Abstract
Background
Epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer predict dramatic clinical responses to tyrosine kinase inhibitor (TKI) treatment. The conclusion on EGFR mutation-specific antibodies by immunohistochemistry (IHC) is not consistent. We evaluated the clinical availability of EGFR mutation-specific antibodies, investigating the prediction role of mutant EGFRs and other IHC markers in TKI therapy in patients with advanced lung adenocarcinoma.
Materials and methods
We analyzed 637 primary lung adenocarcinomas from an unselected Chinese population. For IHC, antibodies against EGFR exon 19 E746_A750 deletions, exon 21 L858R mutations, thyroid transcription factor-1 (TTF-1), and Napsin-A were applied. Positivity was defined as staining score >0.
Results
Specificities of E746_A750 and L858R antibodies were 99.6% and 99.3%, while sensitivities were 86.0% and 82.7%, respectively. Tumors with Napsin-A positivity, TTF-1 positivity, EGFR mutations, and lepidic pattern showed a lower marker of proliferation index (Ki67). Higher expression scores of mutant EGFR protein, TTF-1 positivity, lower Ki67 proliferation index, and lepidic pattern were associated with longer progression-free survival.
Conclusion
High scores of mutant EGFR, Napsin-A positivity, TTF-1 positivity, lower Ki67 index, and lepidic pattern were favorable predictors for TKI therapy in patients with advanced lung adenocarcinoma.
Keywords: epidermal growth factor receptor-tyrosine kinase inhibitor, prognosis, thyroid transcription factor-1, Napsin-A, Ki67, growth pattern, progression-free survival
Introduction
Lung cancer is the leading cause of death from cancer, in both males and females.1 Non-small-cell lung cancer (NSCLC) accounts for ~85% of lung cancer cases.2 In the last several decades, adenocarcinoma has become the predominant type of NSCLC.3 Assessment of epidermal growth factor receptor (EGFR) mutations has become mandatory to choose the most active first-line treatment for patients with advanced primary lung adenocarcinoma.4 Indeed, multiple clinical trials have demonstrated that first-line administration of an EGFR-tyrosine kinase inhibitor (EGFR-TKI) results in a prolonged progression-free survival (PFS) when compared with chemotherapy in patients carrying EGFR mutations.5–7 However, a standardized test for the detection of EGFR mutations in NSCLC has not yet been approved. Amplification-refractory mutation system (ARMS) has been widely used in the People’s Republic of China. However, this method is relatively expensive, time consuming, and not incorporated in routine diagnostic procedures in many departments of pathology. In contrast, immunohistochemistry (IHC) has lower costs, shorter turnaround time, and is available in the majority of laboratories. For these reasons, mutation-specific antibodies might be a relevant alternative for determining EGFR status. So far, there have already been few reports on EGFR IHC in NSCLC,8–11 although no consistent evaluation on IHC has been concluded.
Primary lung adenocarcinoma is a heterogeneous tumor with variations in the pathological profile. In 2011, a new histologic classification of lung adenocarcinoma was proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) to provide uniform terminology and diagnostic criteria for multidisciplinary strategic management. Several studies12,13 have validated the correlation between adenocarcinoma subtypes based on IASLC/ATS/ERS classification and patient outcomes in respective cohorts. However, there have been few reports14 on correlations between IASLC/ATS/ERS classification and clinical outcomes in patients receiving TKI drug therapy.
The aim of this study was to determine the sensitivity and specificity of mutation-specific antibodies in the detection of EGFR mutations in unselected Chinese patients with primary lung adenocarcinoma. Furthermore, to analyze the prognostic utility of lung adenocarcinoma IHC markers, including cytokeratin-7 (CK-7), TTF-1, and Napsin A, tumor cell proliferation index using a marker of the proliferation index (Ki67), the efficacy of mutant EGFR expression in EGFR-TKI treatment (using the current uniform cohort of advanced lung adenocarcinomas harboring EGFR mutations), and their association with IASLC/ATS/ERS classification were evaluated.
Materials and methods
Patients
Details of 637 patients with primary lung adenocarcinoma from the archives in Cancer Center recorded from July 2011 to July 2014 were collected for this study. Clinicopathological variables, including age, sex, histologic type, and pathological stage were collected by reviewing the medical charts and pathology records. Patients were followed up from the date of pathology diagnosis until death or censored on December 31, 2014, which resulted in a follow-up period of 1–41 months (a median of 25 months). The study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. As this was a retrospective study using archived tissue specimens, the Institutional Ethics Committee waived the need for written informed consent.
Tumor sample preparation
Tumor samples were fixed in 10% neutral buffered formalin and embedded in paraffin wax. The paraffin-embedded tumor tissue was sliced into 4 µm sections on microtomes for hematoxylin–eosin (H&E) staining, molecular tests, and IHC.
Immunohistochemistry
Two rabbit monoclonal antibodies specifically against EGFR with L858R point mutation in exon 21 (clone 43B2, catalog no 5354) or E746_A750 deletions in exon 19 (clone 6B6, catalog no 2085; Cell Signaling Technology, Danvers, MA, USA) were used for IHC. Other antibodies, including CK-7 (clone OV-TL 12/30, Maixin, Fuzhou, the People’s Republic of China), Napsin-A (multiclone, Maixin), TTF-1 (clone 8G7G3/1, Maixin), Ki67 (clone MX006, Maixin), and anaplastic lymphoma kinase (ALK, clone D5F3; Cell Signaling Technology) were also employed. Sections of 4 µm thickness cut from formalin-fixed, paraffin-embedded tissue blocks were deparaffinized in xylene and rehydrated through a graded series of ethanol concentrations. The EGFR mutation-specific staining was scored based on membrane staining intensity as previously described:15 0= no staining, 1+ = faint membranous staining in >10% of tumor cells, 2+ = moderate complete membranous staining in >10% of tumor cells, and 3+ = strong membranous staining in >10% of tumor cells. A positive immunoreactivity is defined as an IHC score ≥1+. Criteria for the interpretation of other immunostainings were as follows: distinct nuclear staining of >5% tumor cells defined as positive for TTF-1, and diffuse and intense cytoplasmatic staining of >50% tumor cells defined as positive for CK-7 and Napsin-A. The proliferation index of Ki67 was assessed based on the percentage of distinct nuclear staining in tumor cells.
