Abstract
Ischemia/reperfusion (I/R) injury is the main cause of tissue damage and dysfunction. I/R injury is characterized by Ca2+ overload and production of reactive oxygen species (ROS), which play critical roles in the process of I/R injury to the brain, heart and kidney, but the underlying mechanisms are largely elusive. Recent evidence demonstrates that TRPM2, a Ca2+-permeable cationic channel and ROS sensor, is involved in I/R injury, but whether TRPM2 plays a protective or detrimental role in this process remains controversial. In this review, we discuss the recent progress in understanding the role of TRPM2 in reperfusion process after brain, heart and kidney ischemia and the potential of targeting TRPM2 for the development of therapeutic drugs to treat I/R injury.
Keywords: TRPM2, ischemia/reperfusion injury, cerebral ischemia, cardiac ischemia, renal ischemia, ROS, Ca2+ overload
Introduction
Ischemia is a restriction of the blood supply to tissues, and it results in decreased oxygen and glucose supply to many tissues, such as the brain, heart, kidney, liver, lung, among others1. Ischemia is reversible if the blood flow is restored to the affected tissue, but this causes a secondary effect called ischemia/reperfusion (I/R) injury. I/R injury is characterized by the production of both inflammation factors and reactive oxygen species (ROS), which contributes to tissue damage2,3. In the last several decades, the roles of ROS in I/R injury have been intensively addressed. A large number of studies have demonstrated increased ROS formation during hypoxia/ischemia, and there is ample evidence of a surge in ROS generation with reoxygenation/reperfusion. For example, during the I/R stage, excessive ROS results in cell death by disrupting cellular signaling transduction, activating inflammation factors and inducing lipid peroxidation4,5,6,7. However, the mechanisms for ROS induced cell death during I/R injury are largely elusive.
Recently, emerging evidence indicates that TRPM2, the second member of the TRPM (transient receptor potential melastatin) channel subfamily, is a ROS sensor8,9. As a Ca2+-permeable cationic channel, TRPM2 plays an important role in many physiological and pathological processes by increasing intracellular Ca2+, regulating ROS production or enhancing cytokine production. Although many studies have indicated that TRPM2 performs a detrimental role in I/R injury10,11,12,13, recent studies present evidence to support a role of TRPM2 in protecting the heart from the damage of I/R injury14,15. Due to the complicated function of the TRPM2 channel during the I/R process, it is important to fully understand the intrinsic mechanisms of TRPM2 in I/R injury, which will determine whether the TRPM2 channel is a new therapeutic target for ischemic diseases.
In this review, we mainly focus on the regulation of TRPM2, particularly its role in the reperfusion process after cerebral, cardiac and renal ischemia and its therapeutic potential in I/R injury.
The channel properties of TRPM2
TRPM2 is the second member of the TRPM family of cation channels and is widely expressed in many cell types including brain, heart, hematopoietic, vascular, smooth muscle and endothelial cells8,16,17. As a membrane protein, TRPM2 has not only been described to be present as a Ca2+-permeable channel in plasma membranes, but it has also been found to have a function as a lysosomal calcium release channel in pancreatic β-cells and dendritic cells18,19.
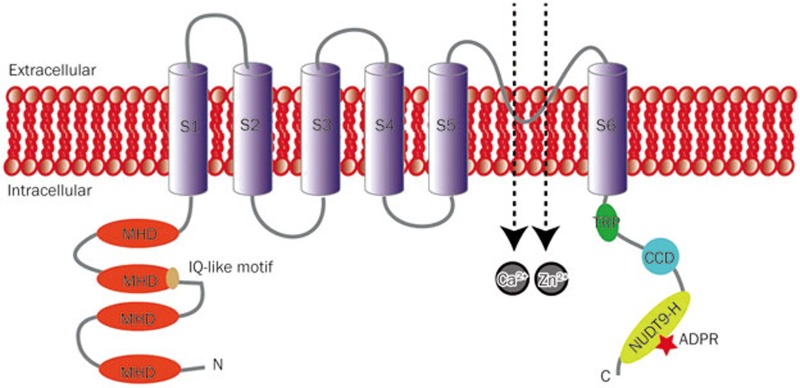
TRPM2 is a homo-tetrameric nonselective cation permeable channel, in which each subunit consists of an intracellular N terminus (approximately 700 amino acids), a region of approximately 300 amino acids containing six transmembrane segments (S1–S6, residues 762-1048) with a pore-forming loop located between the S5 and S6, and a coiled-coil (CC) domain followed by a unique adenosine diphosphate ribose (ADPR) pyrophosphatase homolog domain (NUDT9-H, residues 1236-1503) (Figure 1)8,16,20,21,22. Among these, the N terminus has an IQ-like motif that binds with calmodulin (CaM) located at residues 404-416, and this motif plays an important role in channel facilitation and activation by intracellular calcium (Figure 1)17; the NUDT9-H domain provides the sites for ADPR binding and thus is directly involved in ADPR induced TRPM2 activation (Figure 1)23. In addition to the full-length TRPM2, splicing variants including TRPM2-ΔN, TRPM2-ΔC24, TRPM2-S25, TRPM2-SSF and TRPM2-TE26 have also been identified. Some of these can influence or disrupt the function of the full-length TRPM2, but there is still much uncertainty about the mechanisms by which alternative splicing can alter the isoform expression and the function of these isoforms.
