Abstract
Bipolar disorder (BD) is among the most impairing psychiatric disorders affecting children and adolescents, despite our best psychopharmacological and psychotherapeutic treatments. Cognitive remediation, defined as a behavioral intervention designed to improve cognitive functions so as to reduce psychiatric illness, is an emerging brain-based treatment approach that has thus far not been studied in pediatric BD. The present article reviews the basic principles of cognitive remediation, describes what is known about cognitive remediation in psychiatric disorders, and delineates potential brain/behavior alterations implicated in pediatric BD that might be targets for cognitive remediation. Emerging data shows that cognitive remediation may be useful in children and adults with schizophrenia, ADHD, and anxiety disorders, and in adults with BD. Potential targets for cognitive remediation in pediatric BD include face processing, response inhibition, frustration, and cognitive flexibility. Further study is warranted to determine if cognitive remediation for these targets, or others, may serve as a novel, brain-based treatment for pediatric BD.
INTRODUCTION
Pediatric bipolar disorder (BD) is a significant global health concern, with clinical studies suggesting an increased rate of children diagnosed with the mood disorder during the past few decades. For example, the percentage of minors with a BD diagnosis admitted to German psychiatric hospitals increased 68.5% between 2000 and 2007, whereas those discharged from U.S. psychiatric hospitals surged from less than 10% in the mid-1990s to more than 20% in the mid-2000s (1). Another study showed this increase was not confined to psychiatric hospitals, with a forty-fold rise in the incidence of U.S. outpatient visits for youth diagnosed with BD to providers of all mental health specialties, from 25/100,000 in 1993–1994 to 1003/100,000 in 2002–2003 (2). Moreover, with an estimated overall prevalence of 1.8% (3), and more than 80 million children in the U.S. per the 2000 Census, there are millions of children and adolescents being brought for evaluation/treatment of BD annually (4). Beyond the obvious concern for the sheer number of youth affected by the disorder, pediatric BD results in substantial morbidity and functional impairment for the affected children and their families (5;6) including high rates of suicidal ideation and suicide attempts (7).
With respect to treatments for children and adolescents with BD, studies support a role for both medication (e.g., lithium, atypical neuroleptics, and anti-epileptic drugs) (8–10) and psychotherapy (e.g., family-focused therapy [FFT], and cognitive behavioral therapy [CBT]) (11–14). However, we need better treatments for pediatric BD because despite our best currently available treatments, pediatric BD results in considerable morbidity and mortality, including high rates of suicidality and psychiatric hospitalization (7;15;16). Moreover, these agents may result in serious physical side effects—e.g., extreme weight gain and metabolic syndrome from atypical neuroleptics (17). Finally, there is a need for interventions that can overcome traditional barriers to access, including dearth of specialists, including child psychiatrists and psychologists and pediatricians, who are trained and feel comfortable in evaluating and treating children with serious psychopathology, such as BD.
Cognitive remediation—broadly defined as training impaired cognitive or emotional skills in order to reduce the impairment from a psychiatric illness—is a novel, brain-based treatment approach that may address these needs as part of a comprehensive treatment plan for youth with BD. In the present manuscript, we review cognitive remediation as a possible adjunctive treatment approach for psychiatric conditions, including BD. In particular, after explaining what cognitive remediation is, we discuss recent research on cognitive remediation for psychiatric disorders as well as potential brain-based targets for cognitive remediation in youth with BD.
WHAT IS COGNITIVE REMEDIATION?
Cognitive remediation is a behavioral approach to treatment with basic tenets that involve the following three components. First, cognitive functions representing separable domains (e.g., attention, memory, etc.) can be assessed and treated independently. Second, rehabilitation of impaired cognitive functions is possible given the brain’s capacity for neural plasticity and change in response to drill-and-practice learning. Third, improving those skills may result in reduced illness symptom burden or functional impairment (for excellent review, see Vinaogradov et al.; reference number (18)).