DNA extraction and mutation analysis
DNA was extracted from the archived formalin-fixed, paraffin-embedded tissue samples using QIAamp DNA FFPE tissue Kit (Qiagen NV, Venlo, the Netherlands) according to the protocol in the manufacturer’s instructions, and the concentration and purity of the extracted DNA were assessed by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The extracted DNA with either low concentration or bad purity was excluded from the mutation analysis. Briefly, representative formalin-fixed, paraffin-embedded tissues were selected for the assay. Ten sections of 4 µm thick tumor tissue were cut and, if necessary, macrodissected in order to ensure high percentage of tumor cells. Tissue sections were treated once with xylene, followed by one wash in ethanol. The pellet was resuspended in tissue lysis buffer and treated with proteinase-K overnight at 56°C. After the inactivation of proteinase-K by heating, DNA was extracted. Then, the DNA sample was tested with a proprietary detection kit based on the principle of ARMS, according to the protocol of the EGFR Mutations Detection Kit (Amoy Diagnostics, Xiamen, People’s Republic of China). Polymerase chain reaction was carried out by the Mx3000PtM (Stratagene, La Jolla, USA), and a positive or negative result could be reached if it met the criterion defined in the manufacturer’s instructions.
Statistical analysis
SPSS statistical software package (version 20.0; StataCorp LP, College Station, TX, USA) was used for statistical analyses. Chi-square tests were used to analyze the correlations between EGFR mutation and clinicopathological variables. Kaplan–Meier method was used to determine survival analysis. All statistical tests were conducted at a two-sided level of significance of P<0.05.
Results
Sample characteristics
A total of 637 cases of advanced primary lung adenocarcinomas, including 273 females and 364 males, with a median age of 59 years (range: 23–85 years), were included in this study.
Of those patients, 218 (34.3%) were never smokers and 419 (65.7%) were former or current smokers. According to a new histologic classification of lung adenocarcinoma proposed by IASLC/ATS/ERS in 2011, histologic examination revealed that the main growth pattern consisted of acinar (234 cases), papillary (including micropapillary, 106 cases), lepidic (104 cases), solid (147 cases), variants (including mucinous, fetus, colloid, and intestinal adenocarcinoma, 12 cases), and other low differentiated adenocarcinomas (34 cases). EGFR mutation status analyzed by ARMS revealed exon 19 E746_A750 deletions in 118 patients, exon 21 L858 mutations in 158 patients, and double mutations in four patients. A total of 177 patients had stage III and 460 patients had stage IV tumors. Tumor-node-metastasis stage was determined at the beginning of TKI treatment. No patients had received neoadjuvant chemotherapy, radiotherapy, and EGFR-TKI before the tumor resection or biopsy. In total, 149 patients (53.2%) received TKI drug therapy as first-line, and 131 patients (46.8%) patients received TKI drug therapy as second- or third-line therapy. The characteristics of these 637 patients are listed in Table 1.
Table 1.
Clinicopathological features of the patients (n=637)
| Clinicopathological features | n | (%) |
|---|---|---|
| Median age (range), years | 59 (23–85) | |
| Sex | ||
| Male | 364 | (57) |
| Female | 273 | (43) |
| Smoking | ||
| Never smoker | 218 | (34.3) |
| Former or current smoker | 419 | (65.7) |
| Growth pattern | ||
| Lepidic | 104 | (16.3) |
| Papillary (micropapillary) | 106 | (14.5) |
| Acinar | 234 | (36.7) |
| Solid | 147 | (23.1) |
| Variants | 12 | (0.02) |
| Poorly differentiated | 34 | (0.05) |
| EGFR mutation status | ||
| Exon 19 | 118 | (18.8) |
| Exon 21 | 158 | (25.2) |
| Both | 4 | (<0.1) |
| Wild type | 357 | (55.9) |
| Line of TKI therapy | ||
| First | 149 | (53.2) |
| Second or third | 131 | (46.8) |
| TNM stagea | ||
| IIIa or IIIb | 177 | (27.8) |
| IV | 460 | (82.2) |
Note:
Tumor-node-metastasis (TNM) stage determined at the beginning of TKI treatment.
