Abstract
Aim:
Pirt is a two-transmembrane domain protein that regulates the function of a variety of ion channels. Our previous study indicated that Pirt acts as a positive endogenous regulator of the TRPM8 channel. The aim of this study was to investigate the mechanism underlying the regulation of TRPM8 channel by Pirt.
Methods:
HEK293 cells were transfected with TRPM8+Pirt or TRPM8 alone. Menthol (1 mmol/L) was applied through perfusion to induce TRPM8-mediated voltage-dependent currents, which were recorded using a whole-cell recording technique. PIP2 (10 μmol/L) was added into the electrode pipettes (PI was taken as a control). Additionally, cell-attached single-channel recordings were conducted in CHO cells transfected with TRPM8+Pirt or TRPM8 alone, and menthol (1 mmol/L) was added into the pipette solution.
Results:
Either co-transfection with Pirt or intracellular application of PIP2 (but not PI) significantly enhanced menthol-induced TRPM8 currents. Furthermore, Pirt and PIP2 synergistically modulated menthol-induced TRPM8 currents. Single-channel recordings revealed that co-transfection with Pirt significantly increased the single channel conductance.
Conclusion:
Pirt and PIP2 synergistically enhance TRPM8 channel activity, and Pirt regulates TRPM8 channel activity by increasing the single channel conductance.
Keywords: TRPM8, menthol, Pirt, PIP2, PI, whole-cell recording, single channel conductance
Introduction
TRPM8 was first identified as a Ca2+-permeable nonselective cation channel that can be activated by menthol and temperatures below 25 °C1,2. In the peripheral nervous system (PNS), TRPM8 is expressed in the dorsal root ganglion (DRG) and trigeminal ganglia. The gating behavior of TRPM8 is regulated by several exogenous and endogenous factors. For example, the intracellular pH in the physiological range modulates the activation of TRPM8 by icilin and cold temperatures but not menthol3. The lysophospholipids and polyunsaturated fatty acids from phospholipase A2 (PLA2) mediate TRPM8 sensitization and activation4. Moreover, phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2 or PIP2), a common regulator of different ion channels including the transient receptor potential (TRP) channels5,6,7, participates in the activation of TRPM8 by the cold and menthol8.
Phosphoinositide interacting regulator of TRP (Pirt), a two-transmembrane domain protein specifically expressed in the PNS, was first identified as a positive modulator of TRPV1 via binding to PIP29. Pirt-null mice exhibit impaired pain behavior to capsaicin and noxious heat9. In addition, Pirt also contributes to both non-histamine- and histamine-dependent itch sensations10. Recently, we demonstrated that Pirt functions as an endogenous regulator of TRPM811. Pirt−/− mice displayed decreased behavioral responses to cool temperatures compared with Pirt+/+mice. TRPM8 channel currents that were induced by menthol and cool temperatures were significantly reduced in the Pirt−/− DRG neurons. Pirt also inhibits purinergic receptor P2X3 and reduces bladder overactivity12, but unlike the Pirt positive regulation of TRPV1, this inhibitory effect is mediated through the interaction between the Pirt N-terminal and P2X312.
Pirt promotes TRPM8 channel activity11, but the underlying mechanism remains unclear. Here, we investigated the role of PIP2 and Pirt in the regulation of TRPM8 channel activity in a heterologous cell line. We demonstrated that both Pirt and PIP2 observably increased the voltage-dependent current of TRPM8 and its sensitivity to menthol. Moreover, Pirt and PIP2 displayed a synergistic regulatory effect on TRPM8. Lastly, Pirt enhanced the activity of the TRPM8 channel via increasing its conductance and change in open probability.