Figure 1 outlines the basic steps required to assess the potential for cognitive remediation for a specific disorder or symptom profile. In brief, first studies must determine if there are specific cognitive or emotional processes altered in a particular disorder or associated with a particular symptom profile. Such assessment may include the use of standard pen-and-paper neuropsychological assessments, computerized behavioral tasks, or approaches directly tapping neural function in people, such as functional magnetic resonance imaging (fMRI) or electroencephalography paired with neuroimaging (i.e., magnetoencephalography [MEG]) or with behavioral tasks (i.e., psychophysiology). Then, a drill-and-practice cognitive remediation can be designed to try to ameliorate those deficits. Finally, cognitive remediation must be tested to determine if it can successfully build those skills previously shown deficient, and if patients with those disorders/symptoms can improve, meaning reduced symptoms or impairment from them. For example, studies have demonstrated that adults with schizophrenia have impaired working memory. Therefore, cognitive remediation for schizophrenia might examine if adults with schizophrenia could improve their working memory with repeated drill-and-practice learning, and if such training improved the symptoms of schizophrenia. As would be true of any treatment, key refinements include determining the optimal dose and setting (i.e., at home or in the lab/office) for the cognitive remediation to be delivered as well as the treatment’s durability (i.e., how long the improvements will last) Figure 1).
Figure 1.
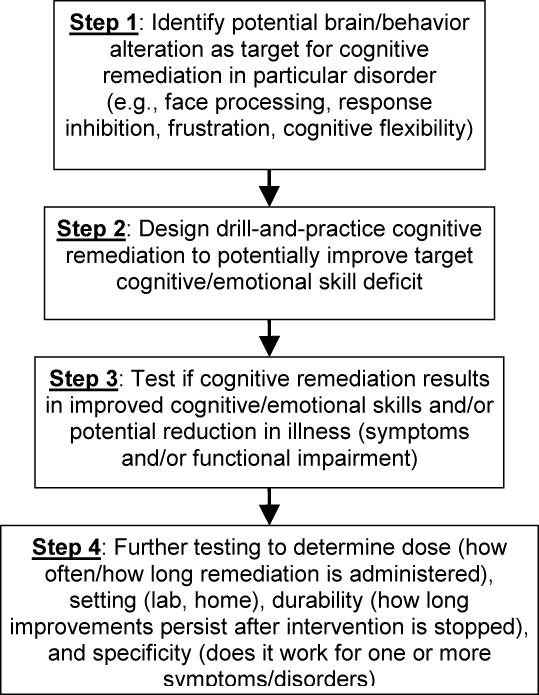
Basic Steps in Assessing Cognitive Remediation as Potential Treatment
Cognitive remediation is neither new nor confined to neuropsychiatric illnesses. Rather, it has been used since the mid-twentieth century in a variety of disorders, including rehabilitation from stroke or traumatic brain injury. Examples of this work include Wagner’s finding that adults with schizophrenia had reduced cognitive function, including attention and abstraction, which could improve with positive reinforcement (18).
Cognitive remediation offers several potential advantages over traditional forms of treatment for psychiatric illness. First and foremost, cognitive remediation may improve patients’ access to care because, unlike other medication and therapy approaches delivered by one practitioner to one patient at a time (or at best to ten group therapy members at a time), cognitive remediation programs are “scalable”—meaning treatment may be delivered to far more patients simultaneously. For example, many patients can simultaneously receive the remediation in a testing center while being monitored by a technician in collaboration with a physician or psychologist as is currently done by neurofeedback centers. At its extreme, potentially unlimited numbers of individuals may receive the remediation simultaneously via internet-based computer assisted cognitive remediation.
Additionally, cognitive remediation may be personalized in that interventions can be precision tailored to meet an individual’s needs. For example, based on their individual and illness-based deficits, one patient with schizophrenia may need remediation for both attention and memory, while another may just need memory.
Finally, cognitive remediation may have fewer side effects than medications used to treat psychiatric illness, such as metabolic syndrome with atypical neuroleptics or sexual dysfunction with selective serotonin reuptake inhibitors. This is not to say that cognitive remediation may be a substitute for psychiatric medications, as most studies to date have examined cognitive remediation’s role augmenting psychotropic medications and/or psychotherapy, rather than as monotherapy for psychiatric illness. It is possible that cognitive remediation may have no side effects, akin to other forms of learning or educational games. However, we are at the early stages of understanding what, if any, side effects cognitive remediation programs may have.
Similarly, there are several important unknowns about cognitive remediation for psychiatric disorders, especially in children where such work is just beginning. First, are there critical windows of neurodevelopment when cognitive remediation is possible or works best? Second, what is the role of cognitive reserve (i.e., the brain’s resilience and ability to cope with stress, illness, and other insults) in determining how children may respond to cognitive remediation, and how can such cognitive reserve be assessed in children (19;20)?
WHAT IS KNOWN ABOUT COGNITIVE REMEDIATION IN CHILD AND ADULT PSYCHIATRIC DISORDERS?