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Concordance between IHC and ARMS on EGFR mutation status in lung adenocarcinoma
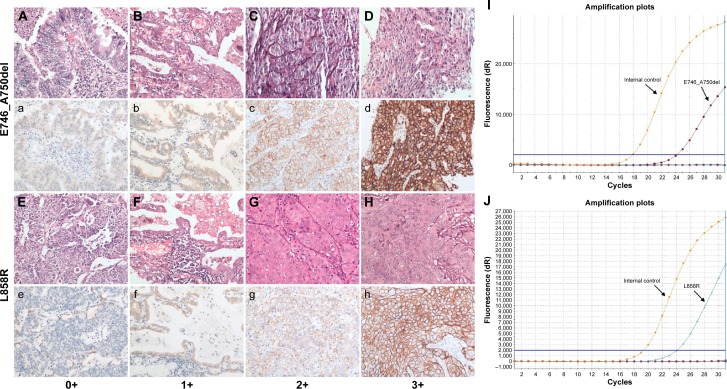
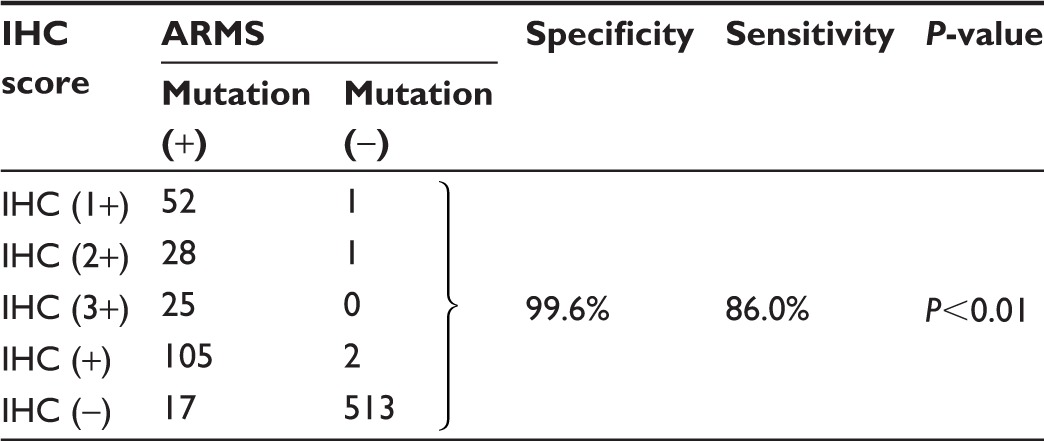
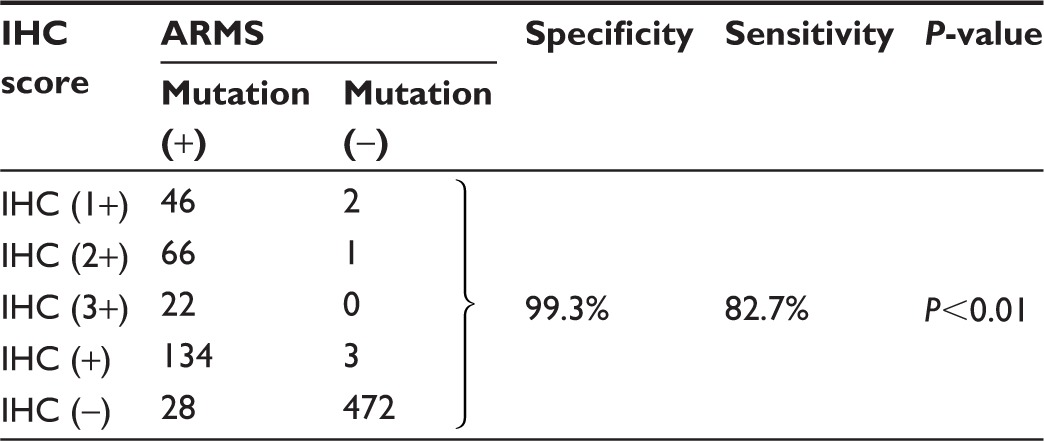
Mutations detected by EGFR IHC and ARMS are summarized in Table 2 (exon 19 E746_A750del) and Table 3 (exon 21 L858R). ARMS analysis revealed 118 patients carrying exon 19 E746_A750 deletions resulting in a frequency of 18.8%, 158 patients carrying exon 21 L858R mutations with a frequency of 25.2%, and four patients carrying both mutations. The characteristics of four patients carrying double mutations are shown in Table 4. The presence of both EGFR E746_A750 del and L858R mutations in the four patients, as detected by ARMS analysis, was also validated by IHC analysis. These patients had a prolonged PFS (12.5–15 months, mean =13.6 months) when compared to those patients carrying the single EGFR mutation (E746_A750del vs L858R: median 11.3 months vs 11.3 months). Of the E746_A750 deletions detected by ARMS, 101 were detected by the exon 19 antibody. Of the exon 21 mutations detected by ARMS, 130 were detected by exon 21 antibody. The specificity of exon 19 antibody was 99.6% (95% confidence interval [CI]: 97.9%–99.9%) and sensitivity was 86.0% (95% CI: 77.9%–91.4%). Specificity of exon 21 antibody was 99.3% (95% CI: 97.6%–99.8%) and sensitivity was 82.7% (95% CI: 75.4%–87.9%). Figure 1 shows representative H&E stainings (Figure 1A–1H) and matching immunostaining images (Figure 1a–1h) for EGFR mutation-specific antibodies in lung adenocarcinoma. Representative results for exon 19 E746_A750del (Figure 1I) and exon 21 L858R (Figure 1J) mutations by the ARMS method are also shown.
Table 2.
Comparison of EGFR exon 19 E746_A750del mutation by ARMS analysis and IHC staining
|
Abbreviations: EGFR, epidermal growth factor receptor; ARMS, amplification- refractory mutation system; IHC, immunohistochemistry.
Table 3.
Comparison of EGFR exon 21 L858R mutation by ARMS analysis and IHC staining
|
Abbreviations: EGFR, epidermal growth factor receptor; ARMS, amplification- refractory mutation system; IHC, immunohistochemistry.
Table 4.