Materials and methods
Cell culture
Human embryonic kidney (HEK) 293 cells were obtained from Dr Paul Worley's laboratory at Johns Hopkins University School of Medicine. The growth medium of HEK293 cells consisted of 90% DMEM, 10% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin-glutamine (Invitrogen). The cells were cultured at 37 °C in the presence of 95% O2 and 5% CO2. HEK293 cells were transfected with TRPM8+Pirt and TRPM8 alone mouse plasmids. An enhanced green fluorescent protein (GFP) cDNA tag was also used to confirm successfully transfected cells. Lipofectamine 2000 (Invitrogen) was used to deliver the cDNA. After 18–24 h, the cells were plated onto glass coverslips coated with poly-D-lysine and laminin.
Chinese hamster ovary (CHO) cells were cultured in a medium consisting of 90% DMEM, 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. GlutaMAX (Gibco 35050-061) was also added. The CHO cells were cultured using the same methods as for the HEK cells. The single-channel recording experiments were performed after an additional 1–2 d.
Electrophysiological recording
Whole-cell recordings were performed in HEK293 cells transfected with TRPM8+Pirt+GFP and TRPM8+GFP. The transfected cells were identified using GFP fluorescence and were selected for recording. To diminish the effect of drugs used in the previous cell recording, only one cell per coverslip was tested. The current signal was collected and measured with an Axon 700B amplifier and the pClamp9.2 software package (Axon Instrument), respectively. The electrodes were pulled (Sutter, model P-97) from borosilicate glass (Sutter Inc) and had a resistance of 2–4 MΩ. The series resistance was usually less than 10 MΩ and was not compensated. All experiments were performed at room temperature (∼25 °C). The electrophysiological recordings were performed by one individual who was blinded to the genotype. After breaking the cell membrane and forming a stable whole-cell recording configuration, the capacitance values were obtained from the amplifier.
For whole-cell voltage-clamp recordings, the train of voltage steps ranged from −100 mV to +100 mV with 20 mV step sizes that lasted 20 ms. To confirm the specificity of the TRPM8 channel to the stimulation of the voltage changes, a train of voltage step stimulations were provided at the peak with 1 mmol/L menthol inducing an inward current. The holding potential was kept at −60 mV when various voltage stimuli were applied to the recorded cells. Whole-cell recording data were sampled at 2 kHz and filtered at 0.5 kHz for analysis.
For singe-channel recording, the cell-attached recording mode was adapted in CHO cells expressing TRPM8+Pirt and TRPM8 alone. Menthol (1 mmol/L) was added into the electrode pipette to detect TRPM8 channel activity, but it was absent from the bath solution. Voltage steps ranging from −80 mV to +80 mV with a 20 mV step size were performed to analyze TRPM8 single-channel activity. The single-channel currents were sampled at 0.5 kHz and filtered with an 8-pole with a low pass Bessel filter at 0.1 kHz. As the dwelling time of TPRM8 single-currents was >0.5 s, the single-channel unitary current (i) was determined from the best-fit Gaussian distribution of amplitude histograms. Single-channel activity was calculated from NPo=I/i, where I is the mean total current in a patch, and i is the unitary current at this voltage. The open probability (Po) for the main conductance is presented in the figures. For single-channel slope conductance, linear fitting was used separately for the positive and negative holding potentials. The single-channel recording data were analyzed with Clampfit 9.2 software. The “event detection” function option in the main menu was used to analyze the single channel current traces, determine the number of channels in the patch (N), and calculate the conductance.
Drugs and solutions
For the whole-cell recordings, HEK293 cells were perfused with an extracellular solution comprising 140 mmol/L NaCl, 4 mmol/L KCl, 2 mmol/L CaCl2, 2 mmol/L MgCl2, 10 mmol/L HEPES, and 5 mmol/L glucose, and the pH was adjusted to 7.38 using NaOH. The intracellular pipette solution comprised 135 mmol/L KCl, 3 mmol/L MgATP, 0.5 mmol/L Na2ATP, 1.1 mmol/L CaCl2, 2 mmol/L EGTA, and 5 mmol/L glucose. The pH was adjusted to 7.38 using KOH, and the osmolarity was adjusted to 300 mOsm using sucrose. For the PIP2 and PI experiments, 10 μmol/L PIP2-diC8 or PI-diC8 was added to the intracellular pipette solution. Menthol was used as a TRPM8 agonist. It was dissolved in ethanol (100 mg/mL), stored at −20 °C, and diluted with extracellular solution to different concentrations. All of the solutions were applied to the cells at a rate of 1 mL/min via a gravity-fed perfusion system (VC-6 Six Channel Valve Controller, Warner Instruments)
For the single-channel recordings, the bath solution for recording CHO cells comprised 100 mmol/L K-gluconate, 4 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L EGTA, 10 mmol/L glucose, and 10 mmol/L HEPES (pH 7.3). The pipette solution for the single-channel recordings included 100 mmol/L Na-gluconate, 10 mmol/L NaCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 10 mmol/L glucose, and 10 mmol/L HEPES (pH 7.3).
