Abstract
The nucleotides comprising the ribosomal decoding center are highly conserved, as they are important for maintaining translational fidelity. The bacterial A-site has a small base variation as compared with the human analogue, allowing aminoglycoside (AG) antibiotics to selectively bind within this region of the ribosome and negatively affect microbial protein synthesis. Here, by using a fluorescence displacement screening assay, we demonstrate that neomycin B (NEO) dimers connected by L-arginine-containing linkers of varying length and composition bind with higher affinity to model A-site RNAs compared to NEO, with IC50 values ranging from ~40–70 nM, and that a certain range of linker lengths demonstrates a clear preference for the bacterial A-site RNA over the human analogue. Furthermore, AG-modifying enzymes (AMEs), such as AG O-phosphotransferases, which are responsible for conferring antibiotic resistance in many types of infectious bacteria, demonstrate markedly reduced activity against several of the L-arginine-linked NEO dimers in vitro. The antimicrobial activity of these dimers against several bacterial strains is weaker than that of the parent NEO.
Introduction
The growing problem of antibiotic resistance presents an urgent need for the development of new therapeutics to treat bacterial infections. One class of commonly prescribed broad-spectrum antibiotics, the aminoglycosides (AGs), combat Gram-positive and Gram-negative bacteria by selectively binding bacterial ribosomes and inhibiting protein synthesis.1, 2 A particularly vulnerable site for antibiotic targeting is the decoding center of the small ribosomal subunit, which is responsible for matching the correct aminoacyl-transfer (t)RNA with the three-nucleotide codon of the messenger (m)RNA. The binding of AGs to the aminoacyl (A)-site of the ribosome interferes with this recognition process, which results in mistranslation of the mRNA.3–6
Bacterial AG antibiotic resistance is commonly conferred via AG-modifying enzymes (AMEs), namely AG O-phosphotransferases (APHs), AG N-acetyltransferases (AACs), and AG O-nucleotidyltransferases (ANTs), which render their substrate molecules unable to bind at their respective ribosomal sites of action.7–10 Resistant bacterial strains carry plasmids containing genes for these enzymes, and any forthcoming AG antibiotics must be able to bypass this troublesome resistance mechanism.
Therefore, when developing novel AGs, it is crucial that (i) they be highly selective for the bacterial A-site over the human A-site counterpart, as mutations in human mitochondrial ribosomes that result in higher affinity for these ligands often lead to drug toxicity,11, 12 and (ii) they evade the action of the AMEs responsible for the majority of resistance to these drugs. In this work, we have combined the two aforementioned molecular design strategies in order to produce novel compounds that are both poor substrates for AMEs and display increased affinities for their RNA targets over their unmodified parent AG. We report the synthesis and screening of a series of neomycin B (NEO) dimers that are joined by L-arginine-containing linkers of varying lengths. NEO dimers with triazole, urea, and thiourea linkages have been shown to be poor substrates for certain AMEs,13 and both NEO dimers and L-arginine-conjugated AGs have been reported to increase ligand affinity for nucleic acids as compared with their unconjugated counterparts.14–21 Other AG homo- and hetero-dimers have also been reported to display promise in enhancing RNA binding, improving antibiotic activity, and/or resisting the action of AMEs.22–29 Our novel dimers display higher affinities for both human and bacterial RNA A-site model constructs than does NEO, as shown by a fluorescence displacement assay,30 and also show slight binding preferences among the bacterial and human constructs used in this study. Significantly, AMEs display very different activity for these L-arginine-linked NEO dimer substrates than they do for NEO, and notably, these dimers are very poor substrates especially for the AG O-phosphotransferases APH(2″)-Ia and APH(3′)-Ia. Despite these collective differences, the dimers’ antimicrobial activities are comparable to those of NEO.