Figure 1.
The topology model of TRPM2 channel. Each subunit of TRPM2 has six transmembrane (TM) spanning domains (S1–S6) with a pore-forming loop between the fifth (S5) and sixth (S6) segments. The intracellular N-terminus has four sections of TRPM subfamily homology domain (MHD) and a IQ-like motif located in the second MHD. The intracellular C-terminus contains a TRP box (TRP) and a coiled-coil domain (CCD). The distal C-terminus has an adenosine diphosphate ribose (ADPR) pyrophosphatase homolog domain (NUDT9-H).
TRPM2 can be activated by many extracellular signals including oxidative stress, tumor necrosis factor-α (TNF-α), amyloid β-peptide (Aβ-42) and concanavalin A (ConA)27, which all result in the production of intracellular ADPR, which, in turn, activates the channel with an EC50 of 1–90 μmol/L and creates substantial permeation to monovalent or divalent cations such as Na+, K+, Zn2+ and Ca2+. The relative permeability of PK/PNa is approximately 1.1, PCa/PNa is approximately 0.9 and PMg/PNa is approximately 0.528. The current displays a characteristic linear current voltage (I-V) relationship with a reversal voltage close to 0 mV, and the single-channel conductance is in the range of 50–80 pS29. In addition to ADPR, some other adenine nucleotide second messengers, which are metabolically related to ADPR, were also reported to have the ability to activate TRPM2, including cyclic ADPR (cADPR; EC50 ∼0.7 mmol/L)9, nicotinic acid adenine dinucleotide phosphate (NAADP; EC50 ∼0.73 mmol/L)30 and nicotinamide adenine dinucleotide (NAD+; EC50 ∼1–1.8 mmol/L)16. However, it still remains unclear whether TRPM2 can be directly activated by these molecules because the contamination with ADPR or the metabolism of the molecules to ADPR under the function of enzyme CD38 may account for the observed TRPM2 activation30,31. Moreover, 2′-O-acetyl-ADP-ribose (OAADPR), a metabolite of the silent information regulator 2 (SIR2) protein deacetylase, can also induce TRPM2 activation by directly binding to the NUDT9-H domain with an EC50 of about 100 μmol/L31.
In addition, Ca2+ plays a critical role in the full activation of TRPM2; in the absence of either external Ca2+ or internal Ca2+, ADPR failed to induce TRPM2 currents17. This is probably due to an increase in the channel sensitivity to ADPR32. It has also been suggested that Ca2+ itself may gate the TRPM2 channel in a concentration-dependent manner with an EC50 of 17 μmol/L; this is possibly a result of conformational changes evoked by a Ca2+-dependent tethering of CaM with the above-mentioned TRPM2 IQ-like motif33.
TRPM2 can also be activated by micromolar levels of H2O2 and agents that produce ROS, which results in a direct link to oxidative stress. However, whether H2O2 directly binds to TRPM2 or gates the channel through H2O2-triggered activation of poly(ADPR)polymerase (PARP)/poly(ADPR) glycohydrolase (PARG) and ADPR, production remains contentious23,24,34,35.
So far, several TRPM2 inhibitors have been identified. Adenosine monophosphate (AMP), generated from ADPR hydrolysis, was the first discovered inhibitor of TRPM2, and it binds to the NUDT9-H domain and competes with ADPR for the binding sites9,18,30,36. Another antagonist, 8-Br-ADPR, can also have a similar competitive inhibitory mechanism, providing strong inhibition of the channel activation by ADPR37. In addition, the inhibition of TRPM2 currents has also been observed with protons and many divalent heavy metal cations including Cu2+, Hg2+, Pb2+, Fe2+, Se2+ and Zn2+, which all target the extracellular pore region38,39,40,41,42,43. Moreover, several structurally unrelated pharmacological agents have been reported to inhibit the TRPM2 channel, including flufenamic acid (FFA), clotrimazole (CTZ), econazole N-(p-amylcinnamoyl) anthranilic acid (ACA) and 2-aminoethoxydiphenyl borate (2-APB)44. However, none of these is specific to the TRPM2 channel. Thus, the development of TRPM2-specific inhibitors is required to better understand the channel functions and for potential therapeutic purposes.