Clinicopathological features of the patients carrying both EGFR exon 19 E746_A750 deletions and exon 21 L858R mutation (n=4)
| Clinicopathological parameters | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (year) | 59 | 72 | 45 | 63 |
| Sex | Male | Female | Female | Female |
| Smoking | Former smoker | Never smoker | Never smoker | Never smoker |
| TNM stage | IIIb | IV | IV | IV |
| IHC markers | ||||
| CK-7 | + | + | + | + |
| TTF-1 | + | + | + | + |
| Napsin-A | + | + | + | + |
| ALK | − | − | − | − |
| Ki67 index (%) | 10 | 20 | 5 | 15 |
| E746_A750del score | 2+ | 2+ | 3+ | 3+ |
| L858R mutation score | 2+ | 2+ | 3+ | 2+ |
| Growth pattern | Acinar | Acinar | Lepidic | Papillary |
| PFS (months) | 12.5 | 13 | 15 | 14.5 |
Abbreviations: EGFR, epidermal growth factor receptor; TNM, tumor-node-metastasis; CK-7, cytokeratin-7; TTF-1, thyroid transcription factor-1; IHC, immunohistochemistry; PFS, progression-free survival; ALK, anaplastic lymphoma kinase.
Figure 1.
Representative pictures for IHC staining and ARMS analysis on mutant EGFRs.
Notes: H&E staining (A–H) and immunohistochemical staining (a–h) for EGFR E746_A750del (A–D for H&E staining, a–d for match IHC staining) and L858R (E–H for H&E staining, e–h for match IHC staining) mutation-specific antibody in tumor samples from patients with primary lung adenocarcinomas, respectively. (A and a, E and e) No IHC staining in malignant glandular epithelium for EGFR mutant protein (0+); (B and b, F and f) Faint IHC membrane staining in. 10% tumor cells (1+); (C and c, G and g) Moderate complete IHC membrane staining in. 10% tumor cells (2+); (D and d, H and h) Strong complete IHC membrane staining in. 10% tumor cells (3+) (magnification, 200×). Representative results for exon 19 E746_A750del (I) and exon 21 L858R (J) mutations by ARMS method are also shown.
Abbreviations: H&E, hematoxylin–eosin; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; ARMS, amplification-refractory mutation system.
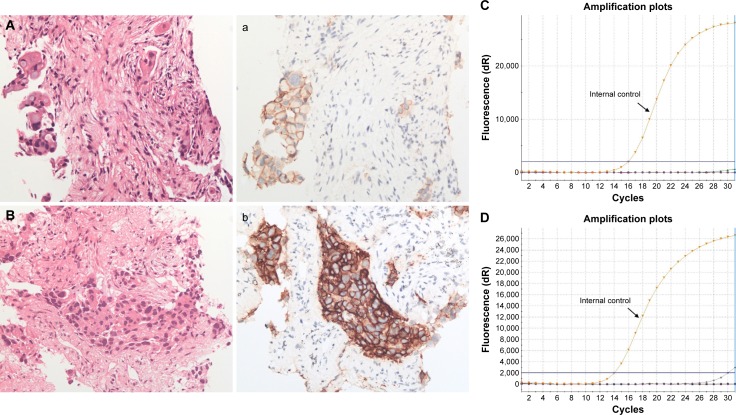
In addition, EGFR IHC detected five cases, including two E746_A750 deletions and three L858R mutations, which were wild type by ARMS analysis. For these cases, IHC and ARMS were performed on the same block. Tumor cell numbers were 100–300 and tumor cell intensity was ~30%; all the samples were biopsy specimens. H&E staining results are shown in Figure 2, of these cases are shown in Figure 2A and B, matching immunostaining images (Figure 2a and 2b), and the corresponding ARMS results (Figure 2C and 2D) for EGFR mutations, including one case of E746_A750 deletions (Figure 2A, 2a, and 2C) and one case of L858R mutations (Figure 2B, 2b, and 2D).
Figure 2.
ARMS analysis failed to detect EGFR mutations while IHC staining demonstrated positivity.
Notes: Lung adenocarcinoma with positive immunohistochemical staining for EGFR E746_A750del (A for H&E staining, a for match IHC staining) and L858R (B for H&E staining, b for match IHC staining) mutation-specific antibody (magnification, 200×). However, ARMS method (C and D) demonstrated neither mutation in these cases.
Abbreviations: EGFR, epidermal growth factor receptor; H&E, hematoxylin–eosin; IHC, immunohistochemistry; ARMS, amplification refractory mutation system.
Correlation between immunoreactivity of EGFR mutants, ALK, CK-7, TTF-1, Napsin-A, and histologic types of lung adenocarcinoma
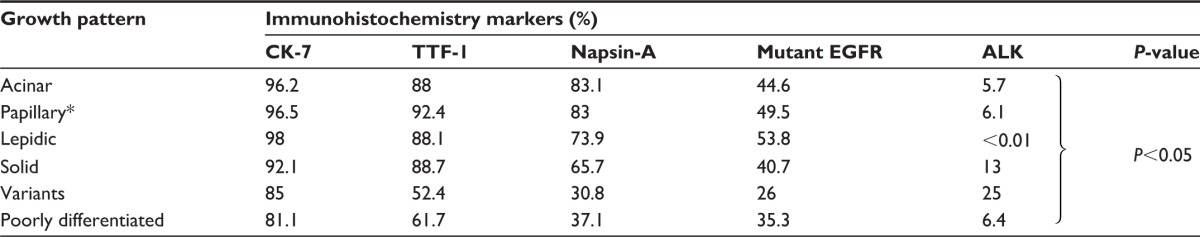
The results of immunostainings for each histologic subtype of adenocarcinomas are summarized in Table 5. The proportion of CK-7 positivity in all subtypes of adenocarcinomas ranged from 81.1% to 98% (median: 94.15%, 95% CI: 84.21%–98.76%). The proportion of TTF-1 positivity in all subtypes of adenocarcinomas ranged from 52.4% to 92.4% (median: 88.05%, 95% CI: 60.72%–96.38%). The proportion of Napsin-A positivity in all subtypes of adenocarcinomas ranged from 30.8% to 83.1% (median: 69.8%, 95% CI: 38.18%–86.36%). All Napsin-A-positive cases were also positive for TTF-1. Mutant EGFRs were also detected, and the proportion of EGFR mutation positivity ranged from 26% to 53.8% (median: 42.65%, 95% CI: 31.11%–52.19%). ALK positivity ranged from 0.01% to 25% (median: 6.25%, 95% CI: 0.24%–18.49%).