Cognitive remediation is an important and emerging form of treatment for psychiatric disorders. However, as this manuscript is being written, there are no currently published studies testing the role of cognitive remediation for BD in children and, in fact, cognitive remediation research in patients with mood disorders (either BD or unipolar major depressive disorder) has lagged behind that in other disorders. In adults, the vast majority of research so far centers on the role of cognitive remediation for schizophrenia, although it has also been studied in a host of other disorders, including attention deficit hyperactivity disorder (ADHD), eating disorders (21–25), and, as aforementioned, to a lesser extent BD (26–30). Moreover, we unfortunately note that far less is known about cognitive remediation for children with psychiatric disorders than in adults, despite the fact that cognitive remediation centers on restoring function at school, work, home, and in social relationships (31;32).
With respect to adults with schizophrenia, cognitive remediation studies have targeted several cognitive processes, especially working memory, but also verbal memory, processing speed, and reasoning (33). For example, schizophrenic adults whose standard vocational rehabilitation was augmented with cognitive remediation had improved cognitive performance at 3 months and greater work outcomes at 2 years vs. those who received only vocational rehabilitation and no cognitive remediation (34). Studies suggest that cognitive remediation can be cost-effective for adults with schizophrenia (35), with notable effects on cognitive functioning, as well as quality of life and self-esteem (33). A meta-analysis of 2,104 patients found that cognitive remediation may result in enduring improvements in functioning (23). Another meta-analysis of 1,151 patients showed that cognitive remediation for adults with schizophrenia was associated with a medium effect size for cognitive performance (0.41), a smaller effect size for psychosocial functioning (0.36), and a small effect size for symptoms (0.28) (22). Guided by this work in adults, a recent study evaluated cognitive remediation for executive function and working memory in adolescents presenting with early onset schizophrenia. They showed not only improved working memory and executive function, but improved daily living skills and global functioning vs. those randomized to receive treatment as usual (36). Thus, the trickle down effect from studies of cognitive remediation in adults with schizophrenia to pediatric-aged samples with schizophrenia and psychosis has begun.
In children, studies have thus far begun to examine cognitive remediation for ADHD and anxiety disorders. With respect to studies of children with ADHD, findings generally support improved functioning (i.e., in the targeted cognitive skill(s) and/or ADHD symptoms) among those youths receiving a cognitive remediation-based intervention (37–40). For example, Van Der Oord et al. showed that latency-aged children randomized to a computerized executive functioning training (i.e., targeting inhibition, cognitive flexibility, and working memory) had improvement in the executive skills targeted, as well as decreased ADHD symptoms of inattention and hyperactivity/impulsivity as rated by parents on the Disruptive Behavior Disorder Rating Scale, compared to youths placed in the waitlist condition (37;41). Interestingly, this study showed these improvements not only at the end of their 25 training sessions, but also at a 9-week post-treatment follow up. Similarly, Gray et al. found that adolescents with comorbid ADHD and learning disorders randomized to a working memory vs. math training program showed significantly improved WM and were rated as less inattentive/hyperactive at home by their parents (39). Children with ADHD who received drill-and-practice cognitive remediation have shown moderate to large effects on academic tasks and parental ratings of ADHD (42;43). Specifically, Klingberg et al. found decreased inattention and hyperactivity/impulsivity on the Conners Parent ADHD rating scale among children randomized to a working memory training vs. control condition, whereas Shalev only found decreased parental ratings of inattention after treatment with an attention training program vs. control condition (42;43).
A recent meta-analytic review of 25 cognitive remediation studies in children with ADHD concluded that while training short-term memory resulted in moderate improvements, similar training programs in attention or mixed executive functions did not result in those domains improving, reduced ADHD symptoms, or improved functioning (44). Working memory training with COGMED (a commercially available software product produced by the Pearson corportation) has yielded mixed results, with some studies showing neither improved working memory nor ADHD symptoms (45), while others show improved verbal and non-verbal working memory storage, but no improvements either in working memory processing/manipulation or in ADHD symptoms (40). Thus, there is a need for ongoing research to examine key aspects of cognitive remediation for ADHD, including whether it is better to have a laser-like focus on training one cognitive skill or to have a shotgun-like broad approach to training multiple domains simultaneously, as well as identifying which domains result in greatest symptom reduction and functional improvement.