The role of TRPM2 in cerebral ischemia/reperfusion
The extracellular glutamate concentration increases during the initial phase of an ischemic attack, and Ca2+ permeable NMDA receptors (NMDARs) are widely accepted as the key effectors during ischemia. Despite the considerable promise of neuroprotection of NMDAR blockers from in vitro and in vivo animal studies, clinical trials on all NMDAR antagonists were halted due to a lack of efficacy45. This has led researchers to consider other non-selective cation channels as therapeutic targets for ischemia, including acid-sensing ion channels46,47 and TRP channels48,49,50,51. Remarkably, recent studies have shown that nonselective cation channels activated by oxidative stress play a crucial role in cerebral ischemia, especially in the reperfusion phase during which the oxidative stress is dramatically increased52. Increasing oxidative stress and the production of ROS during cerebral ischemia may activate nonselective cation channels such as TRPM2 and TRPM7, contributing to neuronal injury after I/R52. Among these TRP channels, the TRPM2 channel is the most explicitly established channel serving as a cellular redox potential sensor, which confers cells with the ability to sense and respond to oxidative stress8,9,29. Accumulating studies on TRPM2 channels have provided insight into their potential role in I/R injury, and this points to the importance of continued studies on TRPM2 channels. Until now, the in vivo and in vitro evidence has consistently shown that TRPM2 is detrimental in brain ischemia10,11,53,54,55,56,57 (Figure 2).
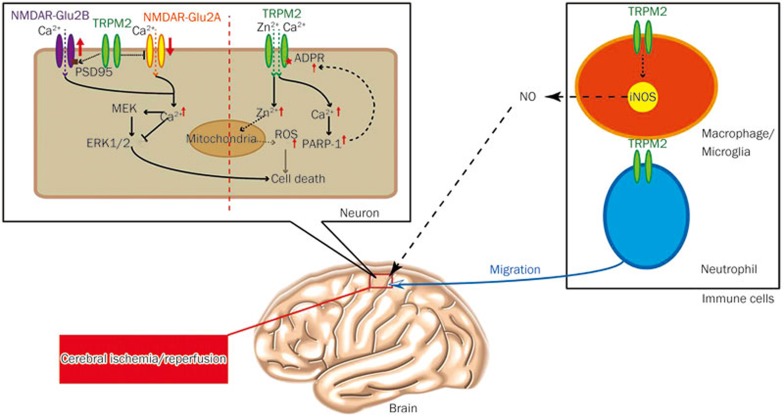
Figure 2.
TRPM2 channels in neurons, microglia and neutrophils play detrimental role in brain ischemic reperfusion injury via multiple signaling pathways.
Although an early study showed upregulated TRPM2 expression weeks after focal cerebral ischemia53, the involvement of TRPM2 channels in ischemic brain injury was first assessed using pharmacological inhibitors and short-hairpin RNA (shRNA)-mediated knockdown of TRPM2 expression, which suggested that TRPM2 was selectively involved in male specific focal ischemic damage54. Later on, by using the patch clamping approach, the same group reported that TRPM2 was selectively activated in male cultured neurons after the reperfusion stage in vitro, which first suggested that TRPM2-mediated reperfusion induced neuronal death55. In addition, a recent study has reported that the inhibition of TRPM2 activity with CTZ reduced male hippocampal CA1-delayed neuronal death when administered with normothermia cardiac arrest for 30 min and followed by a resuscitation (CA/CPR) model of global cerebral ischemia56, and this provided the first evidence for TRPM2 as a target against global cerebral ischemia in the male brain. Afterward, by using the TRPM2 inhibitor CTZ, Shimizu and colleagues reported that the androgen signaling and PARP-1 activity were required for TRPM2-mediated neuronal death in the male brain57.
Because these studies employed non-specific inhibitors or siRNA of TRPM2 to present its role in brain ischemia, recent studies have employed TRPM2-knockout (KO) mice and have also demonstrated that TRPM2 is involved in this process. These studies include the following:
1) Alim and colleagues demonstrated that TRPM2 was responsible for neuronal death induced by transient focal ischemia but not permanent focal ischemia10. This study also showed that the excitability of TRPM2-KO mice was increased compared with that of wild type (WT) mice during redox modulation, which was due to the upregulation of the GluN2A subunit and the downregulation of the GluN2B subunit resulting from TRPM2 deficiency in neurons. Accordingly, they further detected the increase of GluN2A-mediated prosurvival signaling via Akt and the ERK pathway; however, the GluN2B-mediated death signaling pathway was impaired in TRPM2-KO mice. Although this study uncovered a new mechanism for TRPM2 mediated ischemic injury through regulating the expression of NMDARs, the function of TRPM2 itself in ischemia was still elusive.
2) It is well known that intracellular zinc accumulation is critical for delayed CA1 pyramidal neuronal death after global ischemia injury58,59,60,61,62, and previous studies have shown that the Ca2+ permeable AMPA receptor plays an important role in zinc accumulation during global ischemia63,64,65,66. Using a transient global ischemia model in vivo and an oxygen and glucose-deprived model (OGD) in vitro, we have uncovered a new mechanism for TRPM2 involved in ischemia. The TRPM2-KO mice showed a dramatic reduction in CA1 neuronal death and zinc accumulation, and they showed protected learning and memory impairment induced by ischemia injury, indicating that the survived neurons protected by TRPM2 deletion had preserved functional outcomes after ischemia11. Importantly, this finding further clarified that Ca2+-permeable AMPA receptors mediated the increases in the [Zn2+]c during the OGD stage, while the TRPM2 channels were responsible for the increases in both the [Zn2+]c and ROS generation during the reperfusion stage. This supports the notion that Zn2+ induces further ROS production post-ischemia61,64,67,68,69,70,71. This study was the first to provide compelling evidence that TRPM2 activation during reperfusion initiates a vicious positive feedback mechanism that drives the delayed increase in the [Zn2+]c, cell death in CA1 pyramidal neurons, and memory impairment after transient ischemia.
