Abstract
C-kit-negative gastrointestinal stromal tumors (GISTs) are uncommon, and there have been few reports about the diagnosis and treatment of c-kit-negative GISTs in the stomach. We report the case of a patient who was diagnosed with a huge and atypical GIST in the stomach. The GIST was completely resected and finally diagnosed as c-kit-negative GIST based on immunohistochemical staining of tumor cells, which were negative for CD117 and CD34 and positive for Discovered on GIST-1 (DOG1). C-kit-negative GISTs could be treated by complete resection and/or imatinib, which is the same treatment for c-kit-positive GISTs.
Keywords: C-kit protein, DOG1 protein, Gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumor (GIST) is one of the most common sarcomatous tumors detected in gastrointestinal tract.1 The incidence of GIST is 7 to 10 cases per million people in -Eu rope and the USA.2,3 However, in Korea, the incidence is higher than that in Europe or the USA, a 16 to 22 cases per million people.4
GISTs are derived from interstitial cells of Cajal, and they have three histological types, spindled, epithelioid, and mixedtype cells.5 Identifying the expression of CD117, also known as kit, by immunohistochemical (IHC) staining is necessary to diagnose GISTs. CD117 expression can be found in over 95% of GISTs.6 However, CD117 expression is not found in the 5% of c-kit-negative GISTs. Although, in those cases, additional staniing with Discovered on GIST-1 (DOG1, also known as ANO1) can be helpful to confirm a GIST,7 the diagnosis and treatment of c-kit negative GISTs remain obscure.
Here, we have encountered a c-kit-negative GIST case, and we are going to report on the diagnosis and treatment of that tumor.
Case Report
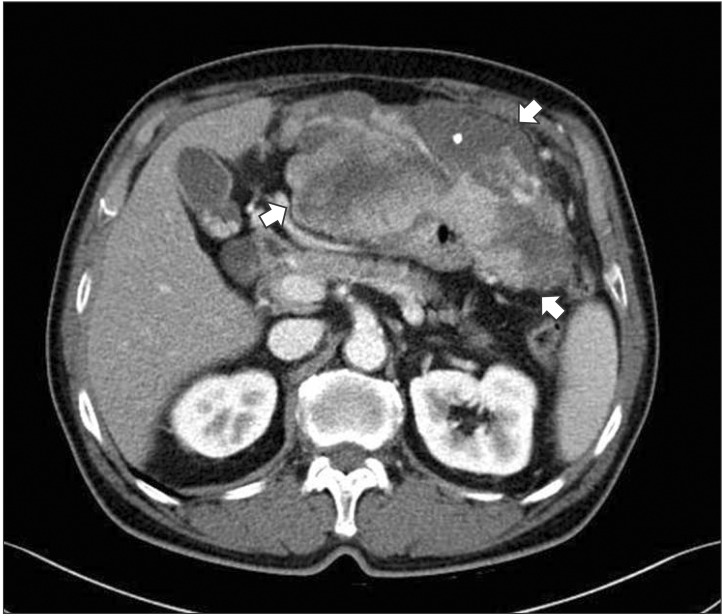
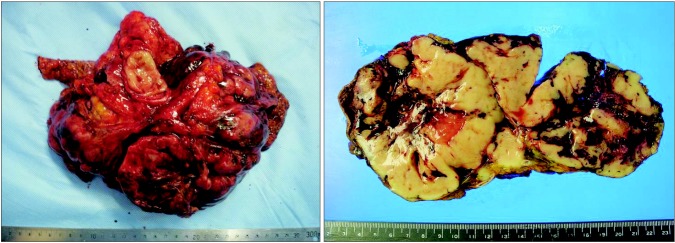
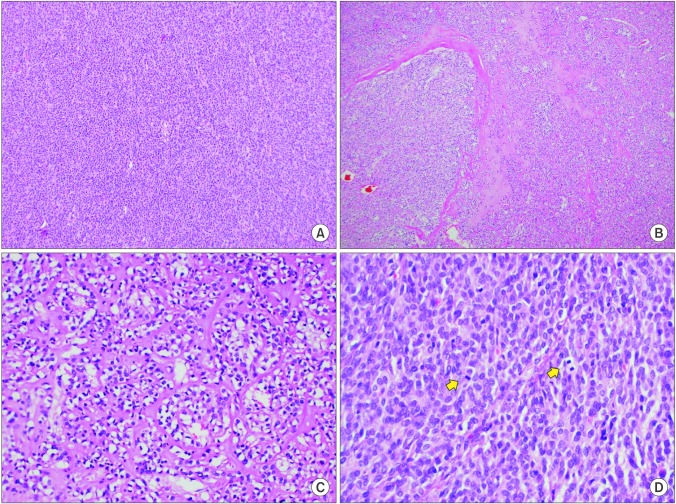
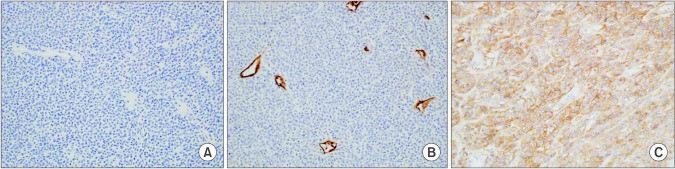
A 73-year-old man presented with abdominal distention and maldigestion for 1 month. The patient had a medical history of diabetes and hypertension. An intraabdominal mass was palpated in the epigastric area, and the patient did not complain of pain and tenderness. Laboratory findings showed no abnormalities, except an elevated fasting glucose level. Contrast-enhanced computed tomography (CT) revealed an 18-cm exophytic mass from the