Results and Discussion
Synthesis of L-Arginine NEO Dimers
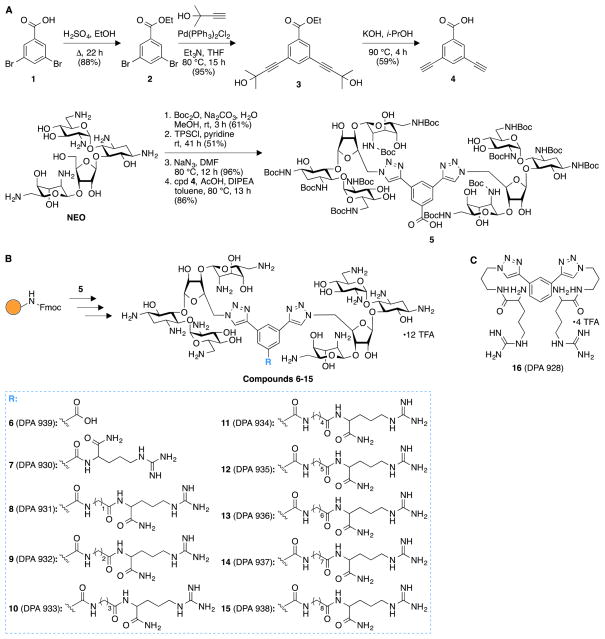
We successfully achieved the synthesis of nine novel triazole-linked L-arginine-NEO dimers (compounds 7-15) by using a high-yielding robust synthetic “click chemistry” approach followed by standard 9-fluorenylmethoxycarbonyl (Fmoc)-based solid-phase peptide synthesis (Fig. 1). Our initial focus was to synthesize the terminal dialkyne linker 4 in order to achieve triazole-linked NEO dimers via click chemistry. To this end, we synthesized compound 4 in two steps from ethyl 3,5- dibromobenzoate (2) via Sonogashira-Hagihara cross-coupling followed by removal of the 2-hydroxyisopropyl group of compound 3 to generate free terminal alkynes in molecule 4 (Fig. 1A). We synthesized the Boc-protected NEO azide from commercially available NEO in three steps31 and further coupled it with the dialkyne compound 4 to successfully produce the triazole-linked NEO dimer 5 via click chemistry (Fig. 1A). The presence of a free carboxyl functional group in 5 proved to be extremely beneficial for further modifications using solid-phase peptide chemistry. To this end, we rapidly generated a library of L-arginine-NEO dimers 7-15 with varying chain lengths by using Fmoc-based solid-phase peptide chemistry (Figs. 1B and S1B). Compound 5 was further deprotected to obtain NEO dimer 6 with a free carboxyl group (Fig. S1A), which was used as a control molecule for the binding study. Detailed experimental procedures and characterization data (NMR spectra and HRMS/MALDI-TOF MS; Figs. S2–S23) for compounds 6-15 are presented in the supporting information.
Fig. 1.
Synthetic schemes for the preparation of A. compound 5, and B. arginine-linked NEO dimers 6-15. C. Structure of compound 16 used as a control in binding studies to model A-sites.
Screening of Compounds 6–16 for Binding to Model A-sites
In order to establish relative affinity of the L-arginine-linked NEO dimers for human and bacterial ribosomal A-sites, we assessed dimers 6-15, NEO, and a control molecule 16 (Fig. 1C), along with a 27-base model A-site RNA oligomers representing E. coli and human rRNA sequences by using a fluorescence displacement assay (Fig. 2).32–34 In this ligand binding assay, F-NEO, a conjugate of NEO and fluorescein, serves as a fluorescent reporter of binding when a ligand displaces it.35 Fluorescence emission intensity of F-NEO is decreased when it is bound within the major groove of RNA, analogously to non-conjugated NEO, as confirmed by docking experiments. Upon displacement from the RNA target oligomer by a competitive binder, the fluorescence of F-NEO increases, thereby reporting on ligand binding.
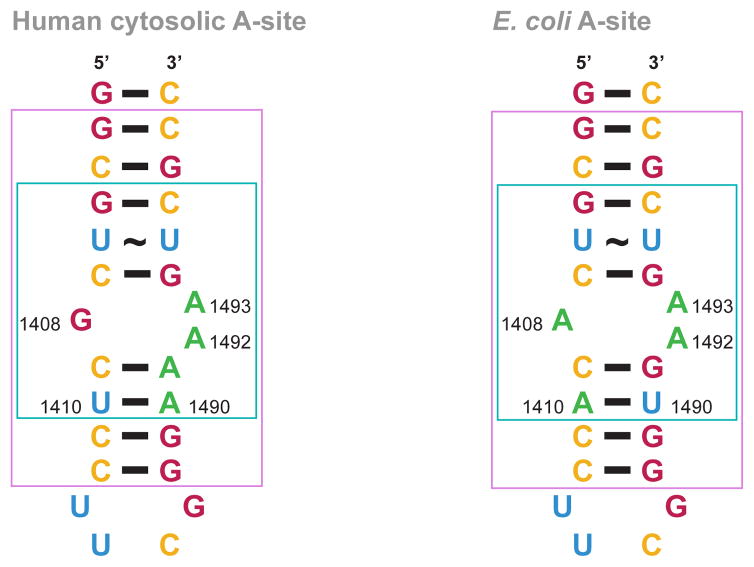
Fig. 2.
rRNA A-site constructs. Nucleotides implicated in AG binding are shown in a turquoise box in the E. coli and human A-site structures. Nucleotides that match part of the crystal structures used for modelling in Fig. 5 are shown in a pink box in the E. coli and human A-site structures. Nucleotides are colored as follow: A = green, G = red, C = yellow, and U = blue.