3) Emerging evidence indicates that inflammatory cells play a role in ischemic brain tissue by extending the brain infarction. As mentioned above, TRPM2 is highly expressed in both microglia and macrophages. Recent studies indicate that the TRPM2 in microglia and macrophages was also involved in ischemia injury72,73. Sakimoto and colleagues generated bone marrow chimeric mice by transplanting bone marrow from GFP-WT or GFP-TRPM2-KO mice into irradiated recipients of both genotypes. Their results showed that both central and peripheral deficiency of TRPM2 in microglia/macrophage improved neurological deficits and further suggested that TRPM2 in microglia/macrophage controlled nitric oxide (NO) release-mediated cerebral ischemia injury. Similarly, Gelderblom and colleagues also demonstrated that TRPM2 in neutrophils and macrophages regulated their migration ability to the ischemic brain and thereby resulted in secondary brain injury72. These findings uncovered a new role of TRPM2 in ischemic injury, in which TRPM2 in immune cells regulates NO release and migration of microglia or macrophage. Taken together, these findings demonstrate that the TRPM2 expressed in both neurons and immune cells is detrimental for brain I/R injury, suggesting that TRPM2 is a promising target of brain ischemia injury.
Several non-specific TRPM2 inhibitors, such as CTZ and ACA, used either before or after ischemia have shown protective effects against ischemia54,56,57,72, which is encouraging in the development of specific TRPM2 inhibitors as ischemic therapeutics in the future. Therefore, further investigations looking for specific, safe, and well-tolerable TRPM2 inhibitors are warranted.
The role of TRPM2 in cardiac ischemia/reperfusion
Like brain cells, cardiac tissue is also one of the most sensitive tissues for ischemia51,74. Lethal reperfusion injury is a type of myocardial injury caused by the restoration of coronary blood flow after an ischemic episode. After reperfusion, the constriction ability of cardiomyocytes is maintained in a decreased situation, and this requires several hours or even days to recover. The specific features of this situation include decreased ventricular end diastolic pressure (VEDP), ventricular peal systolic pressure (VPSP) and the first time derivative of left ventricular (LV) pressure rise (dp/dt) max75. Similar to brain ischemia injury, ROS and Ca2+ overloading are also the main causes of cardiac I/R injury.
Oxidative stress is a significant hallmark of reperfusion injury, and it produces ROS, which strongly induces cell injury and even death76. Several molecules downstream of the ROS pathways, including the Rho family of small GTP binding proteins, the Src family of tyrosine kinase and Ras, are critical for H2O2-activated ERK signaling, which exerts a protective function against apoptosis after oxidative stress77.
Ca2+ overloading is another important part of cardiac I/R injury78. Although under normal conditions, myocytes keep a low level of intercellular Ca2+, intracellular Ca2+ is increased in the reperfusion period, which reduces the membrane potential of mitochondria and Ca2+ uptake. This mitochondrial dysfunction not only reduces ATP production and cardiac myocyte bioenergetics maintenance, but it also generates a caspase cascade to induce autophagy and apoptosis24. Ca2+ and ROS play significant roles in myocardial I/R injury, suggesting that the TRPM2 channel is a key player in this physiological and pathological process79. TRPM2 is mainly located in sarcolemma and transverse tubules in mouse left ventricle myocytes14. Several studies have demonstrated that TRPM2 channels are involved in the exacerbation of myocardial I/R injury (Figure 3A). These studies include the following:
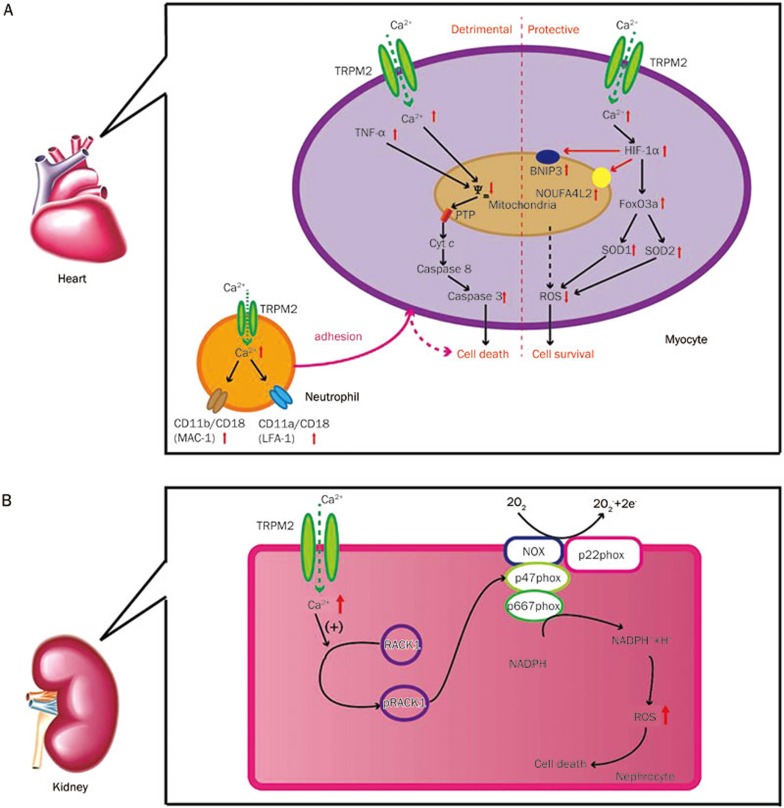
Figure 3.
