Abstract
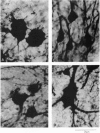
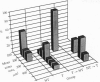
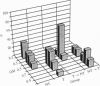
Reproduction in vertebrates is regulated by the hypothalamic-pituitary-gonadal axis via neural and hormonal feedback. This axis is also subject to exogenous influences, particularly social signals. In the African cichlid fish Haplochromis burtoni, gonadal development in males is socially regulated. A small fraction of the males, which are brightly colored, maintain territories and aggressively dominate inconspicuously colored nonterritorial males. Here we show through manipulation of the social and endocrine environment that changes in social status and gonadal state are accompanied by soma size changes in a population of gonadotropin-releasing hormone-containing neurons in the ventral forebrain. In territorial males, these cells are significantly larger than in nonterritorial males. When an animal switches from being territorial to nonterritorial through a change in social situation, these cells shrink; in animals that change from nonterritorial to territorial status, the cells enlarge. These gonadotropin-releasing hormone-containing cells project to the pituitary and are ultimately responsible for regulating gonadal growth. This mechanism of socially induced cell size change provides the potential for relatively quick adaptive changes in the neuron-endocrine system without nerve cell addition or death. Since the structure of this regulatory axis is conserved among all vertebrates, other species with socially modulated reproductive physiology may exhibit a similar form of physiological regulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cattanach B. M., Iddon C. A., Charlton H. M., Chiappa S. A., Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977 Sep 22;269(5626):338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- Davis M. R., Fernald R. D. Social control of neuronal soma size. J Neurobiol. 1990 Dec;21(8):1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- Francis R. C., Jacobson B., Wingfield J. C., Fernald R. D. Hypertrophy of gonadotropin releasing hormone-containing neurons after castration in the teleost, Haplochromis burtoni. J Neurobiol. 1992 Oct;23(8):1084–1093. doi: 10.1002/neu.480230812. [DOI] [PubMed] [Google Scholar]
- Fricke H., Fricke S. Monogamy and sex change by aggressive dominance in coral reef fish. Nature. 1977 Apr 28;266(5605):830–832. doi: 10.1038/266830a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Zoeller R. T., Young W. S., 3rd, Phillips H. S., Nikolics K., Seeburg P. H. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986 Dec 12;234(4782):1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Münz H., Stumpf W. E., Jennes L. LHRH systems in brain of platyfish. Brain Res. 1981 Sep 21;221(1):1–13. doi: 10.1016/0006-8993(81)91059-3. [DOI] [PubMed] [Google Scholar]
- Oka Y., Ichikawa M. Gonadotropin-releasing hormone (GnRH) immunoreactive system in the brain of the dwarf gourami (Colisa lalia) as revealed by light microscopic immunocytochemistry using a monoclonal antibody to common amino acid sequence of GnRH. J Comp Neurol. 1990 Oct 22;300(4):511–522. doi: 10.1002/cne.903000406. [DOI] [PubMed] [Google Scholar]
- Ross R. M., Losey G. S., Diamond M. Sex change in a coral-reef fish: dependence of stimulation and inhibition on relative size. Science. 1983 Aug 5;221(4610):574–575. doi: 10.1126/science.221.4610.574. [DOI] [PubMed] [Google Scholar]
- Schreibman M. P., Halpern L. R., Goos H. J., Margolis-Kazan H. Identification of luteinizing hormone-releasing hormone (LH-RH) in the brain and pituitary gland of a fish by immunocytochemistry. J Exp Zool. 1979 Oct;210(1):153–159. doi: 10.1002/jez.1402100117. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Mason A. J., Young W. S., 3rd, Stewart T. A., Nikolics K. The gene encoding GnRH and its associated peptide GAP: some insights into hypogonadism. J Steroid Biochem. 1989 Oct;33(4B):687–691. doi: 10.1016/0022-4731(89)90479-2. [DOI] [PubMed] [Google Scholar]
- Sohn J. J. Socially induced inhibition of genetically determined maturation in the platyfish, Xiphophorus maculatus. Science. 1977 Jan 14;195(4274):199–201. doi: 10.1126/science.831271. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J. G. Acceleration and inhibition of puberty in female mice by pheromones. J Reprod Fertil Suppl. 1973 Dec;19:411–419. [PubMed] [Google Scholar]