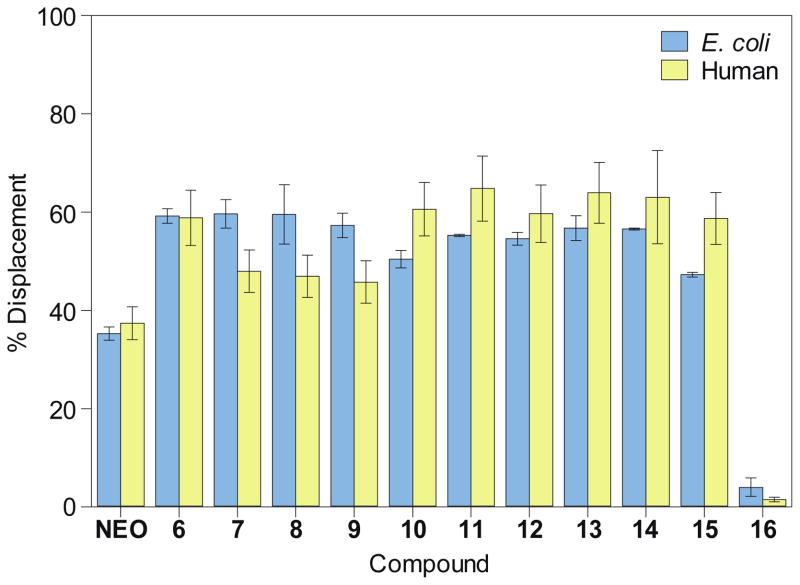
The dissociation constants (Kd) between the F-NEO probe and A-sites measured by direct titrations were 4 ± 1 nM and 23 ± 3 nM for E. coli and human A-sites, respectively. The approximate four-to-six-fold difference between affinities of NEO to E. coli and human A-site has been previously documented.36 We assessed the percentage of displacement of F-NEO by compounds 6-16 by comparison of fluorescence emission of F-NEO (Fig. 3) in the presence of model A-site RNAs and again upon addition of a single concentration of the tested compounds. In order to better distinguish between NEO-like binders to model A-site RNAs, the ligand concentration used in competitive binding experiments was approximately equal to the NEO IC50, where the IC50 concentration is defined as a concentration at which 50% of F-NEO is displaced. No increase in emission due to the displacement of the probe was observed for control molecule 16, indicating that it did not bind appreciably to either of the A-site RNAs. All NEO dimers, 6-15, demonstrated stronger binding preferences for both A-sites as compared with NEO. This result is consistent with previous findings demonstrating that the presence of a single positively-charged arginine enhances the binding ability of NEO dimers to RNAs as compared with NEO alone.31
Fig. 3.
Bar graph showing the percentage of displacement of F-NEO by compounds 6-16. NEO is shown as a control.
We also observed that the linker length affected the binding affinities of the L-arginine-linked NEO dimers to the A-site RNAs as shown by IC50 measurements (Table 1 and Figs. S24–S25). Compounds 13 and 14, with 6 and 7 consecutive carbons within their linkers, respectively, demonstrated the strongest affinity for the E. coli A-site of all the compounds tested. IC50 values were also measured for human A-site. Measured IC50 values of these compounds for the human A-site were similar to those measured for the E. coli A-site (Table 1). The affinities of compounds 7, 11, 12, and 13 were higher for the E. coli A-site than for the human homologue, indicating that the consecutive carbons chains within the arginine linkers have an optimum length of 4–6 carbons, and that the presence of this carbon chain assists in distinction between two A-sites. Since the reported IC50 values were determined by F-NEO displacement and are therefore relative to F-NEO affinity for each RNA, and since F-NEO has a ~5.8-fold larger affinity for the E. coli versus the human A-site RNA, we can report that all of the L-arginine-linked NEO dimers have a higher affinity for the E. coli A-site than does NEO, and most likely preferences for one A-site for another are actually larger than the reported IC50 values imply. Therefore, we have calculated selectivity factor that indicates the preferential affinity ratio of each compound for the E. coli A-site as compared with the human A-site. The affinity of NEO for the E. coli A-site is five times stronger than for the human homologue.