Data analysis
The electrophysiological data were analyzed and fitted using Clampfit (Axon instruments, Foster city, CA, USA) and Origin Pro 8 (Origin Lab, USA) software. All of the data were analyzed with an unpaired Student's t-test and expressed as the mean±standard errors of the means (SEM). The statistical significance was set at P<0.05.
Results
Pirt enhances TRPM8-mediated voltage-dependent currents via PIP2
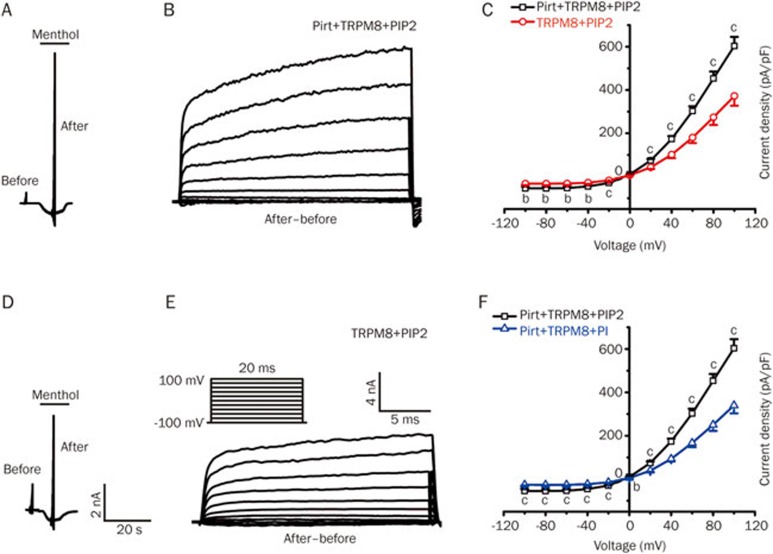
We previously demonstrated that Pirt increased TRPM8-mediated currents via positively charged amino acids residues in its C terminal11. To investigate the effect of PIP2 on Pirt-mediated regulation of TRPM8, we increased the intracellular PIP2 concentration by adding 10 μmol/L of PIP2 into the electrode pipette. A 10 μmol/L PI solution was used as a control. We measured the current–voltage relationships in HEK293 cells expressing TRPM8+Pirt and TRPM8 alone. In the recordings, the depolarizing voltage steps ranging from −100 mV to +100 mV were applied to induce currents in the presence of 1 mmol/L menthol. The background current was examined prior to the addition of menthol, and an identical protocol was performed when the inward current reached the maximum menthol perfusion, which indicated the maximum opening state of the TRPM8 channel (Figure 1A and 1D). The subtraction of two currents that were evoked by the background current (before) and the menthol inward currents (after) should identify a relatively pure TRPM8-mediated voltage-dependent current (Figure 1B and 1E). We observed that the current density was significantly larger in TRPM8+Pirt cells than in TRPM8 cells at potentials ranging from −100 mV to +100 mV (Figure 1C). In cells transfected with TRPM8+Pirt, the current density was significantly greater with the addition of PIP2 than with the addition of PI (Figure 1F). Taken together, these results indicate that Pirt may enhance TRPM8-mediated voltage-dependent currents via PIP2.