Table 5.
Correlation between growth pattern and immunohistochemistry markers, including CK-7, TTF-1, Napsin-A, mutant EGFR, and ALK
|
Notes:
Including micro-papillary.
Abbreviations: CK-7, cytokeratin-7; TTF-1, thyroid transcription factor-1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase. The acinar, papillary, lepidic, and solid patterns had similar expression ratios on CK-7, TTF-1, Napsin-A, and mutant EGFRs, except ALK. The variant adenocarcinoma and poorly differentiated adenocarcinoma had lower expression ratios of CK-7, TTF-1, Napsin-A, and mutant EGFRs when compared to the acinar, papillary, lepidic, and solid patterns. The lepidic pattern had the lowest expression ratio of ALK, while the variant adenocarcinoma had the highest expression ratio of ALK.
Ki67 proliferation status of lung adenocarcinoma in different subtypes categorized by pathological markers
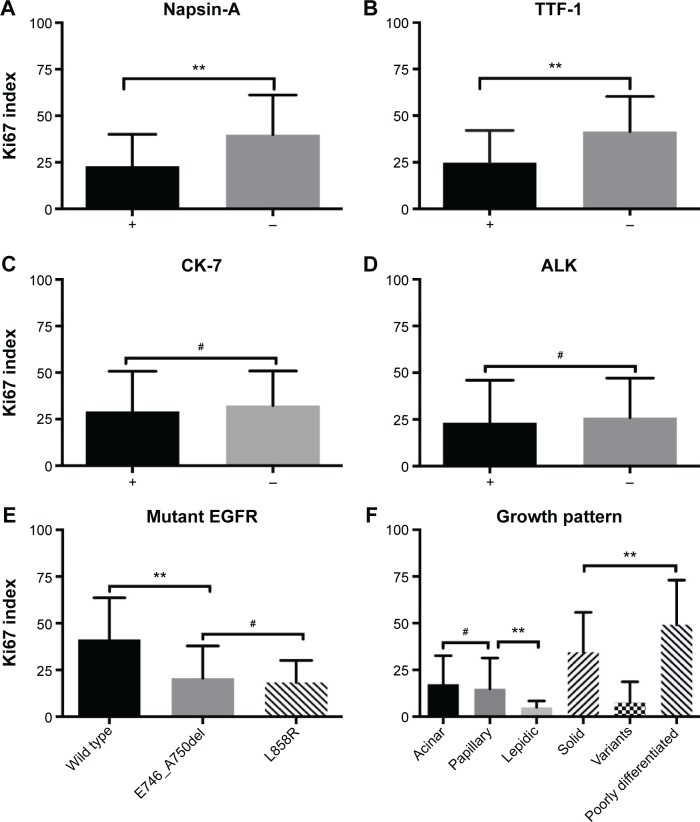
Based on these results, we further compared the Ki67 proliferation index in different subtypes. The mean values of Ki67 index were 22.84 (standard deviation [SD] =17.2) in Napsin-A-positive adenocarcinomatous cells and 39.84 (SD =21.32) in Napsin-A-negative adenocarcinomas cells (P<0.01) (Figure 3A). The mean of Ki67 index in TTF-1-positive adenocarcinomatous cells was 24.8 (SD =17.24), while it was 41.49 (SD =18.78) in the TTF-1-negative group (P<0.01) (Figure 3B). There was no statistical difference between CK-7 or ALK-positive and CK-7 or ALK-negative adenocarcinomatous cells (mean ± SD: CK-7-positive vs -negative: 29.12±21.63 vs 32.27±18.62, ALK-positive vs -negative: 23.29±22.63 vs 25.98±21.03) (Figure 3C and D). The mean of Ki67 index in EGFR wild-type adenocarcinomas was 41.42 (SD =22.26), and in EGFR mutant adenocarcinomas, the mean Ki67 index values were 20.62 (SD =17.29) for E746_A750del mutation and 18.4 (SD =11.71) for L858R mutation (P<0.01, when E746_A750del is compared to wild type; P>0.05, when E746_A750del is compared to L858R; Figure 3E). Moreover, the mean values of Ki67 index in different growth patterns were 17.44 (SD =15.22) for acinar pattern, 14.92 (SD =16.46) for papillary pattern, 5 (SD =3.46) for lepidic pattern, 34.47 (SD =21.34) for solid pattern, 7.5 (SD =11.17) for variants in adenocarcinomas, and 49.14 (SD =23.87) for poorly differentiated adenocarcinoma (Figure 3F). There was no significant difference in the Ki67 index between the acinar and papillary patterns. The expression of Ki67 index was lower in the lepidic pattern than that in the other groups (P<0.01).
Figure 3.
Ki67 proliferation index grouped by the results of different pathological markers, including Napsin-A (A), TTF-1 (B), CK-7 (C), ALK (D), mutant EGFR (E) staining, and growth pattern (F).