Table 1.
IC50 values (nM) and selectivity factors for E. coli over human A-site of compounds 6-15 and NEO.
| Compound | E. coli A-site | Human A-site | Selectivity factor |
|---|---|---|---|
| NEO | 87 ± 6 | 76 ± 13 | 5.0 |
| 6 | 51 ± 6 | 50 ± 5 | 5.6 |
| 7 | 55 ± 1 | 71 ± 1 | 7.4 |
| 8 | 62 ± 2 | 58 ± 2 | 5.3 |
| 9 | 70 ± 3 | 58 ± 1 | 4.7 |
| 10 | 56 ± 8 | 52 ± 3 | 5.3 |
| 11 | 52 ± 2 | 70 ± 8 | 7.4 |
| 12 | 54 ± 4 | 68 ± 6 | 7.2 |
| 13 | 44 ± 2 | 56 ± 1 | 7.3 |
| 14 | 48 ± 2 | 43 ± 1 | 5.2 |
| 15 | 60 ± 3 | 62 ± 3 | 5.9 |
Antibacterial Activity
Having studied the binding of our compounds to model A-sites, we next investigated our L-arginine-linked NEO dimers 6-15 for their inhibition of antimicrobial growth against three bacterial strains: Bacillus cereus ATCC 11778, Staphylococcus epidermidis ATCC 12228, and Staphylococcus aureus ATCC 25923. The minimum inhibitory concentration (MIC) values for dimers 6-15 and NEO, which served as a control, are summarized in Table 2. Due to the large molecular weight of our compounds (ranging from 2,818 to 3,284 g/mol) we provide the MIC values in μM, which we feel is best suited for larger molecules. However, as it is standard practice to provide MIC values in μg/mL in microbiology, we also included these values into parentheses in Table 2. A range is reported for the concentrations at which a growth inflection point was not observed. While most of the NEO dimers showed little activity against S. epidermidis and S. aureus, all dimers had a profound inhibitory effect on the growth of the spore-forming B. cereus, demonstrating the selectivity of our compounds towards certain bacterial strains. Interestingly, compound 6, which does not contain arginine, showed better inhibition of S. aureus and S. epidermidis than the L-arginine-containing NEO dimers 7-15. All dimers showed promising activity (MIC values ranging from 0.8–13 μM) against B. cereus, with dimers 8 and 9 being the best with MIC values of 1.6 μM and 0.8 μM, respectively. As observed previously, the positive charge of the L-arginine group in the linker is likely to assist with bacterial membrane penetration and binding to the negatively charged RNA.
Table 2.
MIC values in μM (and in μg/mL into parentheses) of compounds 6-15 and NEO.
| Compound |
B. cereus ATCC 11778 |
S. epidermidis ATCC 12228 |
S. aureus ATCC 25923 |
|---|---|---|---|
| NEO | ≤ 0.1 (≤ 0.1) | 0.2 (0.2) | 0.2 (0.2) |
| 6 | 3–6 (9–18) | 13 (35) | 13 (35) |
| 7 | 3–6. (10–19) | > 50 (> 154) | > 50 (> 154) |
| 8 | 1.6 (5) | > 50 (> 157) | 50 (157) |
| 9 | 0.8 (2.5) | 50 (158) | 50 (158) |
| 10 | 1.6–3 (5–10) | > 50 (> 159) | > 50 (> 159) |
| 11 | 3–13 (10–40) | > 50 (> 159) | > 50 (> 159) |
| 12 | 1.6–3 (5–10) | > 50 (> 160) | > 50 (> 160) |
| 13 | 3 (10) | > 50 (> 161) | > 50 (> 161) |
| 14 | 3–6 (10–19) | 25 (81) | > 50 (> 161) |