Beyond ADHD, an emerging literature has examined attention bias modification treatment (ABMT) for children and adults with anxiety disorders. ABMT is based on considerable literature demonstrating that children with anxiety disorders as well as related conditions, such as behavioral inhibition, have brain/behavior alterations in how they respond to threat and fearful stimuli (46–50). For example, young adults found to have high levels of behavioral inhibition when they were children have greater neural connectivity between the amygdala and dorsal-lateral prefrontal cortex (DLPFC) when watching threat-related stimuli (51).
In this vein, ABMT seeks to reverse this attention bias—meaning the tendency of cues in a person’s environment to preferentially engage and hold a person’s attention. Specifically, ABMT for anxiety might involve having a child complete a computerized task whereby pairs of face stimuli—one happy and one threatening—are simultaneously displayed followed by a star on only one side, and the child would have to indicate by key press which side the star was on. To train away from threat, the game would be configured to have more trials where the star was on the side of the happy face rather than the threatening face. Thus, by drill-and-practice, the anxious child would have reduced attention bias for threat and better ability to attend to the star regardless of whether the preceding same-sided image was threatening or not.
Thus far, studies of ABMT in anxious youths have demonstrated that the largest reduction in the number and severity of anxiety symptoms came from active ABMT compared to either a placebo ABMT that did not train away from threat or an ABMT that used neutral, rather than threatening, stimuli (52). A recent study showed that ABMT when combined with cognitive behavioral therapy (CBT) may result in reduced anxiety symptoms by self-report and interview compared to a placebo ABMT plus CBT (53)). These early studies show that ABMT has promise as part of the treatment of anxiety disorders in children, with emerging work examining the role of dose (i.e., how often ABMT must be administered), durability (i.e., how long after ABMT anxiety symptoms will remain diminished), and interaction with other treatments, such as psychotropic medication and psychotherapy.
Similarly, and certainly relevant to considering the role of cognitive remediation for youth with BD, ABMT has been used with individuals diagnosed with depression. For example, Yang et al. showed decreased depressive symptoms among college students randomized to the ABMT condition vs. placebo or control conditions at post-treatment and 3-month follow-up (54). However, Baert et al. suggested the effect of cognitive remediation on depressive symptoms might depend on the initial symptom severity. That is, they found that symptoms decreased post-ABMT for young adults initially presenting with mild to moderate depression whereas symptoms actually increased for adults presenting with more severe depression and while receiving inpatient psychiatric care compared to those receiving outpatient psychiatric treatment (55).
In adults with BD, primary targets for cognitive remediation have also included attention, memory, and executive functions. For example, Deckersbach et al. showed fewer residual depressive symptoms and improved occupational functioning at post-treatment and 3-month follow-up for adults diagnosed with either BD I or II provided 14 individual sessions of cognitive remediation (26). Sole et al. found significant improvement in overall psychosocial functioning at post-treatment for euthymic BD II adults provided 21 weeks of group formatted cognitive remediation (29). The first randomized controlled trial assessing the effect of cognitive remediation for adults with continued cognitive difficulties despite remission from BD, is currently underway and will likely prove valuable in leading child-focused efforts in the future (28).
WHAT ARE POTENTIAL TARGETS FOR COGNITIVE REMEDIATION AMONG CHILDREN/ADOLESCENTS WITH BIPOLAR DISORDER?
In contrast to the abovementioned disorders, research on the potential of cognitive remediation for BD among children and adolescents is in its infancy. However, such work will build on a robust and growing literature about the pathophysiology of pediatric BD. In particular, studies grounded in affective neuroscience—the study of brain/behavior interactions underlying emotion and neuropsychiatric disorders—has shown that children and adolescents with BD have aberrant functioning in at least four cognitive/emotional processes: emotional face processing, response inhibition, frustration, and cognitive flexibility. As outlined below, each cognitive process and its underlying circuitry is a potential target for cognitive remediation. However, without further research, the following remain important knowns: (1) Are any of these processes amenable to change/remediation? (2) Will remediation result in symptom improvement? (3) Is a laser-like remediation, focusing on only one process, more or less likely to result in both remediation and functional improvement compared to a shotgun-like remediation, which trains aspects of all of these (and potentially other) processes?