(A) The schema model of TRPM2 channels mediated detrimental or protective signaling pathways in cardiac ischemic reperfusion injury; (B) TRPM2 channels play a detrimental role in renal ischemic reperfusion injury through regulating RAC1 dependent NADPH oxidase activity.
1) Yang and colleagues reported that TRPM2 and PARP-1 activation were involved in oxidative stress-induced cardiomyocyte death80. By combining treatments with TRPM2 and PARP-1 inhibitors, oxidative stress activated TRPM2 in cultured rat cardiomyocytes, which induced mitochondrial Na+ and Ca2+ overloading, resulting in mitochondrial membrane disruption, cytochrome c release, and caspase-3-dependent apoptosis of cell death.
2) Later, Roberge and colleagues reported another role of TRPM2 in ischemia-induced myocyte death81. As a main factor of ROS, TNF-α induced a non-selective cation current, which is inhibited by TRPM2 inhibitors, caspase-8 inhibitors, PARP-1 inhibitors and antioxidants in isolated myocytes, suggesting that TNF-α activated TRPM2 channels led to a ROS increase, PARP-1 activation and ADPR production by inducing caspase-8 activation. CTZ and TRPM2 inhibitory antibodies decreased TNF-α-induced cardiomyocyte death, suggesting that TRPM2 is involved in TNF-α-mediated myocyte injury. These findings suggest that TRPM2 in myocyte ischemia injury was mainly involved in Ca2+ overloading, which promoted mitochondrial damage and activated an apoptosis signaling pathway.
3) Recently, Hiroi and colleagues showed that TRPM2-KO mice exerted reduced myocardial infarct and improved cardiac contractile function in I/R injury but in not ischemia injury compared with TRPM2 WT mice. Neutrophil activation and infiltration is a hallmark in reperfusion injury, and TRPM2 is highly expressed in polymorphonuclear leucocytes (PMNs)12. To clarify whether neutrophil and/or myocardial TRPM2 channels are implicated in reperfusion injury, hearts isolated from the TRPM2 WT or TRPM2-KO mice were perfused with PMNs from either type of mice. Interestingly, only PMNs from the WT mice resulted in an enlargement in infarct size in both WT and TRPM2-KO hearts, which indicates that TRPM2 activation in PMNs was responsible for cardiac I/R injury12. These findings suggest that TRPM2 is detrimental in cardiac I/R injury.
Nevertheless, recent studies have argued that TRPM2 channels exert a protective function in cardiomyocyte I/R injury (Figure 3A). At rest, there were no differences between the TRPM2-KO and WT mice in body weight and cardiac functions. After I/R injury was induced by occluding the left anterior descending coronary artery for 30 min, infarct size in TRPM2-KO and WT mice was similar, while TRPM2-KO mice exerted lower +dp/dt compared with the WT mice, which indicates that TRPM2 channels protect cardiomyocytes from I/R injury in heart function14. Furthermore, they observed a reduced Ca2+ influx; decreased expression of HIF-1α, FOXOs and SODs; and an increased ROS level in the TRPM2-KO mice after I/R injury, suggesting that a Ca2+ influx mediated by TRPM2 plays an important role in regulating ROS level by controlling the expression of HIF-1α, FOXOs, and SODs in this process. Most recently, the same group has shown that TRPM2-mediated Ca2+ entry protects the heart from I/R injury by not only sustaining mitochondrial function and reducing mitochondrial ROS but also by promoting calcineurin-RACK1-related survival signals15.
Thus far, it is still difficult to determine whether TRPM2 is detrimental or beneficial in cardiac I/R injury. For example, two groups reported opposite results by using TRPM2-KO mice, which might be due to differences in the deleting sequence of TRPM2 in KO mice, differences in the animal models such as time of ischemia (45 vs 30 min) and reperfusion (24 vs 72 h), the anesthesia approach (pentobarbital vs isoflurane), cell types (neutrophil vs myocyte) or different cell lines.