Figure 1.
Pirt increases the TRPM8-mediated voltage-dependent currents via PIP2. (A) Representative current traces from TRPM8 stably expressed in HEK293 cells co-transfected with Pirt (Pirt+TRPM8+PIP2, n=21), where PIP2 (10 μmol/L) was added into the electrode pipette. Whole-cell inward currents evoked by 1 mmol/L menthol were analyzed using a series of 20-ms step pulses (−100 mV to +100 mV in 20 mV steps from −60 mV holding potential; the protocol is shown in Figure 1E). Before, background currents; after, currents elicited during menthol perfusion. (B) The net currents mediated by TRPM8 were calculated from (A). (C) I-V curves constructed from TRPM8-mediated net currents (after–before) for HEK293 cells stably expressing TPRM8 alone (TRPM8+PIP2, n=21, red line) or co-transfected with Pirt (Pirt+TRPM8+PIP2, n=21, black line). PIP2 (10 μmol/L) was added into the electrode pipette. (D) Menthol (1 mmol/L)-evoked inward current responses of HEK293 cells stably expressing TRPM8 alone (TRPM8+PIP2, n=21) were analyzed with the same protocol described in (A), where PIP2 (10 μmol/L) was added into the electrode pipette. (E) The net currents mediated by TRPM8 were calculated from (D). (F) I-V curves constructed from TRPM8-mediated net currents (after–before) for HEK293 cells stably expressing TPRM8 co-transfected with Pirt. PIP2 (10 μmol/L, Pirt+TRPM8+PIP2, n=21, black line) and PI (10 μmol/L, Pirt+TRPM8+PI, n=26, blue line, the representative current traces are not shown) were added into the electrode pipette. The reversal potentials for groups (C and F) of cells were close to 0 mV, characteristic of a TRPM8-mediated current. Each value is presented as the mean±SEM. bP<0.05, cP<0.01, unpaired t-test.
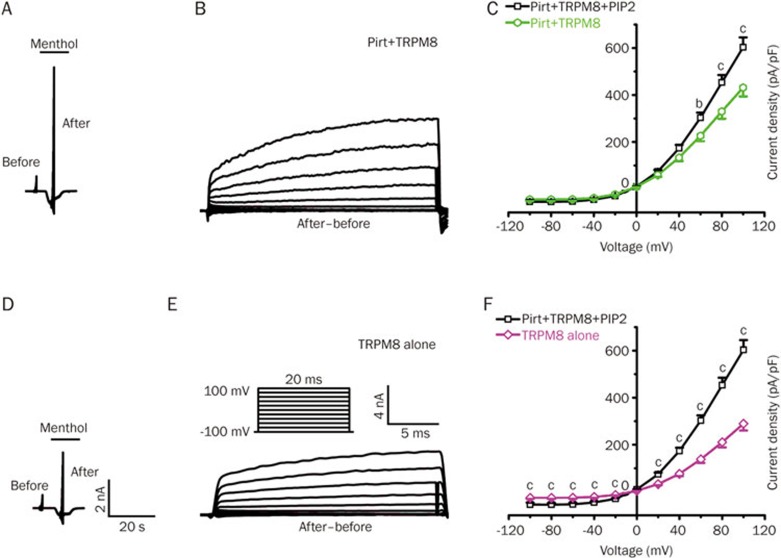
Pirt and PIP2 contribute synergistically to the TRPM8-mediated voltage-dependent current
As we previously demonstrated that Pirt could bind PIP2 and form a complex with TRPM89, we hypothesized that Pirt and PIP2 contributed synergistically to TRPM8-mediated voltage-dependent activity. We observed that Pirt-overexpressing cells exhibited a significantly higher TRPM8-mediated voltage-dependent current density in the presence of PIP2 compared to the PI control (Figure 2A, 2B and 2C). Furthermore, compared with TRPM8 alone, Pirt+TRPM8+PIP2 exhibited a significantly larger current density in the whole range of holding voltage potentials except at 0 mV, which indicates the reversal potential of most TRP channels (Figure 2D, 2E and 2F). Taken together, these data suggest that Pirt and PIP2 contribute synergistically to modulate the TRPM8-mediated voltage-dependent current.