| 15 | 3–6 (10–20) | 50 (164) | > 50 (> 164) |
Aminoglycoside-Modifying Enzyme (AME) Activity Assays
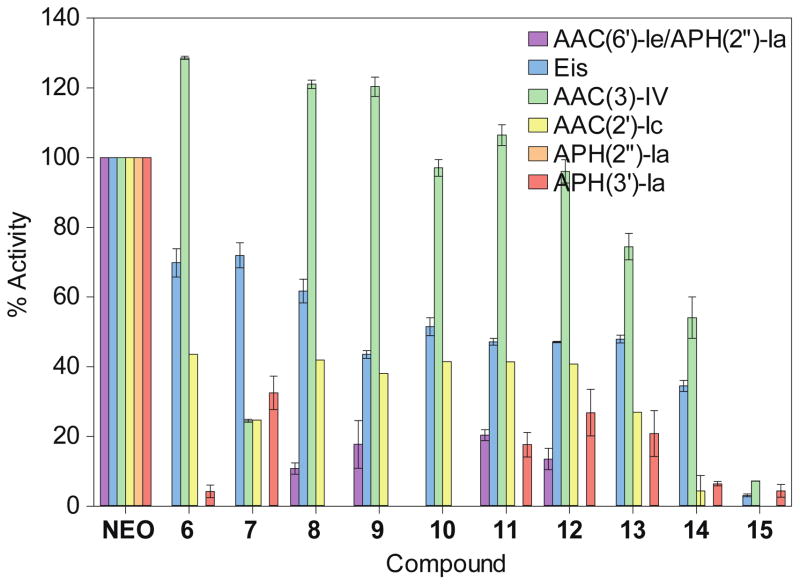
Since the alteration of AG functional groups by AMEs is the most common mechanism of bacterial AG resistance, we finally investigated our NEO dimers 6-15 as potential substrates of N-acetyltransferase (AAC) and O-phosphotransferase (APH) resistance enzymes (Fig. 4). For the AACs, we selected AAC(2′)-Ic from Mycobacterium tuberculosis,37–39 AAC(3)-IV from Escherichia coli,40, 41 and AAC(6′)-Ie from the bifunctional AAC(6′)-Ie/APH(2″)-Ia from S. aureus,40, 42–44 which are known to acetylate NEO at the 2′-, 3, and 6′-position, respectively. We also elected to test against Eis, a unique AAC found in various bacterial strains, which is known to multi-acetylate AGs at a variety of positions.39, 45–49 For the APHs, we selected APH(2″)-Ia and APH(3′)-Ia,13, 50 which commonly phosphorylate NEO. The activity of the enzymes for each compound was normalized to that of NEO. It is important to note that we did not test our NEO dimers 6-15 against ANTs due to the facts that (i) most ANTs modify streptomycin or spectinomycin,7 and (ii) the bacterial strains against which these compounds were tested in this study are not known to contain ANTs that modify NEO.13
Fig. 4.
Bar graph showing the relative initial rates of the listed AMEs with NEO and L-arginine-linked NEO dimers 6-15. All rates were normalized to the parent NEO.
Interestingly, the AAC(3)-IV enzyme that acetylates the 3-amino group of NEO was found to be similarly or more active against certain dimers (6 and 8-12) than NEO. Its activity was found to be inversely proportional to linker length, and gradually decreased with increasing linker length for L-arginine-containing linkers. Compound 7 (n = 0), was modified by AAC(3)-IV at approximately 30% of the rate when compared to the NEO modification.
All other AMEs tested demonstrated much lower enzymatic activity towards compounds 6-15, which were found to be poorer substrates for these enzymes than NEO. The most likely explanation for these positive results is that steric hindrance from the bulky second NEO and/or the increase in molecular dynamics afforded by the flexible linker prevents proper and/or stable binding of the NEO group at the active site of the enzyme. In support of this argument, compound 15 with longest L-arginine tail is the least susceptible substrate for the AMEs tested.
Overall, the O-phosphotransferases APH(2″)-Ia and APH(3′)-Ia, as well as AAC(6′)-Ie/APH-(2″)-Ia showed greatly reduced activity against the dimers. Since phosphorylation of AG functional groups by APH(3′)-Ia is one of the most commonly encountered AG antibiotic resistance mechanisms,10 these results indicate that our L-arginine-linked dimers are a promising avenue for future development as antimicrobial therapeutics.