Face processing provides a window into basic aspects of emotional function because humans are hard-wired from birth to attend to faces and to identify familiar (e.g., one’s own mother) vs. novel (e.g., a stranger’s) faces (56;57). Face processing is often evaluated using emotional face identification tasks, whereby participants must identify which emotion a picture of a person is showing (i.e., happy, angry, sad, neutral, etc.). Variants of this type of task include facial morphing, whereby face stimuli represent gradations in intensity of emotion by blending them with neutral or other faces (i.e., combining a happy and neutral face so that you get 10% happy, 20% happy, or 30% happy, etc.). Another variant often used with fMRI is to compare neural activity when a participant is attending to an emotional (i.e., “how angry?”) vs. non-emotional (i.e., “how wide is the nose?) of facial stimuli.
Out-of-scanner behavioral studies using the Diagnostic Assessment of Non-Verbal Accuracy (DANVA) have demonstrated that youth with BD make significantly more errors categorizing emotional faces than both typically-developing children (TDC) without psychopathology and also vs. those with anxiety disorders (58) and vs. youth with primary ADHD, especially on low-intensity happy faces (59). Similar deficits have been identified in youth at familial risk for BD (60). With respect to neuroimaging, fMRI studies from several research groups across the United States indicate that youth with BD have abnormal prefrontal cortex (PFC)–amygdala–striatal neural activation compared with TDC children when viewing faces with happy, angry, or neutral emotions (61–63). Other fMRI studies suggest that the neural circuitry mediating face processing in youth with BD with episodes of mania may be different than the underlying circuitry in youths with chronic, non-episodic, functionally-disabling irritability meeting Leibenluft et al.’s research criteria for severe mood dysregulation (SMD) that served as the basis for the new Diagnostic and Statistical Manual 5th edition diagnosis known as disruptive mood dysregulation disorder (DMDD) (64–68).
Response inhibition refers to the ability to stop actions that interfere with goal-directed behavior because they are incorrect or inappropriate (69). Response inhibition is linked to the symptoms of distractibility and impulsivity that are most linked to ADHD, which is commonly comorbid to BD. Furthermore, distractibility is an explicit diagnostic criteria for a manic episode. Response inhibition can be tested in several ways, including stop signal and go/no-go tasks. These tasks have some differences, but their core feature is to prime a participant to execute a motor response to one stimulus which is more frequent (i.e., press the “1” when you see an “X” which happens on 70% of trials), but then to sometimes require them to inhibit that response (i.e., if you see an “O” do not press anything, which happens on 30% of trials) (70).
With respect to response inhibition, one study found that youth with BD had a reduced striatal “error signal” during failed motor inhibition compared to controls (71), a deficit which might contribute to the patients’ inability to effectively inhibit. Another study by Singh et al. found that youth with BD had greater neural activation than TDCs in the right DLPFC during no-go vs. go trials, suggesting greater reliance on cognitive control areas to maintain adequate behavioral performance (72). Given its links to core features of BD and comorbid ADHD, response inhibition may be an important target for cognitive remediation in youth with BD.
Frustration may be defined from an affective neuroscience perspective as “reactions elicited in response to withdrawal or prevention of reward”. Frustration is relevant to BD via Blair’s somatic marker hypothesis, which posits that children less able to adapt to social rewards (e.g., praise or reprimand from parents/peers) may feel frustrated (defined as “affective response to blocked goal attainment”) and show symptoms of irritability and aggression, which are found in both manic episodes and depressive episodes (73–75). Frustration can be studied in the laboratory or with neuroimaging using tasks involving rigged feedback, meaning feedback that is not connected with an individual’s actual responses. For example, telling a participant that they were incorrect when they correctly responded that eight minus five is three.
Among youth with BD, the Leibenluft group has conducted a series of studies on frustration using the affective Posner task. At its core, this is an attention task, requiring participants to indicate if a target shape was on the left or right side of the screen. However, the task involves three stages that manipulate feedback. An initial stage provides accurate feedback based on performance (“you are correct” or “you are incorrect” for correctly/incorrectly indicating which side the target was on). A second stage adds accurate monetary reward (i.e., win/lose money based on correct/incorrect responses). A third stage uses rigged feedback (i.e., “Too slow! Lose 25 cents!” for providing the correct response).
In this series of studies, pairing the affective Posner task with EEG psychophysiology monitoring, Rich et al. showed that youth with BD had altered P3 EEG amplitude vs. both TDC and SMD participants with chronic, non-episodic irritability, potentially indicating that youth with BD had impaired executive attention. In contrast, regardless of condition, SMD youths had lower N1 amplitude than either BD or TDC participants, indicating impairments in the initial stage of attention (76). Another study pairing the affective Posner task with MEG showed that youth with BD had greater superior frontal gyrus activation and decreased insula activation than after negative feedback than TDC or SMD youths, while SMD participants had greater anterior cingulate cortex and medial frontal gyrus activation than TDCs (77). Taken together, these studies suggest that specific alterations in the brain/behavior processes underlying frustration may offer useful targets for cognitive remediation in children and adolescents with BD.