Figure 2.
Pirt and PIP2 both modulate the TRPM8-mediated voltage-dependent current. (A) Representative current traces from HEK293 cells stably expressing TRPM8 co-transfected with Pirt (Pirt+TRPM8, n=29). (B) The net currents mediated by TRPM8 were calculated from (A). (C) I-V curves constructed from TRPM8-mediated net currents (after–before) for HEK293 cells stably expressing TPRM8 co-transfected with Pirt. PIP2 was added into the electrode pipette (Pirt+TRPM8+PIP2, n=21, black line) or not (Pirt+TRPM8, n=29, green line). (D) Menthol (1 mmol/L)-evoked inward current responses of HEK293 cells stably expressing TRPM8 alone (TRPM8 alone, n=28). (E) The net currents mediated by TRPM8 were calculated from (D). (F) I-V curves constructed from TRPM8-mediated net currents (after–before) for HEK293 cells stably expressing TPRM8 between Pirt+TRPM8+PIP2 (n=21, black line) and TRPM8 alone (n=28, purple line). Similar to Figure 1, The reversal potentials for groups (C and F) of cells were close to 0 mV. Each value is presented as the mean±SEM. bP<0.05, cP<0.01, unpaired t-test.
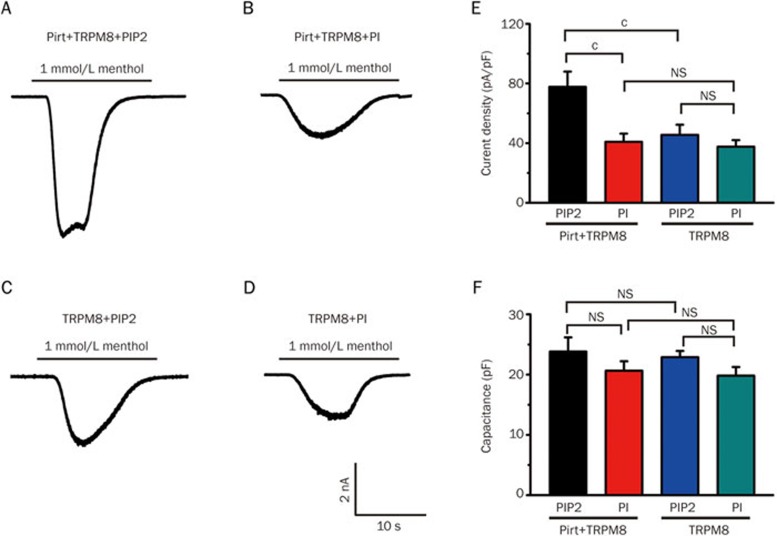
PIP2 but not PI participates in the regulation of Pirt-mediated TRPM8
As we observed that Pirt and PIP2 enhanced the excitability of the TRPM8 channel in a voltage-dependent manner, we speculated that Pirt and PIP2 could form a complex that regulated TRPM8 function. We measured the TRPM8-mediated inward current in the presence of 1 mmol/L menthol applied to the bath solution. In this test, all recording cells were held at −60 mV at voltage-clamp. Similar to the previous test, PI was added into the electrode pipette solution during the recording process, and the same stimulus protocol was adopted. The current density did not significantly differ between TRPM8+Pirt and TRPM8 alone after PI was added into the electrode pipette solution (Figure 3B, 3D and 3E). Similarly, in the TRPM8 alone overexpression cells, PIP2 and PI did not cause observable differences in the inward current (Figure 3E). In addition, we measured the capacitance of all recorded cells and observed no significant difference among these groups (Figure 3F). However, the inward current induced by menthol was significantly larger in the presence of PIP2 than in the presence of PI (Figure 3A, 3B and 3E). When the intracellular solution included PIP2, the inward current was significantly larger in the cells overexpressing TRPM8+Pirt than in those overexpressing TRPM8 alone (Figure 3A, 3C and 3E). Thus, these data suggest that Pirt enhances TRPM8 activity in response to menthol via PIP2 but not PI.