anterior wall of the lower body of the stomach without regional enlarged lymph nodes (Fig. 1). The patient underwent mass removal by gastric wedge resection. The resected specimen was an 18.0×15.0 cm, irregularly shaped, multi-lobulated, yellow, soft, and fleshy mass with a 3.5×2.5 cm wedge-resected portion of the stomach (Fig. 2). The tumor directly invaded the adjacent adipose tissue, grossly. Microscopically, a routine H&E-stained section showed a hypercellular tumor lesion with diffuse sheet-like growth patterns under low-power magnification (Fig. 3A). Some portions of the tumor showed hyalinized stroma with slit-like vessels and sinusoidal capillaries around the tumor cell nests (Fig. 3B). At higher magnification, tumor cells were epithelioid and had prominent cytoplasmic vacuoles and uniform nuclei with moderate nuclear pleomorphisms (Fig. 3C). Mitotic figures were counted in the most active area ('hot spots'), and 37 mitoses per 50 high-power fields (HPFs) were observed (Fig. 3D). By IHC staining, tumor cells were negative for CD117 (Fig. 4A) and CD34 (Fig. 4B). However, DOG1 was observed in the membrane and cytoplasm of the tumor cells (Fig. 4C). There was no involvement of the resected margins. Consequently, this tumor was diagnosed as a c-kit negative GIST with a high risk of malignant behavior. Post-operative recovery was uneventful, and the patient was discharged on post-operative day 7.
Fig. 1. Computed tomography shows an 18.0×15.0 cm irregular enhancing soft tissue mass (arrows) with exophytic growth on the anterior wall of the stomach.
Fig. 2. The resected specimen reveals a multi-lobulated, yellow, soft, and fleshy mass with focal involvement of adjacent adipose tissue.
Fig. 3. Microscopic features. (A) At lower magnification, a hypercellular tumor lesion with diffuse sheet-like growth pattern is identified (H&E, ×100). (B) There are focal areas of hyalinized stroma with slit-like vessels and some capillaries (H&E, ×100). (C) At higher magnification, epithelioid tumor cells with prominent cytoplasmic vacuoles and moderately pleomorphic nuclei are identified (H&E, ×400). (D) Frequent mitotic figures (arrows) are observed (H&E, ×400).
Fig. 4. Immunohistochemical staining (×200). Tumor cells are negative for CD117 (A) and CD34 (B). (C) The membrane and cytoplasm of tumor cells are stained for DOG1.