In addition, previous pharmacological and genetic deletion studies have shown the controversial roles of TRPM2 in I/R injury, which could be due to compensatory mechanisms or indirectly regulating other protein expression levels during development. Tissue specific and drug-inducible fine controlling of the TRPM2 expression by the conditional knockout mice approach will be helpful to determine the function of TRPM2 in cardiac I/R injury in the future. Alternatively because treatment with non-specific TRPM2 inhibitors such as ACA improved cardiac function after I/R injury, it will also be helpful to investigate the role of TRPM2 in this process once the specific TRPM2 inhibitor is available in the future.
The role of TRPM2 in renal ischemia/reperfusion
Kidney I/R injury is common in kidney transplants and hemorrhagic shock, which is always followed by acute kidney injury (AKI) and is associated with serious complications and a high mortality rate82. The pathology of I/R injury in kidneys is almost the same as that in the brain and heart, including the generation of ROS, alteration in nucleotide metabolism, and endothelial cell-mediated apoptosis. Immunofluorescence indicated the expression of TRPM2 in renal proximal tubular epithelial cells mainly in the cytoplasm and intracellular organelles but not on the plasma membrane13. Unlike the heart, a recent study showed that the kidney of TRPM2-knockout mice was resistant to ischemia reperfusion injury, as reflected by decreased blood urea nitrogen (BUN) and serum creatinine (SCR) compared with the WT mice13. Several inflammatory indicators were also observed to be decreased in the KO mice, such as lipocalin 2, a marker in kidney injury. As mentioned above, neutrophil infiltration is one of the causes of ischemia injury in organs including the kidney6. In TRPM2-KO mice, there were significantly fewer neutrophils infiltrating the kidney tissue compared with the WT mice after I/R injury. Pharmacological inhibitors of TRPM2 channels demonstrated similar protective effects in kidney I/R injury13,83,84. These observations indicate that the TRPM2 channels have a harmful function in kidney I/R injury. This study showed that TRPM2 promoted RAC1 activation, and it activated RAC1 to physically interact with TRPM2 following kidney ischemia. Then, activated RAC1 increased NADPH oxidase activity after renal I/R injury, which initiated a positive feedback loop to activate TRPM2 and RAC1 and increased the production of ROS, finally resulting in apoptotic cell death (Figure 3B)85. These findings indicate that TRPM2-dependent RAC1 activation increases NADPH oxidase activity, and they suggest that the therapeutic targeting of TRPM2 may be effective in alleviating ischemic kidney injury86.
Conclusion
Although substantial progress has been made in the prevention of ischemia injury in the past decades, there is still a lack of effective drugs for stroke patients. Is TRPM2 a bad or good guy in I/R injury? As discussed above, although accumulating evidence indicates TRPM2 is involved in cell death induced by I/R injury in the brain, heart and kidney, the role of TRPM2 in cardiac ischemia injury may still not be answered concisely yet. Otherwise, because the signals participating in TRPM2-mediated I/R injury vary in different tissues, it will be interesting to investigate whether these signals play the same role in different tissues. It is worth noting that pharmacological inhibition of TRPM2 prevents the brain, heart and kidney from I/R injury, which suggests that TRPM2 is a promising target for the development of neuroprotective drugs in the future. Once a selective antagonist is available for TRPM2, further extensive preclinical testing is necessary to assess the therapeutic potential of the TRPM2 inhibitor in ischemic injury.
Acknowledgments
We thank Dr Lin-hua JIANG for reading this manuscript. This work was supported by grants from the National Basic Research Program of China (2013CB910204) and the National Natural Science Foundation of China (81371302 and 81571127) to Wei YANG and the National Basic Research Program of China (2014CB910300) to Jian-hong LUO.