Figure 3.
Pirt enhances TRPM8 sensitivity in response to menthol via PIP2 but not PI. (A) Representative inward current response to 1 mmol/L menthol in HEK293 cells stably expressing TRPM8 co-transfected with Pirt, where PIP2 (10 μmol/L) was added into the electrode pipette (Pirt+TRPM8+PIP2, n=12). (B) Representative inward current response to 1 mmol/L menthol in HEK293 cells stably expressing TRPM8 co-transfected with Pirt, where PI (10 μmol/L) was added into the electrode pipette (Pirt+TRPM8+PI, n=17). (C) Representative inward current response to 1 mmol/L menthol in HEK293 cells stably expressing TRPM8 without Pirt, where PIP2 (10 μmol/L) was added into electrode pipette (TRPM8+PIP2, n=13). (D) Representative inward current response to 1 mmol/L menthol in TRPM8 stably transfected HEK293 cells without Pirt, where the PI (10 μmol/L) was added into the electrode pipette (TRPM8+PI, n=12). (E) The current density among groups (A, B, C, and D) calculated from the current amplitude normalized to the cell capacitance was statistically compared. (F) The cell capacitance for four groups (A, B, C, and D) was statistically compared. The value is presented as the mean±SEM. cP<0.01, unpaired t-test. NS, non-significant difference.
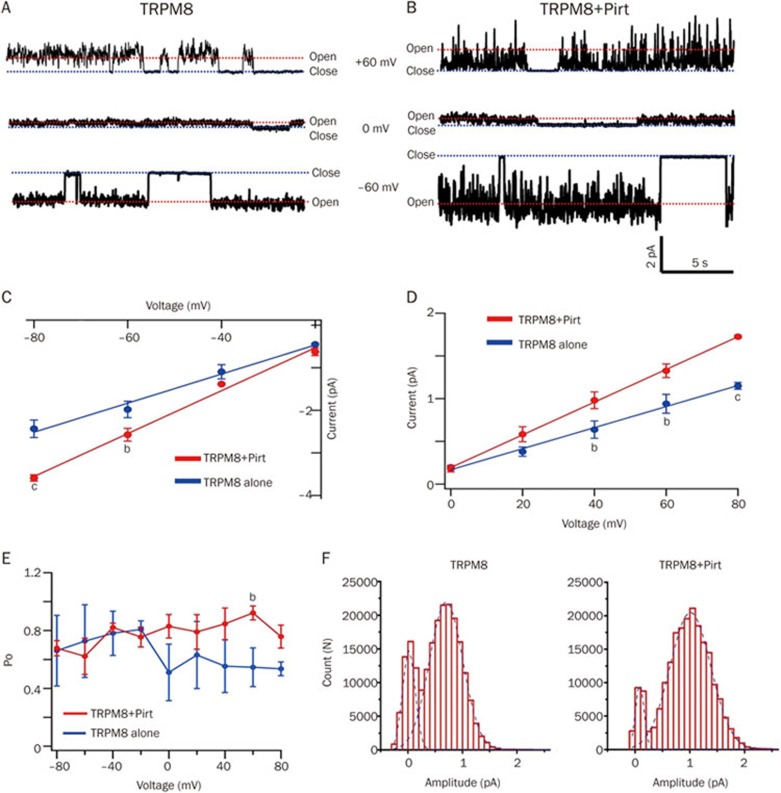
Pirt increases the conductance of TRPM8 at a single channel level
We previously demonstrated that Pirt increased the TRPM8 channel activity in response to electrical and chemical stimuli, but it did not affect the expression level of the TRPM8 protein11. Therefore, we hypothesized that Pirt might modulate the gating properties of the TRPM8 channel. To test this hypothesis, we performed cell-attached single channel recordings in CHO cells overexpressing TRPM8+Pirt and TRPM8 alone. As expected, when 1 mmol/L of menthol was added to the patch electrodes solution, various unitary currents were observed in both the cells overexpressing TRPM8+Pirt and TRPM8 alone at various holding potentials (Figure 4A and 4B). In contrast to TRPM8 alone cells, the unitary current was robustly enhanced in cells overexpressing TRPM8+Pirt at −60 mV and +60 mV (Figure 4A and 4B). At negative holding voltage ranges, the channel conductance was significantly larger in TRPM8+Pirt cells than in TRPM8 alone cells at −80 and −60 mV holding potentials (Figure 4C). The conductance was also significantly larger in TRPM8+Pirt cells than in TRPM8 alone cells at +40 mV to + 80 mV holding potentials (Figure 4D). These data indicate that Pirt increases TRPM8 single channel conductance. In regard to the open probability of the channel, we did not observe significant differences between TRPM8+Pirt cells and TRPM8 alone cells at most holding potentials except at +60 mV (Figure 4E). The number of open channels in the TRPM8+Pirt group and the TRPM8 alone group were plotted as Gaussian distributions. Pirt observably shifted the fitted curve to the right (Figure 4F). Taken together, these data suggest that Pirt increases the activity of TRPM8 by changing the conductance of the channel.