Cognitive flexibility is defined as the ability to adapt one’s thinking and behavior in response to changing rewards and punishments (78). Cognitive flexibility is relevant to BD because clinical features of BD may reflect specific alterations in how reward inaccurately shapes behavior—i.e., hyper-hedonia in mania (e.g., excessive involvement in pleasurable activities with high potential for painful consequences) and hypo-hedonia in depression (e.g., anhedonia) (78). Cognitive flexibility can be studied using reversal learning tasks. In these, subjects use trial-and-error learning to determine first, that an object is initially rewarded, and then, that this stimulus/reward relationship had switched, so that the previously rewarded object is now punished and vice versa.
Thus far, studies suggest that youth with BD may have specific alterations in the brain/behavior interactions mediating cognitive flexibility and reversal learning. For example, youth with BD have impaired cognitive flexibility and reversal learning vs. both TDC participants and also SMD youths (79–82). This remains true when using a second behavioral task that increases task difficulty by adding probabilistic feedback—i.e., blocks of trials when a participant receives accurate feedback 80% of the time but inaccurate feedback 20% of the time (79–82). FMRI studies have shown that youth with BD had the opposite neural response as TDC participants during reversal learning (83). While controls show the expected pattern (greater activation when acquiring the initial stimulus/response relationship vs. the reversal), pediatric BD participants had the exact opposite neural pattern (greater activation in reversal than acquisition). Another study showed that this pattern in youth with BD differed from that among SMD youths (84).
In sum, each of these four cognitive processes—emotional face processing, response inhibition, frustration, and cognitive flexibility—holds promise as a potential target for cognitive remediation in youth with BD. Future work is required to design cognitive remediation strategies for each of these processes, and to test if such programs engage the underlying circuitry proximal to these processes and result in distal improvements in symptoms or functioning.
CONCLUSION
In conclusion, cognitive remediation for psychiatric disorders is a burgeoning area of research. Leveraging what is known about the brain/behavior interactions underlying BD in children and adolescents, there are many potential cognitive processes that might be amenable to retraining. Guided by cognitive remediation studies in other psychiatric disorders and also in adults, cognitive remediation is likely to play an important role in the treatment of pediatric BD in the future. Such work represents a synergistic union of affective neuroscience techniques and treatment studies as well as between academic and industry research, united towards improving the lives of children and families struggling with BD.
Acknowledgments
Disclosure information:
Dan Dickstein has the following disclosures:
NIMH, Researcher, Grant (5R21MH096850)
NIMH, Researcher, Grant (5R01MH087513)
Footnotes
Grace Cushman, Kerri Kim, Alexandra Weissman and Ezra Wegbreit have nothing to disclose.
Reference List
- 1.Blader JC, Carlson GA. Increased Rates of Bipolar Disorder Diagnoses Among U.S. Child, Adolescent, and Adult Inpatients, 1996–2004. Biol Psychiatry. 2007;62(2):107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 3.Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011 doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Ruan WJ, et al. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(10):1205–1215. doi: 10.4088/jcp.v66n1001. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein DP, Rich BA, Binstock AB, Pradella AG, Towbin KE, Pine DS, et al. Comorbid anxiety in phenotypes of pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2005;15(4):534–548. doi: 10.1089/cap.2005.15.534. [DOI] [PubMed] [Google Scholar]
- 6.Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein TR, Ha W, Axelson DA, Goldstein BI, Liao F, Gill MK, et al. Predictors of prospectively examined suicide attempts among youth with bipolar disorder. Arch Gen Psychiatry. 2012;69(11):1113–1122. doi: 10.1001/archgenpsychiatry.2012.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M, DelBello MP, Pandina G, Kushner S, Van H I, Augustyns I, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2009;11(7):687–700. doi: 10.1111/j.1399-5618.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner KD, Redden L, Kowatch RA, Wilens TE, Segal S, Chang K, et al. A double-blind, randomized, placebo-controlled trial of divalproex extended-release in the treatment of bipolar disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48(5):519–532. doi: 10.1097/CHI.0b013e31819c55ec. [DOI] [PubMed] [Google Scholar]
- 10.Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry. 2012;69(5):515–528. doi: 10.1001/archgenpsychiatry.2011.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miklowitz DJ, George EL, Axelson DA, Kim EY, Birmaher B, Schneck C, et al. Family-focused treatment for adolescents with bipolar disorder. J Affect Disord. 2004;82(Suppl 1):S113–S128. doi: 10.1016/j.jad.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. Child-and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004;43(5):528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Fristad MA. Psychoeducational treatment for school-aged children with bipolar disorder. Dev Psychopathol. 2006;18(4):1289–1306. doi: 10.1017/S0954579406060627. [DOI] [PubMed] [Google Scholar]