A Computer Generated Model of Dimer 11 Bound to the Two RNA A-sites
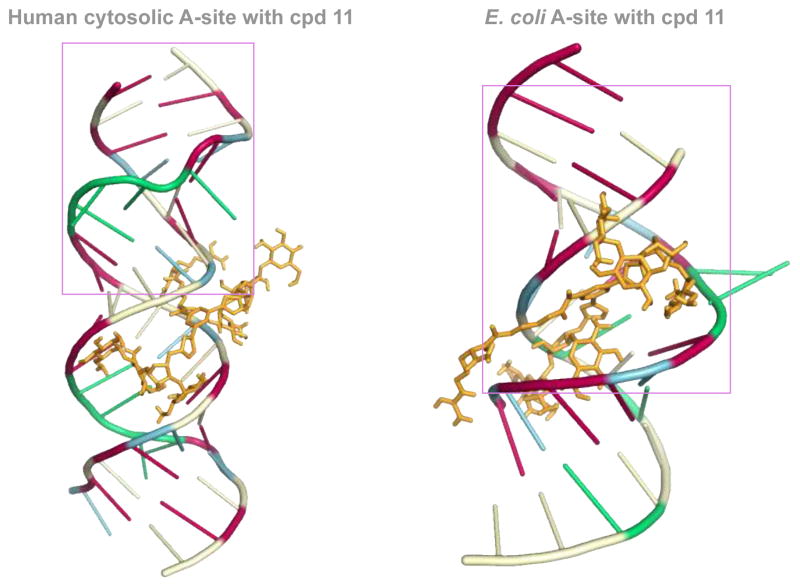
Docking experiments using Auto Dock were performed with dimer 11, as it showed a significant difference in binding between the two A-sites. These docking experiments were performed to offer a potential explanation of our experimental findings. Indeed, different binding modes were observed when we docked 11 with E. coli as well as human RNA A-sites. In the 11-E. coli RNA A-site complex, NEO rings interact in the same region of the RNA (Fig. 5, please see pink boxed region), as established for AG (NEO) binding with E. coli RNA A-site (Fig. 2). However, in the binding of 11 with human RNA A-site, NEO rings interact with RNA bases away from adenines A1490–A1493 (Fig. 5). The difference in binding sites may also cause the observed difference in binding site of the L-arginine containing side chain of 11 (Fig. 5). Together, these two factors likely contribute to the seven-fold difference in binding of 11 with E. coli RNA A-site as compared to human RNA A-site.
Fig. 5.
Docked structures of the L-arginine-linked NEO dimer 11 (depicted as orange stick) with the E. coli (right) and human RNA (left) A-sites. The nucleoside bases are colored as follow: A = green, G = red, C = pale yellow, and U = blue. The pink box represent the residues that are identical to those presented in Fig. 2.
Conclusions
In summary, we have designed a series of NEO dimers with L-arginine-containing linkers of varying lengths to target the ribosomal A-site. These dimers displayed higher affinity for both human and bacterial RNA A-site model constructs than the parent NEO does, as shown by a fluorescence displacement assay. These compounds also showed slight binding preferences among the bacterial and human A-site model constructs, with a carbon linker length of 4–6 being optimal for E. coli A-site preference over the human sequence. The dimers displayed antimicrobial effects that were weaker than their parent AG, NEO. It is interesting to note that dimer 6 also displayed significant antibacterial activity. Significantly, AMEs showed very different activity for the arginine-linked NEO dimer substrates than they do for NEO. As compared with NEO, our dimers are very poor substrates for several AMEs, especially for the phosphotransferases APH(2″)-Ia and APH(3′)-Ia, as well as for the AAC(6′)-Ie/APH-(2″)-Ia. Our L-arginine-linked NEO dimers therefore present a potential avenue for the development of therapeutics to combat AG antibiotic-resistant infections.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants 2R42GM097917 (D.P.A) and AI090048 (S.G.-T.) and by startup funds from the College of Pharmacy at the University of Kentucky (S.G.-T.). We thank Dr. Sayantan Bhaduri for assistance with the description of the synthetic scheme. We thank Dr. Souvik Sur for modelling.
Footnotes
Electronic supplementary information (ESI) available: A figure showing the preparation of compounds 6-15 (Fig. S1) is presented. Experimental procedures and images of spectroscopic characterization of all newly synthesized compounds (Figs. S2–S23) and of IC50 plots (Figs. S24–S25) are also provided.
DPA conceived and designed the dimer compounds and RNA binding experiments. YJ synthesized the dimers. KDG, under SGT supervision, performed MIC values determination against bacterial strains and experiments against AMEs. NND assisted with manuscript preparation. NND and DW performed RNA screening assays. MNS, SGT, and DPA wrote the manuscript. SGT prepared all figures.
Contributor Information
Sylvie Garneau-Tsodikova, Email: sylviegtsodikova@uky.edu.