References
- 1Kerrigan CL, Stotland MA. Ischemia reperfusion injury: a review. Microsurgery 1993; 14: 165–75. [DOI] [PubMed] [Google Scholar]
- 2Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190: 255–66. [DOI] [PubMed] [Google Scholar]
- 3Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest 2003; 111: 583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005; 38: 1433–44. [DOI] [PubMed] [Google Scholar]
- 5Chen XM, Chen HS, Xu MJ, Shen JG. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin 2013; 34: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury: from pathophysiology to treatment. J Renal Inj Prev 2015; 4: 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Davidson SM, Yellon DM, Murphy MP, Duchen MR. Slow calcium waves and redox changes precede mitochondrial permeability transition pore opening in the intact heart during hypoxia and reoxygenation. Cardiovasc Res 2012; 93: 445–53. [DOI] [PubMed] [Google Scholar]
- 8Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001; 411: 595–9. [DOI] [PubMed] [Google Scholar]
- 9Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 2005; 18: 61–9. [DOI] [PubMed] [Google Scholar]
- 10Alim I, Teves L, Li R, Mori Y, Tymianski M. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J Neurosci 2013; 33: 17264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Ye M, Yang W, Ainscough JF, Hu XP, Li X, Sedo A, et al. TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 2014; 5: e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, et al. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc Res 2013; 97: 271–81. [DOI] [PubMed] [Google Scholar]
- 13Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, et al. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 2014; 124: 4989–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Miller BA, Wang J, Hirschler-Laszkiewicz I, Gao E, Song J, Zhang XQ, et al. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2013; 304: H1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Hoffman NE, Miller BA, Wang J, Elrod JW, Rajan S, Gao E, et al. Ca2+ entry via TRPM2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol 2015; 308: H637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001; 293: 1327–30. [DOI] [PubMed] [Google Scholar]
- 17McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 2003; 278: 11002–6. [DOI] [PubMed] [Google Scholar]
- 18Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2009; 2: ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 2011; 589: 1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Perraud AL, Schmitz C, Scharenberg AM. TRPM2 Ca2+ permeable cation channels: from gene to biological function. Cell Calcium 2003; 33: 519–31. [DOI] [PubMed] [Google Scholar]
- 21Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci 2004; 25: 633–9. [DOI] [PubMed] [Google Scholar]
- 22Fleig A, Penner R. Emerging roles of TRPM channels. Novartis Found Symp 2004; 258: 248–58. [PubMed] [Google Scholar]
- 23Kuhn FJ, Luckhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J Biol Chem 2004; 279: 46431–7. [DOI] [PubMed] [Google Scholar]
- 24Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 2002; 277: 23150–6. [DOI] [PubMed] [Google Scholar]
- 25Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 2003; 278: 16222–9. [DOI] [PubMed] [Google Scholar]
- 26Orfanelli U, Wenke AK, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res 2008; 18: 1128–40. [DOI] [PubMed] [Google Scholar]
- 27Faouzi M, Penner R. TRPM2. Handb Exp Pharmacol 2014; 222: 403–26. [DOI] [PubMed] [Google Scholar]
- 28Xia R, Mei ZZ, Mao HJ, Yang W, Dong L, Bradley H, et al. Identification of pore residues engaged in determining divalent cationic permeation in transient receptor potential melastatin subtype channel 2. J Biol Chem 2008; 283: 27426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Jiang LH, Yang W, Zou J, Beech DJ. TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets 2010; 14: 973–88. [DOI] [PubMed] [Google Scholar]
- 30Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J 2006; 20: 962–4. [DOI] [PubMed] [Google Scholar]
- 31Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL, et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem 2006; 281: 14057–65. [DOI] [PubMed] [Google Scholar]
- 32Starkus J, Beck A, Fleig A, Penner R. Regulation of TRPM2 by extra- and intracellular calcium. J Gen Physiol 2007; 130: 427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci U S A 2009; 106: 7239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Ayub K, Hallett MB. The mitochondrial ADPR link between Ca2+ store release and Ca2+ influx channel opening in immune cells. FASEB J 2004; 18: 1335–8. [DOI] [PubMed] [Google Scholar]
- 35Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 2005; 280: 6138–48. [DOI] [PubMed] [Google Scholar]
- 36Toth B, Csanady L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem 2010; 285: 30091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol 2007; 179: 7827–39. [DOI] [PubMed] [Google Scholar]
- 38Du J, Xie J, Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol 2009; 134: 471–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Starkus JG, Fleig A, Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol 2010; 588: 1227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Yang W, Zou J, Xia R, Vaal ML, Seymour VA, Luo J, et al. State-dependent inhibition of TRPM2 channel by acidic pH. J Biol Chem 2010; 285: 30411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Yang W, Manna PT, Zou J, Luo J, Beech DJ, Sivaprasadarao A, et al. Zinc inactivates melastatin transient receptor potential 2 channels via the outer pore. J Biol Chem 2011; 286: 23789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Yu W, Jiang LH, Zheng Y, Hu X, Luo J, Yang W. Inactivation of TRPM2 channels by extracellular divalent copper. PLoS One 2014; 9: e112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Zeng B, Chen GL, Xu SZ. Divalent copper is a potent extracellular blocker for TRPM2 channel. Biochem Biophys Res Commun 2012; 424: 279–84. [DOI] [PubMed] [Google Scholar]
- 44Pantaler E, Luckhoff A. Inhibitors of TRP channels reveal stimulus-dependent differential activation of Ca2+ influx pathways in human neutrophil granulocytes. Naunyn Schmiedebergs Arch Pharmacol 2009; 380: 497–507. [DOI] [PubMed] [Google Scholar]
