. Martin-Garcia for phospho-STAT3 staining (INSERM U955, Créteil, France) and C. Boéchat (Lausanne) for FISH analyses. We thank V. Conan-Charlet, P. Caverivière, C. Charon, A. Dubois-Gordeeff, F. Berger, C. Sagan, M.S. Soubeyrand, G. Barneon, J.L. Bouzigues, I. Liolios, O. Languille, H. Vacheret and W. Hu for iALCL samples. We thank the INCa expert group: B. Asselain, J. Chopier, C. Ivaldi, Y. Kirova, C. Malhaire, T. Molina, F. Reyal, R. Sinna, M. Talagas and H. Tilly. This work was supported in part by institutional grants from the Institut National du Cancer (INCA), the Laboratoire d'Excellence Toulouse Cancer (TOUCAN) (contract ANR11-LABX), the Programme Hospitalo-Universitaire en Cancérologie CAPTOR (contract ANR11-PHUC0001) and the Institut Carnot Lymphome (CALYM).
references
- 1.Talwalkar SS, Miranda RN, Valbuena JR et al. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol 2008; 32: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 2.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin's lymphomas of the female breast. Cancer 1992; 69: 725–735. [DOI] [PubMed] [Google Scholar]
- 3.Validire P, Capovilla M, Asselain B et al. Primary breast non-Hodgkin's lymphoma: a large single center study of initial characteristics, natural history, and prognostic factors. Am J Hematol 2009; 84: 133–139. [DOI] [PubMed] [Google Scholar]
- 4.Martinelli G, Ryan G, Seymour JF et al. Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2009; 20: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 5.Gualco G, Chioato L, Harrington WJ et al. Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases. Appl Immunohistochem Mol Morphol 2009; 17: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keech JA, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997; 100: 554–555. [DOI] [PubMed] [Google Scholar]
- 7.Gaudet G, Friedberg JW, Weng A et al. Breast lymphoma associated with breast implants: two case-reports and a review of the literature. Leuk Lymphoma 2002; 43: 115–119. [DOI] [PubMed] [Google Scholar]
- 8.de Jong D, Vasmel WL, de Boer JP et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA 2008; 300: 2030–2035. [DOI] [PubMed] [Google Scholar]
- 9.Roden AC, Macon WR, Keeney GL et al. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol 2008; 21: 455–463. [DOI] [PubMed] [Google Scholar]
- 10.Lazzeri D, Agostini T, Giannotti G et al. Null-type anaplastic lymphoma kinase-negative anaplastic large cell lymphoma arising in a silicone breast implant capsule. Plast Reconstr Surg 2011; 127: 159e–162e. [DOI] [PubMed] [Google Scholar]
- 11.Carty MJ, Pribaz JJ, Antin JH et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg 2011; 128: 112e–118e. [DOI] [PubMed] [Google Scholar]
- 12.Kim B, Roth C, Chung KC et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg 2011; 127: 2141–2150. [DOI] [PubMed] [Google Scholar]
- 13.Aladily TN, Medeiros LJ, Amin MB et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol 2012; 36: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CR, Siddiqi IN, Brody GS. Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. Appl Immunohistochem Mol Morphol 2013; 21: 13–20. [DOI] [PubMed] [Google Scholar]
- 15.Story SK, Schowalter MK, Geskin LJ. Breast implant-associated ALCL: a unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist 2013; 18: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George EV, Pharm J, Houston C et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol 2013; 6: 1631–1642. [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda RN, Aladily TN, Prince HM et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 2014; 32: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gidengil CA, Predmore Z, Mattke S et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg 2015; 135: 713–720. [DOI] [PubMed] [Google Scholar]
- 19.Alobeid B, Sevilla DW, El-Tamer MB et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma 2009; 50: 831–833. [DOI] [PubMed] [Google Scholar]
- 20.Sahoo S, Rosen PP, Feddersen RM et al. Anaplastic large cell lymphoma arising in a silicone breast implant capsule: a case report and review of the literature. Arch Pathol Lab Med 2003; 127: e115–e118. [DOI] [PubMed] [Google Scholar]
- 21.Brody GS, Deapen D, Taylor CR et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015; 135: 695–705. [DOI] [PubMed] [Google Scholar]
- 22.de Leval L, Parrens M, Le Bras F et al. Angioimmunoblastic T-cell lymphoma is the most common T-cell lymphoma in two distinct French information data sets. Haematologica 2015; 100: e361–e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent C, Do C, Gascoyne RD et al. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol 2009; 27: 4211–4216. [DOI] [PubMed] [Google Scholar]
- 24.van Dongen JJ, Langerak AW, Brüggemann M et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia 2003; 17: 2257–2317. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AL, Law ME, Inwards DJ et al. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod Pathol 2010; 23: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadin M, Xu H, Pavlov I, Epstein A. Breast implant associated ALCL closely resembles primary cutaneous ALCL. Lab Invest 2012; 92: 320–386.22157719 [Google Scholar]
- 27.Vase M, Friis S, Bautz A et al. Breast implants and anaplastic large-cell lymphoma: a Danish population-based cohort study. Cancer Epidemiol Biomarkers Prev 2013; 22: 2126–2129. [DOI] [PubMed] [Google Scholar]
- 28.Sibon D, Fournier M, Brière J et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 2012; 30: 3939–3946. [DOI] [PubMed] [Google Scholar]
- 29.Kim B, Predmore ZS, Mattke S et al. Breast implant-associated anaplastic large cell lymphoma: updated results from a structured expert consultation process. Plast Reconstr Surg Glob Open 2015; 3: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrilla Castellar ER, Jaffe ES, Said JW et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014; 124: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crescenzo R, Abate F, Lasorsa E et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 2015; 27: 516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiarle R, Simmons WJ, Cai H et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med 2005; 11: 623–629. [DOI] [PubMed] [Google Scholar]
- 33.Katzin WE, Centeno JA, Feng LJ et al. Pathology of lymph nodes from patients with breast implants: a histologic and spectroscopic evaluation. Am J Surg Pathol 2005; 29: 506–511. [DOI] [PubMed] [Google Scholar]
- 34.Shanklin DR, Smalley DL. The immunopathology of siliconosis. History, clinical presentation, and relation to silicosis and the chemistry of silicon and silicone. Immunol Res 1998; 18: 125–173. [DOI] [PubMed] [Google Scholar]
- 35.Seyhan H, Kopp J, Beier JP et al. Smooth and textured silicone surfaces of modified gel mammary prostheses cause a different impact on fibroproliferative properties of dermal fibroblasts. J Plast Reconstr Aesthet Surg 2011; 64: e60–e66. [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Jacombs A, Vickery K et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg 2015; 135: 319–329. [DOI] [PubMed] [Google Scholar]
- 37.Lamant L, Pileri S, Sabattini E et al. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: evidence for an association in five cases. Haematologica 2010; 95: 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.