Dev P. Arya, Email: dparya@clemson.edu.
Notes and references
- 1.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. ChemBio Chem. 2010;11:880–902. doi: 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 2.Fosso MY, Li Y, Garneau-Tsodikova S. MedChemComm. 2014;5:1075–1091. doi: 10.1039/C4MD00163J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazed D, Noller HF. J Mol Biol. 1990;211:135–145. doi: 10.1016/0022-2836(90)90016-F. [DOI] [PubMed] [Google Scholar]
- 4.Moazed D, Noller HF. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 5.De Stasio EA, Moazed D, Noller HF, Dahlberg AE. EMBO J. 1989;8:1213–1216. doi: 10.1002/j.1460-2075.1989.tb03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CH, Hendrix M, Priestley ES, Greenberg WA. Chem Biol. 1998;5:397–406. doi: 10.1016/s1074-5521(98)90073-4. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez MS, Tolmasky ME. Drug Resist Updates. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CA, Baker EN. Curr Drug Targets Infect Disord. 2002;2:143–160. doi: 10.2174/1568005023342533. [DOI] [PubMed] [Google Scholar]
- 9.Labby KJ, Garneau-Tsodikova S. Future Med Chem. 2013;5:1285–1309. doi: 10.4155/fmc.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garneau-Tsodikova S, Labby KJ. MedChemComm. 2015 doi: 10.1039/C5MD00344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Harris KA, Fanning KD, Sarachan KL, Frohlich KM, Agris PF. J Biol Chem. 2015;290:19273–19286. doi: 10.1074/jbc.M115.655092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Fanning K, Harris K, Frohlich K, Agris P. FASEB J. 2015;29:575.520. [Google Scholar]
- 13.Watkins D, Kumar S, Green KD, Arya DP, Garneau-Tsodikova S. Antimicrob Agents Chemother. 2015;59:3899–3905. doi: 10.1128/AAC.00861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litovchick A, Evdokimov AG, Lapidot A. Biochemistry. 2000;39:2838–2852. doi: 10.1021/bi9917885. [DOI] [PubMed] [Google Scholar]
- 15.Litovchick A, Evdokimov AG, Lapidot A. FEBS Lett. 1999;445:73–79. doi: 10.1016/s0014-5793(99)00092-7. [DOI] [PubMed] [Google Scholar]
- 16.Berchanski A, Lapidot A. Bioconjugate Chem. 2008;19:1896–1906. doi: 10.1021/bc800191u. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera C, Gutierrez A, Barretina J, Blanco J, Litovchick A, Lapidot A, Clotet B, Este JA. Antiviral Res. 2002;53:1–8. doi: 10.1016/s0166-3542(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Xue L, Arya DP. J Am Chem Soc. 2011;133:7361–7375. doi: 10.1021/ja108118v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Kellish P, Robinson WE, Jr, Wang D, Appella DH, Arya DP. Biochemistry. 2012;51:2331–2347. doi: 10.1021/bi201657k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Spano MN, Arya DP. Bioorg Med Chem. 2015;23:3105–3109. doi: 10.1016/j.bmc.2015.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King A, Watkins D, Kumar S, Ranjan N, Gong C, Whitlock J, Arya DP. Antimicrob Agents Chemother. 2013;57:4717–4726. doi: 10.1128/AAC.00671-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael K, Wang H, Tor Y. Bioorg Med Chem. 1999;7:1361–1371. doi: 10.1016/s0968-0896(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 23.Sucheck SJ, Wong AL, Koeller KM, Boehr DD, Draker KA, Sears P, Wright GD, Wong CH. J Am Chem Soc. 2000;122:5230–5231. [Google Scholar]
- 24.Agnelli F, Sucheck SJ, Marby KA, Rabuka D, Yao SL, Sears PS, Liang FS, Wong CH. Angew Chem. 2004;43:1562–1566. doi: 10.1002/anie.200353225. [DOI] [PubMed] [Google Scholar]
- 25.Luedtke NW, Liu Q, Tor Y. Biochemistry. 2003;42:11391–11403. doi: 10.1021/bi034766y. [DOI] [PubMed] [Google Scholar]
- 26.Bodlenner A, Alix A, Weibel JM, Pale P, Ennifar E, Paillart JC, Walter P, Marquet R, Dumas P. Org Lett. 2007;9:4415–4418. doi: 10.1021/ol701760k. [DOI] [PubMed] [Google Scholar]