Notes: **P<0.05, statistical significance; #P>0.05, no statistical significance.
Abbreviations: TTF-1, thyroid transcription factor-1; CK-7, cytokeratin-7; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
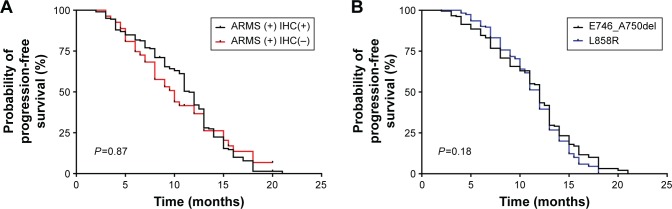
IHC staining scores and the correlation with clinical outcomes
All patients with positive results by ARMS received EGFR-TKI therapy between July 2011 and December 2014. Kaplan–Meier analysis was used to determine the PFS of EGFR-TKI treatment for the entire cohort carrying the activating EGFR mutations, and the median PFS in patients with positive results by both ARMS and IHC was 11.5 months (95% CI: 9.9–11.9), whereas there was no significant difference in the PFS between the two groups with positive results by ARMS (ARMS + IHC+ vs ARMS + IHC−: median 11.5 vs 10.2 months; P=0.87; Figure 4A). The median PFS in patients with E746_A750del was similar to that in patients with L858R mutations (E746_A750del vs L858R: median 11.3 vs 11.3 months; P=0.18; Figure 4B).
Figure 4.
PFS in patients with different IHC results.
Notes: (A) Progression-free survival (PFS) in two groups categorized by the results of ARMS analysis and IHC staining. There was no significant difference in PFS between the two groups after TKI therapy (ARMS + IHC+ vs ARMS + IHC−: median 11.5 vs 10.2 months; P=0.87). (B) The median PFS in patients with EGFR E746_A750del was similar to that in patients with EGFR L858R mutation (E746_A750del vs L858R: median 11.3 vs 11.3 months; P=0.18).
Abbreviations: ARMS, amplification-refractory mutation system; IHC, immunohistochemistry; TKI, tyrosine kinase inhibitor; EGFR, epidermal growth factor receptor.
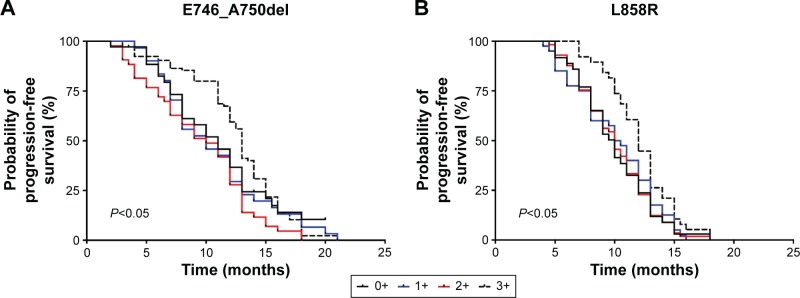
We also evaluated the possible relations between the expression scores of mutant EGFR by IHC analysis and clinical outcomes. Higher expression score (IHC score =3+) of mutant EGFR indicated a longer PFS (13 months; 95% CI =12.0–13.5; P=0.008 for E746_A750del mutations, Figure 5A; 12 months; 95% CI =11.0–13.0; P=0.04 for L858R mutations, Figure 5B), whereas there was no significant difference in the PFS between the groups with lower expression scores by IHC analysis (IHC 1+ vs 2+: median 10.2 vs 10.2 months; P=0.17 for E746_A750del mutations, Figure 5A; IHC 1+ vs 2+: median 10.0 vs 10.25 months; P=0.67 for L858R mutations, Figure 5B).
Figure 5.
PFS in different groups categorized by IHC scores detected by EGFR E746_A750del (A) and L858R (B) mutation-specific antibodies.
Notes: Patients with a high staining score (3+) gained a longer PFS than those from other groups. (A) Median 10.8, 10.2, 10.2, 13.0 for 0+,1+, 2+, 3+, respectively; P<0.05; (B) median 10.0, 10.0, 10.25, 12.0 for 0+,1+, 2+, 3+, respectively; P<0.05.
Abbreviations: PFS, progression-free survival; IHC, immunohistochemistry; EGFR, epidermal growth factor receptor.
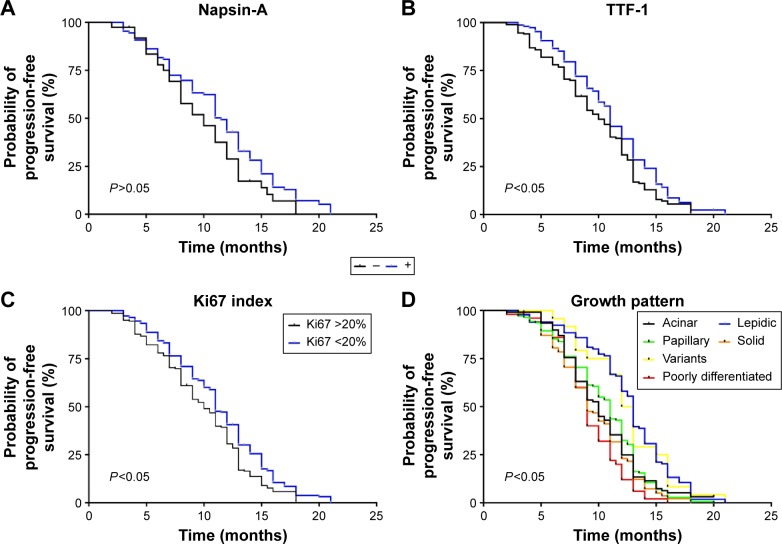
TTF-1 positivity (P=0.014), lower cellular proliferation index (Ki67 index; P=0.018), and lepidic pattern (P<0.01) were associated with longer PFS. Napsin-A positivity (P=0.069) was also a good prognostic factor for PFS, although there was no statistical significance (P=0.069). Survival curves are shown in Figure 6.