- 14.Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Arch Gen Psychiatry. 2009;66(9):1013–1021. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- 15.Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 16.Berry EA, Heaton PT, Kelton CM. National estimates of the inpatient burden of pediatric bipolar disorder in the United States. J Ment Health Policy Econ. 2011;14(3):115–123. [PubMed] [Google Scholar]
- 17.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinogradov S, Fisher M, Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37(1):43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Y, Zarahn E, Hilton HJ, Flynn J, DeLaPaz R, Rakitin B. Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):691–701. doi: 10.1076/jcen.25.5.691.14573. [DOI] [PubMed] [Google Scholar]
- 20.Kontis D, Huddy V, Reeder C, Landau S, Wykes T. Effects of age and cognitive reserve on cognitive remediation therapy outcome in patients with schizophrenia. Am J Geriatr Psychiatry. 2013;21(3):218–230. doi: 10.1016/j.jagp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 21.White HA, Shah P. Training attention-switching ability in adults with ADHD. J Atten Disord. 2006;10(1):44–53. doi: 10.1177/1087054705286063. [DOI] [PubMed] [Google Scholar]
- 22.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 24.Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: a proof of principle study. Psychol Med. 2007;37(9):1229–1238. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- 25.Tchanturia K, Davies H, Campbell IC. Cognitive remediation therapy for patients with anorexia nervosa: preliminary findings. Ann Gen Psychiatry. 2007;6:14. doi: 10.1186/1744-859X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deckersbach T, Nierenberg AA, Kessler R, Lund HG, Ametrano RM, Sachs G, et al. RESEARCH: Cognitive rehabilitation for bipolar disorder: An open trial for employed patients with residual depressive symptoms. CNS Neurosci Ther. 2010;16(5):298–307. doi: 10.1111/j.1755-5949.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowie CR, Gupta M, Holshausen K. Cognitive remediation therapy for mood disorders: rationale, early evidence, and future directions. Can J Psychiatry. 2013;58(6):319–325. doi: 10.1177/070674371305800603. [DOI] [PubMed] [Google Scholar]
- 28.Demant KM, Almer GM, Vinberg M, Kessing LV, Miskowiak KW. Effects of cognitive remediation on cognitive dysfunction in partially or fully remitted patients with bipolar disorder: study protocol for a randomized controlled trial. Trials. 2013;14:378. doi: 10.1186/1745-6215-14-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sole B, Bonnin CM, Mayoral M, Amann BL, Torres I, Gonzalez-Pinto A, et al. Functional remediation for patients with bipolar II disorder: Improvement of functioning and subsyndromal symptoms. Eur Neuropsychopharmacol. 2014 doi: 10.1016/j.euroneuro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Correa MS, da Silveira EM, de Lima DB, Balardin JB, Walz JC, Kapczinski F, et al. The role of encoding strategies in contextual memory deficits in patients with bipolar disorder. Neuropsychol Rehabil. 2015;25(1):122–136. doi: 10.1080/09602011.2014.969281. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 32.Cavedini P, Zorzi C, Bassi T, Gorini A, Baraldi C, Ubbiali A, et al. Decision-making functioning as a predictor of treatment outcome in anorexia nervosa. Psychiatry Res. 2006;145(2–3):179–187. doi: 10.1016/j.psychres.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Garrido G, Barrios M, Penades R, Enriquez M, Garolera M, Aragay N, et al. Computer-assisted cognitive remediation therapy: cognition, self-esteem and quality of life in schizophrenia. Schizophr Res. 2013;150(2–3):563–569. doi: 10.1016/j.schres.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 34.McGurk SR, Mueser KT, DeRosa TJ, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophr Bull. 2009;35(2):319–335. doi: 10.1093/schbul/sbn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel A, Knapp M, Romeo R, Reeder C, Matthiasson P, Everitt B, et al. Cognitive remediation therapy in schizophrenia: cost-effectiveness analysis. Schizophr Res. 2010;120(1–3):217–224. doi: 10.1016/j.schres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Puig O, Penades R, Baeza I, De la SE, Sanchez-Gistau V, Bernardo M, et al. Cognitive remediation therapy in adolescents with early-onset schizophrenia: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53(8):859–868. doi: 10.1016/j.jaac.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Oord SV, Ponsioen AJ, Geurts HM, Brink EL, Prins PJ. A Pilot Study of the Efficacy of a Computerized Executive Functioning Remediation Training With Game Elements for Children With ADHD in an Outpatient Setting: Outcome on Parent- and Teacher-Rated Executive Functioning and ADHD Behavior. J Atten Disord. 2012 doi: 10.1177/1087054712453167. [DOI] [PubMed] [Google Scholar]