Figure 4.
The unitary current trace of TPRM8 in transfected CHO cells at different holding potentials. (A) Representative single-channel trace of CHO cells stably expressing TRPM8 without Pirt at voltages ranging from +60 mV to −60 mV (n=5). Red and blue dashed lines represent the open and closed conditions of TRPM8, respectively. (B) Representative unitary trace of TRPM8 with Pirt (n=5). (C) At negative holding potential ranges from −80 mV to −20 mV, single channel current-voltage plots of TRPM8 without Pirt (blue line) and TRPM8 with Pirt (red line). The data are fitted to a linear function with a slope of 37.31±1.2 ps (TRPM8 without Pirt, n=5) and 50.36±1.95 ps (TRPM8 with Pirt, n=5). (D) At positive holding potential ranges from +20 mV to +80 mV, single channel current-voltage plots of TRPM8 without Pirt (blue line, n=5) and TRPM8 with Pirt (red line, n=5). (E) The open probability of the TRPM8 channel co-transfected with Pirt (red line, n=5) or not (blue line, n=5) at different holding potentials. (F) All-point histogram of the TRPM8 channel co-transfected with Pirt (right, n=5) or without (left, n=5). The mean current amplitude at each point is obtained through the all-point histogram and fit with a Gaussian distribution. Each value is presented as the mean±SEM. bP<0.01, cP<0.01, unpaired t-test.
Discussion
PIP2 has been shown to modulate the physiological function of a variety of ion channels by acting as the substrate for cleavage by the enzyme phospholipase C (PLC)5,6. PIP2 has been proposed to underlie the regulation of inwardly rectifying K+ (Kir) channels by diverse factors such as G protein, sodium, and magnesium ions, phosphorylation, and pH13,14,15,16. PIP2 also regulates various TRP channels by numerous exogenous and endogenous ligands and physical stimuli17,18,19,20,21,22,23,24. We previously demonstrated that Pirt directly enhanced the activity of the TRPM8 channel as an endogenous regulatory factor11. In the present study, we observed that PIP2 robustly increased the Pirt's regulatory function of TRPM8. Although we know that PIP2 and Pirt could interact with each other, it was not clear how a PIP2–Pirt enhanced the activity of TRPM8. PIP2 and Pirt may form a complex that binds to the TRP domain of TRPM8 channel to regulate the function of TRPM8 by changing the properties of the gated channel.