- 27.Santana AG, Batisda A, Del Campo TM, Asensio JL, Revuelta J. Synlett. 2011;2:219–222. [Google Scholar]
- 28.Hanessian S, Maianti JP, Matias RD, Feeney LA, Armstrong ES. Org Lett. 2011;13:6476–6479. doi: 10.1021/ol2027703. [DOI] [PubMed] [Google Scholar]
- 29.Berkov-Zrihen Y, Green KD, Labby KJ, Feldman M, Garneau-Tsodikova S, Fridman M. J Med Chem. 2013;56:5613–5625. doi: 10.1021/jm400707f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins D, Jiang L, Arya DP. PloS One. 2015 doi: 10.1371/journal.pone.0144251. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang L, Watkins D, Jin Y, Gong C, King A, Washington AZ, Green KD, Garneau-Tsodikova S, Oyelere AK, Arya DP. ACS Chem Biol. 2015;10:1278–1289. doi: 10.1021/cb5010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francois B, Russell RJ, Murray JB, Aboul-ela F, Masquida B, Vicens Q, Westhof E. Nucl Acids Res. 2005;33:5677–5690. doi: 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recht MI, Douthwaite S, Dahlquist KD, Puglisi JD. J Mol Biol. 1999;286:33–43. doi: 10.1006/jmbi.1998.2446. [DOI] [PubMed] [Google Scholar]
- 34.Recht MI, Puglisi JD. Antimicrob Agents Chemother. 2001;45:2414–2419. doi: 10.1128/AAC.45.9.2414-2419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins D, Norris FA, Kumar S, Arya DP. Anal Biochem. 2013;434:300–307. doi: 10.1016/j.ab.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu DH, Rando RR. Bioorg Med Chen. 2001;9:2601–2608. doi: 10.1016/s0968-0896(01)00034-7. [DOI] [PubMed] [Google Scholar]
- 37.Ainsa JA, Perez E, Pelicic V, Berthet FX, Gicquel B, Martin C. Mol Microbiol. 1997;24:431–441. doi: 10.1046/j.1365-2958.1997.3471717.x. [DOI] [PubMed] [Google Scholar]
- 38.Vetting MW, Hegde SS, Javid-Majd F, Blanchard JS, Roderick SL. Nat Struct Biol. 2002;9:653–658. doi: 10.1038/nsb830. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S. Proc Natl Acad Sci U S A. 2011;108:9804–9808. doi: 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green KD, Chen W, Houghton JL, Fridman M, Garneau-Tsodikova S. ChemBioChem. 2010;11:119–126. doi: 10.1002/cbic.200900584. [DOI] [PubMed] [Google Scholar]
- 41.Magalhaes ML, Blanchard JS. Biochemistry. 2005;44:16275–16283. doi: 10.1021/bi051777d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daigle DM, Hughes DW, Wright GD. Chem Biol. 1999;6:99–110. doi: 10.1016/S1074-5521(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 43.Boehr DD, Daigle DM, Wright GD. Biochemistry. 2004;43:9846–9855. doi: 10.1021/bi049135y. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell SJ, Berghuis AM. Antimicrob Agents Chemother. 2012;56:1899–1906. doi: 10.1128/AAC.06378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green KD, Biswas T, Chang C, Wu R, Chen W, Janes BK, Chalupska D, Gornicki P, Hanna PC, Tsodikov OV, Joachimiak A, Garneau-Tsodikova S. Biochemistry. 2015;54:3197–3206. doi: 10.1021/acs.biochem.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green KD, Pricer RE, Stewart MN, Garneau-Tsodikova S. ACS Infect Dis. 2015;1:272–283. doi: 10.1021/acsinfecdis.5b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsodikov OV, Green KD, Garneau-Tsodikova S. PLoS One. 2014;9:e92370. doi: 10.1371/journal.pone.0092370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houghton JL, Biswas T, Chen W, Tsodikov OV, Garneau-Tsodikova S. ChemBioChem. 2013;14:2127–2135. doi: 10.1002/cbic.201300359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pricer RE, Houghton JL, Green KD, Mayhoub AS, Garneau-Tsodikova S. Mol BioSyst. 2012;8:3305–3313. doi: 10.1039/c2mb25341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stogios PJ, Spanogiannopoulos P, Evdokimova E, Egorova O, Shakya T, Todorovic N, Capretta A, Wright GD, Savchenko A. Biochem J. 2013;454:191–200. doi: 10.1042/BJ20130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.