- 38.Green CT, Long DL, Green D, Iosif AM, Dixon JF, Miller MR, et al. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics. 2012;9(3):639–648. doi: 10.1007/s13311-012-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray SA, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, et al. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. J Child Psychol Psychiatry. 2012;53(12):1277–1284. doi: 10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 40.Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A, et al. A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. J Child Psychol Psychiatry. 2014;55(3):247–255. doi: 10.1111/jcpp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelham WE, Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31(2):210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD–a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: effective direct intervention for children with ADHD. Child Neuropsychol. 2007;13(4):382–388. doi: 10.1080/09297040600770787. [DOI] [PubMed] [Google Scholar]
- 44.Rapport MD, Orban SA, Kofler MJ, Friedman LM. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes Clin Psychol Rev. 2013;33(8):1237–1252. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Dongen-Boomsma M, Vollebregt MA, Buitelaar JK, Slaats-Willemse D. Working memory training in young children with ADHD: a randomized placebo-controlled trial. J Child Psychol Psychiatry. 2014;55(8):886–896. doi: 10.1111/jcpp.12218. [DOI] [PubMed] [Google Scholar]
- 46.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 47.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24(2):417–425. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 49.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 50.Lindstrom KM, Guyer AE, Mogg K, Bradley BP, Fox NA, Ernst M, et al. Normative data on development of neural and behavioral mechanisms underlying attention orienting toward social-emotional stimuli: an exploratory study. Brain Res. 2009;1292:61–70. doi: 10.1016/j.brainres.2009.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, et al. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shechner T, Rimon-Chakir A, Britton JC, Lotan D, Apter A, Bliese PD, et al. Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53(1):61–71. doi: 10.1016/j.jaac.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Ding Z, Dai T, Peng F, Zhang JX. Attention Bias Modification training in individuals with depressive symptoms: A randomized controlled trial. J Behav Ther Exp Psychiatry. 2014 doi: 10.1016/j.jbtep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Baert S, De Raedt R, Schacht R, Koster EH. Attentional bias training in depression: therapeutic effects depend on depression severity. J Behav Ther Exp Psychiatry. 2010;41(3):265–274. doi: 10.1016/j.jbtep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1–2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 57.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proc Natl Acad Sci U S A. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48(9):863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 59.Seymour KE, Pescosolido MF, Reidy BL, Galvan T, Kim KL, Young M, et al. Emotional Face Identification in Youths with Primary Bipolar Disorder or Primary ADHD. J Am Acad Child Adolesc Psychiatry. 2013 doi: 10.1016/j.jaac.2013.03.011. In Press 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165(3):385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 61.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(6):636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 65.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20(2):529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas LA, Bones BL, Milch HS, Lindstrom KM, Reynolds RC, Marsh AA, et al. Neural engagement to emotinal faces: Bipolar disorder differs from controls and severe mood dysregulation. Development and Psychopathology. 2011 In Press. [Google Scholar]
- 68.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, V.A.: American Psychiatric Association; 2013. [Google Scholar]
- 69.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20(5):751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 70.Verbruggen F, Logan GD. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. J Exp Psychol Gen. 2008;137(4):649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 72.Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, et al. Neural correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20(1):15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123(Pt 6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 74.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 75.Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br J Psychol. 2010;101(Pt 3):383–399. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- 76.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164(2):309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 77.Rich BA, Carver FW, Holroyd T, Rosen HR, Mendoza JK, Cornwell BR, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011 doi: 10.1016/j.jpsychires.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55(1):32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 80.Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162(10):1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 81.Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(3):341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 82.Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, et al. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40(7):1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1173–1185. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