We previously determined that Pirt positively regulates the activity of TRPM8 but does not change the expression level of the TRPM8 protein11. Therefore, we propose that Pirt improves the voltage-dependent activity of TRPM8 upon menthol stimulation by changing the gating properties of the TRPM8 channel. In our cell-attached single channel recording test, we obtained single-channel data from CHO cells overexpressing TRPM8+Pirt and TRPM8 alone and analyzed the gating properties of TRPM8. We observed that the conductance of the TRPM8 channel was significantly augmented in cells overexpressing TRPM8+Pirt compared with cells overexpressing TRPM8 alone (Figure 4C and 4D), indicating that the ion channel pore size was enlarged in the presence of a Pirt–TRPM8 interaction. However, the open probability of the TRPM8 channel did not change observably at different pipette potentials from −80 mV to +80 mV, except at +60 mV (Figure 4E). In addition, the Gauss distribution of the channel open probability was also shifted to the right in TRPM8+Pirt cells (Figure 4F), indicating that more channels were not fully opened in TRPM8+Pirt cells than in TRPM8 alone cells. These multiple sub-conductance characteristics likely cause the larger current observed in TRPM8+Pirt overexpressing cells. Therefore, Pirt enhances the current of the TRPM8 channel mainly through raising the conductance.
In summary, PIP2 and Pirt together robustly increased the excitability of the TRPM8 channel likely through forming a complex. Moreover, Pirt enhanced the current of TRPM8 mainly through improving the conductance of the channel.
Author contribution
Min TANG performed the experiment and wrote the manuscript; Guang-yi WU helped to perform the electrophysiological experiment; Xin-zhong DONG and Zong-xiang TANG designed the study; Zong-xiang TANG wrote the final manuscript.
Acknowledgments
This work was supported by the National Science Foundation of China to Zong-xiang TANG (31271181 and 31471007), by Oversea, Hong Kong & Macao Scholars Collaborated Researching Fund to Xin-zhong DONG and Zong-xiang TANG (31328012), from the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), sponsored by “Qing Lan Project” in Jiangsu Province and from the Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine (TCM) Prevention and Treatment of Tumor, Nanjing University of Chinese Medicine, China. This work was also supported by Cooperative Innovation Center for Molecular Target New Drug Study, Research and Innovation Project of Hunan Province (CX2015B553) to Min TANG
References
- 1McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–8. [DOI] [PubMed] [Google Scholar]
- 2Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–15. [DOI] [PubMed] [Google Scholar]
- 3Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci 2004; 24: 5364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 2007; 27: 3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001; 2001: re19. [DOI] [PubMed] [Google Scholar]
- 6Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 2008; 37: 175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J 2008; 27: 2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci 2010; 30: 12526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, et al. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 2008; 133: 475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Patel KN, Liu Q, Meeker S, Undem BJ, Dong X. Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One 2011; 6: e20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, et al. Pirt functions as an endogenous regulator of TRPM8. Nat Commun 2013; 4: 2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Gao XF, Feng JF, Wang W, Xiang ZH, Liu XJ, Zhu C, et al. Pirt reduces bladder overactivity by inhibiting purinergic receptor P2X3. Nat Commun 2015; 6: 7650. [DOI] [PubMed] [Google Scholar]
- 13Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 1998; 391: 803–6. [DOI] [PubMed] [Google Scholar]
- 14Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci U S A 1998; 95: 1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol 1999; 1: 183–8. [DOI] [PubMed] [Google Scholar]
- 16Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem 2004; 279: 37271–81. [DOI] [PubMed] [Google Scholar]
- 17Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006; 68: 619–47. [DOI] [PubMed] [Google Scholar]
- 18Hardie RC. Regulation of TRP channels via lipid second messengers. Annu Rev Physiol 2003; 65: 735–59. [DOI] [PubMed] [Google Scholar]
- 19Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 2002; 4: 329–36. [DOI] [PubMed] [Google Scholar]
- 20Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A 2003; 100: 15160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001; 411: 957–62. [DOI] [PubMed] [Google Scholar]
- 22Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 2003; 300: 1284–8. [DOI] [PubMed] [Google Scholar]
- 23Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 2006; 128: 509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 2007; 27: 7070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]