Figure 6.
Kaplan–Meier survival curves according to the results of IHC analysis by different pathology markers in patients receiving TKI therapy, including Napsin-A (A), TTF-1 (B), Ki67 index (C), and growth pattern (D).
Abbreviations: IHC, immunohistochemistry; TKI, tyrosine kinase inhibitor; TTF-1, thyroid transcription factor-1.
Discussion
In this study, we demonstrated a high specificity of IHC using EGFR mutation-specific antibodies in advanced lung adenocarcinoma and the correlation between expression scores of mutant EGFRs and efficacy of EGFR-TKI treatment. Moreover, we evaluated the prognostic utility of histologic subtypes of lung adenocarcinoma and other adenocarcinoma markers, including TTF-1, Napsin-A, CK-7 expression, and Ki67 index, for EGFR-TKI treatment in patients with EGFR-activating mutations and their association with IASLC/ATS/ERS classification.
Determination of mutational status in pulmonary adenocarcinoma has entered routine clinical practice, and it is now an integral part of pathological evaluation. We evaluated the availability of EGFR mutation-specific antibodies, targeted to two hot spots: the in-frame deletions in exon 19 centered around codons 746–750 (E746_A750 del) and the point mutation at codon 858 (L858R) in exon 21.16,17
Based on an IHC score of ≥1+ to serve as a criterion of positivity, each antibody showed high specificity for E746_A750 del and L858R (99.6% and 99.3%, respectively), although sensitivity (86.0% and 82.7%, respectively) was not satisfactory. These findings are consistent with those of previous reports using the same mutant-specific antibodies (clone 6B6 and clone 43B2).9,11,18–20 Thus, the excellent specificity of IHC using the two mutation-specific antibodies is clearly demonstrated.
The reason for the high rate of false negativity is mainly due to different expression abundance of mutant EGFR proteins in tumors and the binding efficiency of those antibodies by IHC staining. Besides, the coloring method of IHC staining by diaminobenzidine is a possible reason. The purpose of the present study is to evaluate the availability of mutation-specific antibodies and use it as a prescreening and alternative method in case of tissue shortage in biopsy samples.
In the present study, there were five cases that showed positive reactions to mutation-specific antibodies, but they were EGFR wild type as detected by ARMS analysis. All those cases were biopsy samples with limited tumors and could not be subjected to further analysis by the third method. These patients were excluded from EGFR-TKI therapy due to the EGFR wild-type results by ARMS analysis. However, Kitamura et al21 reported that a patient who showed positive reactions to mutation-specific antibodies, but was EGFR wild type, showed a good clinical response to gefitinib. The possible reasons could be proposed as follows: 1) the limited tumor cells led to the failure of ARMS analysis, 2) the expression of mutation-specific proteins in these patients is due to EGFR mutants that are always not detected by the ARMS method, and 3) there is another mechanism for the expression of mutant proteins. Further studies are needed to explore the possible underlying mechanisms in more similar cases.
In the present study, there were four patients carrying double mutations, as detected by ARMS analysis, which were also validated by IHC analysis. These patients had a prolonged PFS (12.5–15 months, mean =13.6 months) when compared to those patients carrying a single EGFR mutation (E746_A750del vs L858R: median 11.3 vs 11.3 months). The prolonged PFS data demonstrated that these patients benefited more from EGFR-TKI therapy.
The patients harboring mutant EGFRs with a higher expression score (IHC score =3+) had a longer PFS than the other groups (P<0.05). Considering the high concordance between IHC and ARMS, we concluded a strategy on choosing EGFR-TKI treatment for advanced lung adenocarcinoma. The patients harboring mutant EGFRs with an IHC score 3+ could be subjected to EGFR-TKI treatment without further confirmation by other methods, while the other patients with IHC scores, 2+ need a methodological reevaluation for considering EGFR-TKI therapy. However, a further multicenter clinical outcome-based retrospective study is needed to clarify the prediction value of mutation-specific antibodies in EGFR-TKI treatment.
The present study retrospected on the relationship between growth patterns and IHC markers. The acinar, papillary, lepidic, and solid patterns had similar expression ratios on CK-7, TTF-1, Napsin-A, and mutant EGFRs, except ALK. The variant adenocarcinoma and poorly differentiated adenocarcinoma had lower expression ratios on CK-7, TTF-1, Napsin-A, and mutant EGFRs when compared to the acinar, papillary, lepidic, and solid patterns. CK-7, TTF-1, Napsin-A, and mutant EGFRs were tissue-specific markers9,22 for well-differentiated lung adenocarcinomas, which were also consistent with the growth patterns. The lepidic pattern had the lowest expression ratio of ALK, while the variant adenocarcinoma had the highest expression ratio of ALK. Invasive mucinous adenocarcinoma was a variant of lung adenocarcinoma, and most ALK protein positivity was mainly found in solid predominant mucinous adenocarcinoma with a mucin production subtype, as in previous reports.23,24
The prognostic utility of TTF-1,25 Napsin-A,26 Ki67, and growth pattern12–14 for different aspects of lung adenocarcinoma has been previously reported. Only a few studies suggested that IHC markers and histologic features have a predictive role associated with the effectiveness of a specific treatment for lung adenocarcinoma patients, especially the use of EGFR-TKIs for patients with lung adenocarcinoma harboring EGFR mutations. The present study demonstrated that Napsin-A positivity, TTF-1 positivity, lower Ki67 proliferation index, and lepidic pattern were good prognostic factors for EGFR-TKI therapy. Our findings would help physicians potentially select patients who will benefit most from TKI drug treatments, especially when patients face multiple targeted therapies.