- 45Bittigau P, Sifringer M, Felderhoff-Mueser U, Hansen HH, Ikonomidou C. Neuropathological and biochemical features of traumatic injury in the developing brain. Neurotox Res 2003; 5: 475–90. [DOI] [PubMed] [Google Scholar]
- 46Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 2005; 48: 635–46. [DOI] [PubMed] [Google Scholar]
- 47Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 2006; 209: 59–68. [DOI] [PubMed] [Google Scholar]
- 48Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003; 115: 863–77. [DOI] [PubMed] [Google Scholar]
- 49Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol 2011; 704: 531–44. [DOI] [PubMed] [Google Scholar]
- 50Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta 2007; 1772: 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther 2008; 118: 337–51. [DOI] [PubMed] [Google Scholar]
- 52Weilinger NL, Maslieieva V, Bialecki J, Sridharan SS, Tang PL, Thompson RJ. Ionotropic receptors and ion channels in ischemic neuronal death and dysfunction. Acta Pharmacol Sin 2013; 34: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Fonfria E, Mattei C, Hill K, Brown JT, Randall A, Benham CD, et al. TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J Recept Signal Transduct Res 2006; 26: 179–98. [DOI] [PubMed] [Google Scholar]
- 54Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, et al. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab 2011; 31: 2160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Verma S, Quillinan N, Yang YF, Nakayama S, Cheng J, Kelley MH, et al. TRPM2 channel activation following in vitro ischemia contributes to male hippocampal cell death. Neurosci Lett 2012; 530: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J Mol Neurosci 2013; 51: 92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ, et al. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab 2013; 33: 1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996; 272: 1013–6. [DOI] [PubMed] [Google Scholar]
- 59Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci 1998; 21: 347–75. [DOI] [PubMed] [Google Scholar]
- 60Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem 2003; 85: 563–70. [DOI] [PubMed] [Google Scholar]
- 61Stork CJ, Li YV. Rising zinc: a significant cause of ischemic neuronal death in the CA1 region of rat hippocampus. J Cereb Blood Flow Metab 2009; 29: 1399–408. [DOI] [PubMed] [Google Scholar]
- 62Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 2009; 10: 780–91. [DOI] [PubMed] [Google Scholar]
- 63Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A 1999; 96: 2414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Yin HZ, Sensi SL, Ogoshi F, Weiss JH. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J Neurosci 2002; 22: 1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci U S A 2005; 102: 12230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Mony L, Berger TK, Isacoff EY. A specialized molecular motion opens the Hv1 voltage-gated proton channel. Nat Struct Mol Biol 2015; 22: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 2013; 47: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Noh KM, Kim YH, Koh JY. Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J Neurochem 1999; 72: 1609–16. [DOI] [PubMed] [Google Scholar]
- 69Kim YH, Koh JY. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol 2002; 177: 407–18. [DOI] [PubMed] [Google Scholar]
- 70Leng T, Shi Y, Xiong ZG, Sun D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog Neurobiol 2014; 115: 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Man HY. GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity? Curr Opin Neurobiol 2011; 21: 291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Gelderblom M, Melzer N, Schattling B, Gob E, Hicking G, Arunachalam P, et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke 2014; 45: 3395–402. [DOI] [PubMed] [Google Scholar]
- 73Shirakawa H, Sakimoto S, Nakagawa T, Kaneko S. Pathophysiology of immune cells during the progression of cerebral ischemic injury – involvement of TRPM2-mediated induction of iNOS in microglia/macrophage. Nihon Yakurigaku Zasshi 2014; 144: 104–9. [DOI] [PubMed] [Google Scholar]
- 74Rowell J, Koitabashi N, Kass DA. TRP-ing up heart and vessels: canonical transient receptor potential channels and cardiovascular disease. J Cardiovasc Transl Res 2010; 3: 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Chalkias A, Xanthos T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev 2012; 17: 117–28. [DOI] [PubMed] [Google Scholar]
- 76Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxid Redox Signal 2003; 5: 789–94. [DOI] [PubMed] [Google Scholar]
- 77Kleinbongard P, Schulz R, Heusch G. TNFalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev 2011; 16: 49–69. [DOI] [PubMed] [Google Scholar]
- 78Fauconnier J, Roberge S, Saint N, Lacampagne A. Type 2 ryanodine receptor: a novel therapeutic target in myocardial ischemia/reperfusion. Pharmacol Ther 2013; 138: 323–32. [DOI] [PubMed] [Google Scholar]
- 79Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 2007; 1772: 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, et al. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ 2006; 13: 1815–26. [DOI] [PubMed] [Google Scholar]
- 81Roberge S, Roussel J, Andersson DC, Meli AC, Vidal B, Blandel F, et al. TNF-alpha-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovasc Res 2014; 103: 90–9. [DOI] [PubMed] [Google Scholar]
- 82Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc 2008; 40: 3279–88. [DOI] [PubMed] [Google Scholar]
- 83Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, et al. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 2013; 84: 920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Raz L, Zhang QG, Zhou CF, Han D, Gulati P, Yang LC, et al. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS One 2010; 5: e12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Katano M, Numata T, Aguan K, Hara Y, Kiyonaka S, Yamamoto S, et al. The juvenile myoclonic epilepsy-related protein EFHC1 interacts with the redox-sensitive TRPM2 channel linked to cell death. Cell Calcium 2012; 51: 179–85. [DOI] [PubMed] [Google Scholar]
- 86Dusmez D, Cengiz B, Yumrutas O, Demir T, Oztuzcu S, Demiryurek S, et al. Effect of verapamil and lidocaine on TRPM and NaV1.9 gene expressions in renal ischemia-reperfusion. Transplant Proc 2014; 46: 33–9. [DOI] [PubMed] [Google Scholar]