Discussion
The stomach is the most common organ (50% to 60%) in which GISTs are found. The other organs are the small intestine (30% to 35%), colon (5%), and esophagus (<1%). Less than 5% of GISTs are found in the omentum, mesentery, and retroperitoneum, and those tumors are called extra-GISTs (E-GISTs). EGISTs occur in relatively high proportions in Korea (10%).8
In fact, the identification KIT or PDGFRA mutation by gene analysis is needed to make a confirmatory diagnosis. In general, the identification of CD117 expression by IHC staining is enough to diagnosis GISTs, clinically. Moreover, CD117 or CD3 4 expression is found in more than 90% of GISTs.8 However, they are not expressed in some GISTs. In CD117- or CD34-negative cases, DOG1 expression could be identified if the patients are clinically suspected of having GISTs. DOG1 expression is detected in many c-kit-negative GISTs,8 and one previous study reported that more than 90% of c-kit-negative GISTs were positive for DOG1.9 Several studies have reported that DOG1 is more sensitive than CD117 and more helpful, especially in ckit- negative GISTs.10,11,12 Thus, for patients who are clinically su-s pected of having c-kit negative GISTs, DOG1 expression should be checked.
C-kit-positive GISTs are treated with surgery and/or imatinib. Imatinib is prescribed for patients with GISTs based on their risk of recurrence or metastasis, which is estimated using tumor size and the number of mitoses. In the stomach, for low-risk GISTs, which are less than 2 cm and have less than 5 mitoses per 50 HPF, a complete (R0) resection without rupturing is recommended.13 Lymph node dissection is usually not recommended. For high-risk GISTs, which are larger than 2 cm or have more than 5 mitoses per 50 HPF, a complete resection, if possible, is recommended following adjuvant imatinib. For unresectable GISTs, neoadjuvant or palliative imatinib and/or surgery is recommended.14 For c-kit-positive GISTs, 400 mg daily imatinib can be used for neoadjuvant, adjuvant, and palliative treatment.15,16
Despite these treatment modalities, the 5-year survival rate o f c-kit-positive GISTs is known roughly to be 48% to 65%, and the tumors usually recur. The prognosis of GISTs depends on tumor size, the number of mitoses, chemotherapy, and complete resection.17 On the other hand, the prognoses and predictive fa-c tors for prognosis of c-kit-negative GISTs remain unclear.
However, complete resection and adjuvant imatinib, if possible, should be considered for c-kit-negative GISTs also because of the possibility of KIT or PDGFRA mutation, as in c-kitpositive GISTs.18 Imatinib is a competitive inhibitor of tyrosine kinases, such as KIT and PDGFRA,19 and several studies have shown that the c-kit-negative GISTs still have mutated KIT or PDGFRA despite the absence of IHC expression.18 Therefore, surgery and imatinib should be used as a therapeutic strategy for c-kit-negative GISTs. In addition, some studies reported the effectiveness of imatinib.7 However, c-kit-negative GISTs commonly have PDGFRA D842 mutations, and in such cases, imatinib resistance develops.9 Thus, clinicians should consider this mutation when considering imatinib treatment for c-kitnegative GISTs.
We reported on our patient with a c-kit-negative GIST. The tumor, which was located in the stomach, was completely resected in spite of its large size. The histological diagnosis was confirmed with DOG1 IHC staining. According to the indications for imatinib, the patient should have been treated with adjuvant imatinib, based on the risks of the tumor. However, the patient refused further evaluation and imatinib treatment for rpivate reasons. Recurrence has not yet developed, as of 9 months after surgery. If the patient is treated with adjuvant imatinib, a lower recurrence rate can be expected.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Pèoc'h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson B, Bümming P, Meis-Kindblom JM, Odèn A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 4.Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010;25:853–862. doi: 10.3346/jkms.2010.25.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 6.Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–894. doi: 10.1097/00000478-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 9.Kang GH, Srivastava A, Kim YE, Park HJ, Park CK, Sohn TS, et al. DOG1 and PKC-θ are useful in the diagnosis of KIT negative gastrointestinal stromal tumors. Mod Pathol. 2011;24:866–875. doi: 10.1038/modpathol.2011.11. [DOI] [PubMed] [Google Scholar]
- 10.Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 11.Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 13.Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97:1854–1859. doi: 10.1002/bjs.7222. [DOI] [PubMed] [Google Scholar]
- 14.Deshaies I, Cherenfant J, Gusani NJ, Jiang Y, Harvey HA, Kimchi ET, et al. Gastrointestinal stromal tumor (GIST) recurrence following surgery: review of the clinical utility of imatinib treatment. Ther Clin Risk Manag. 2010;6:453–458. doi: 10.2147/TCRM.S5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren, M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 16.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, doubleblind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]