Our study also had some limitations. First, it was limited by a small patient number in the ARMS-IHC+ group, with only five cases. Further study might consider a larger sample size to investigate the underlying mechanisms. Second, we did not strictly comply with the 2011 IASLC/ATS/ERS classification. In order to ensure that there were sufficient numbers of patients in each histologic subgroup for a stable analysis, a more detailed classification based on acinar, papillary, and solid patterns was not performed in the invasive adenocarcinoma subgroup. Third, as a clinic-based retrospective study, various characteristics, including sex, age, stage, and line of TKI therapy, were not well balanced between groups. We analyzed only the PFS data in patients. Further study might consider the long-term observations such as the overall survival rate.
Conclusion
In conclusion, patients with advanced lung adenocarcinoma harboring the mutant EGFR protein (IHC score: 3+), Napsin-A positivity, TTF-1 positivity, lower Ki67 proliferation index, and lepidic pattern would benefit from EGFR-TKI therapy than the other cohorts.
Acknowledgments
This work was supported by the National Natural Science Foundation of People’s Republic of China (Grant Nos 81200104 and 81302020) and Medical Scientific Research Foundation of Hubei Province (Grant No JX3B07). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(6):548–582. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 3.International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 4.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 8.Fan X, Liu B, Xu H, et al. Immunostaining with EGFR mutation-specific antibodies: a reliable screening method for lung adenocarcinomas harboring EGFR mutation in biopsy and resection samples. Hum Pathol. 2013;44(8):1499–1507. doi: 10.1016/j.humpath.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Wen YH, Brogi E, Hasanovic A, et al. Immunohistochemical staining with EGFR mutation-specific antibodies: high specificity as a diagnostic marker for lung adenocarcinoma. Mod Pathol. 2013;26(9):1197–1203. doi: 10.1038/modpathol.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo AN, Park TI, Jin Y, et al. Novel EGFR mutation-specific antibodies for lung adenocarcinoma: highly specific but not sensitive detection of an E746_A750 deletion in exon 19 and an L858R mutation in exon 21 by immunohistochemistry. Lung Cancer. 2014;83(3):316–323. doi: 10.1016/j.lungcan.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang G, Hao Y, et al. A comparison of ARMS and mutation specific IHC for common activating EGFR mutations analysis in small biopsy and cytology specimens of advanced non small cell lung cancer. Int J Clin Exp Pathol. 2014;7(7):4310–4316. [PMC free article] [PubMed] [Google Scholar]
- 12.Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6(9):1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Liu Y, Lian F, et al. Lepidic and micropapillary growth pattern and expression of Napsin A can stratify patients of stage I lung adenocarcinoma into different prognostic subgroup. Int J Clin Exp Pathol. 2014;7(4):1459–1468. [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida T, Ishii G, Goto K, et al. Solid predominant histology predicts EGFR tyrosine kinase inhibitor response in patients with EGFR mutation-positive lung adenocarcinoma. J Cancer Res Clin Oncol. 2013;139(10):1691–1700. doi: 10.1007/s00432-013-1495-0. [DOI] [PubMed] [Google Scholar]
- 15.Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010;12(2):169–176. doi: 10.2353/jmoldx.2010.090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 17.Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 18.Bondgaard AL, Hogdall E, Mellemgaard A, et al. High specificity but low sensitivity of mutation-specific antibodies against EGFR mutations in non-small-cell lung cancer. Mod Pathol. 2014;27(12):1590–1598. doi: 10.1038/modpathol.2014.67. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Wang X, Xue L, et al. The use of mutation-specific antibodies in predicting the effect of EGFR-TKIs in patients with non-small-cell lung cancer. J Cancer Res Clin Oncol. 2014;140(5):849–857. doi: 10.1007/s00432-014-1618-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Liu HB, Yu CH, et al. Diagnostic value of mutation-specific antibodies for immunohistochemical detection of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. PLoS One. 2014;9(9):e105940. doi: 10.1371/journal.pone.0105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura A, Hosoda W, Sasaki E, et al. Immunohistochemical detection of EGFR mutation using mutation-specific antibodies in lung cancer. Clin Cancer Res. 2010;16(13):3349–3355. doi: 10.1158/1078-0432.CCR-10-0129. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Li X, Yin J, et al. The high diagnostic accuracy of combined test of thyroid transcription factor 1 and Napsin A to distinguish between lung adenocarcinoma and squamous cell carcinoma: a meta-analysis. PLoS One. 2014;9(7):e100837. doi: 10.1371/journal.pone.0100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Pan Y, Li Y, et al. Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. Onco Targets Ther. 2014;7:1423–1437. doi: 10.2147/OTT.S58900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y, Zhao D, Mu J, et al. Prognostic analysis of primary mucin-producing adenocarcinoma of the lung: a comprehensive retrospective study. Tumour Biol. 2015 Aug 9; doi: 10.1007/s13277-015-3869-1. Epub. [DOI] [PubMed] [Google Scholar]
- 25.Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13(8B):1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JG, Kim S, Shim HS. Napsin A is an independent prognostic factor in surgically resected adenocarcinoma of the lung. Lung Cancer. 2012;77(1):156–161. doi: 10.1016/j.lungcan.2012.02.013. [DOI] [PubMed] [Google Scholar]