Abstract
Background
Delirium and frailty – both potentially reversible geriatric syndromes – are seldom studied together, although they often occur jointly in older patients discharged from hospitals. This study aimed to explore the relationship between delirium and frailty in older adults discharged from hospitals.
Methods
Of the 221 patients aged >65 years, who were invited to participate, only 114 gave their consent to participate in this study. Delirium was assessed using the confusion assessment method, in which patients were classified dichotomously as delirious or nondelirious according to its algorithm. Frailty was assessed using the Edmonton Frailty Scale, which classifies patients dichotomously as frail or nonfrail. In addition to the sociodemographic characteristics, covariates such as scores from the Mini-Mental State Examination, Instrumental Activities of Daily Living scale, and Cumulative Illness Rating Scale for Geriatrics and details regarding polymedication were collected. A multidimensional linear regression model was used for analysis.
Results
Almost 20% of participants had delirium (n=22), and 76.3% were classified as frail (n=87); 31.5% of the variance in the delirium score was explained by frailty (R2=0.315). Age; polymedication; scores of the Confusion Assessment Method (CAM), instrumental activities of daily living, and Cumulative Illness Rating Scale for Geriatrics; and frailty increased the predictability of the variance of delirium by 32% to 64% (R2=0.64).
Conclusion
Frailty is strongly related to delirium in older patients after discharge from the hospital.
Keywords: Edmonton Frailty Scale, delirium risk factors, cognitive impairment, physical impairment
Introduction
Delirium is a mental disorder with an acute onset and fluctuating course, characterized by disturbances of consciousness, orientation, memory, thought, perception, and behavior.1 This syndrome represents a serious problem in acute care hospitals and is associated with decreased functional status, longer periods of time before recovery and discharge, institutionalization, premature mortality, and increased health care costs. The etiology of delirium is considered to be multifactorial, with an occurrence rate as high as 83% in hospitals.2 Multicomponent delirium prevention strategies have been shown in intervention studies to consistently reduce the occurrence of delirium.3 Based on this evidence base, the National Institute for Health and Care Excellence has advocated the adoption of multicomponent delirium prevention interventions into the routine care for older inpatients.4 However, despite successful reductions in incident delirium by about a third, anticipated reductions in mortality or admissions to long-term care have not been conclusively observed.5 One-third of older patients still have undetected and unresolved symptoms of delirium at hospital discharge.6
Frailty is a geriatric condition characterized by diminished strength, endurance, and physiological functions that increase an individual’s vulnerability to adverse outcomes such as falls, sarcopenia, institutionalization, worsened disability, and premature death.7 Frailty was recently defined as a state of increased vulnerability related to a poor level of homeostasis after a stressful event, with a corresponding increased risk of adverse outcomes, including falls, delirium, and disability.7 Frailty has been linked to the development and progression of many age-related diseases and syndromes.8
However, little is known about the relationship of frailty as a precipitating risk factor for specific geriatric syndromes in hospitalized older patients.9 Frailty associated with hospitalization may occur in one-third of older patients and can be triggered even when the illness causing hospitalization is successfully treated.7 Frailty has a considerable influence not only on outpatients’ independence and quality of life but also on their use of health care services.10
Delirium and frailty appear to be distinct clinical geriatric conditions. However, in vulnerable older adults, both syndromes can appear simultaneously in response to a stressor.9 Besides the elevated rates of delirium during hospitalization, studies have reported about the important consequences of frailty among older adults discharged from hospital, including increased dependence as shown on the Instrumental Activities of Daily Living (IADL) scale, weight loss, and cognitive impairment.11 These consequences result from the acute syndrome of delirium itself and are also related to the management of patient care during hospitalization. They can lead to a state of severe frailty at discharge, with a high risk of premature death.12
Delirium has been associated with increased long-term cognitive impairment and acceleration of existing cognitive decline. Furthermore, it may delay physical and cognitive recovery, ultimately resulting in new or increasing frailty and long-term disability and institutionalization. Both frailty and delirium in older patients can affect independent functioning and the ability to live at home.9 A study on older patients who had undergone cardiac surgery and then developed postoperative delirium showed that delirium was associated with an approximately twofold increase in the risk of frailty after 1 month.13 However, few studies have examined the relationship between frailty and delirium among older patients.14,15
Therefore, we hypothesized that delirium and frailty were associated in a sample of older adult patients discharged from a hospital.
Methods and materials
Data were sourced from a prospective clinical trial entitled “Effect estimation of an innovative nursing intervention to improve delirium among home-dwelling older adults,” which was conducted in 2012 and published elsewhere.16 The trial protocol was approved by the cantonal medical ethics committee of Valais, Switzerland (CCVEM 030/11). Written informed consent was obtained from all participants or from their closest relatives if they scored <15 points on the Mini-Mental State Examination (MMSE).17
Participants
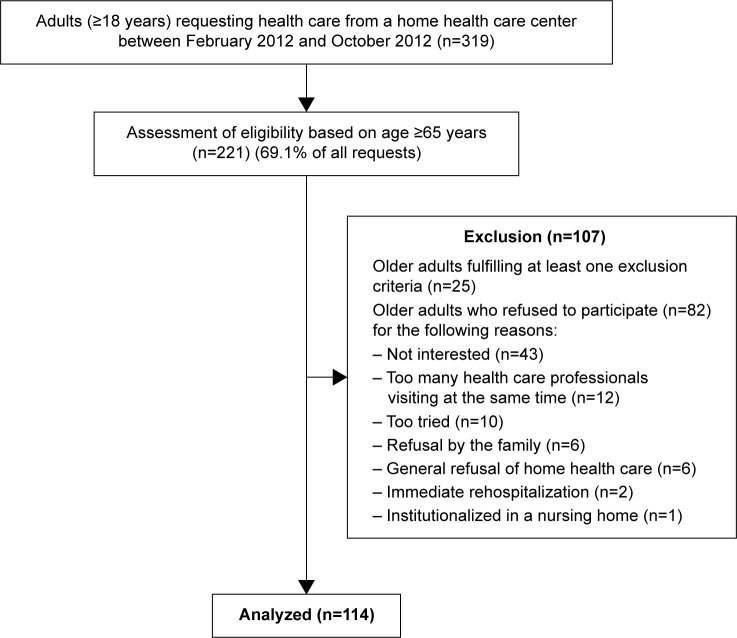
In the original study, a nonprobabilistic sample of 221 eligible participants was contacted within 48 hours of their hospital discharge, in collaboration with a home health care service, in order to request their participation. For different reasons, 107 older adults refused to participate, resulting in a final sample of 114 participants. Patients with a medical prescription for home health care were eligible to participate if they were 1) aged 65 years or older, 2) discharged after hospitalization of at least ≥48 hours, and 3) capable of understanding and answering questions in French. Figure 1 presents the recruitment procedure.
Figure 1.
Recruitment of the participants.
Measurements
Assessment of delirium
Delirium was assessed using the validated French version of the Confusion Assessment Method (CAM).18 The CAM is an instrument developed to assist clinicians in identifying patients with delirium. It has been considered suitable for bedside use.19 The psychometric properties of the CAM have been documented as excellent, with 94% sensitivity, 89% specificity, and a Kappa’s inter-rater reliability between 0.70 and 1.00.19 The research nurse or the principal investigator (PI) completed the CAM form based on a structured interview, patient records, and clinical observation of the symptoms/signs described in the CAM. Data were subsequently analyzed categorically by using the CAM algorithm. The inter-rater reliability between the PI and the research nurse (trained in CAM assessment) showed a satisfactory Kappa Cohen’s coefficient of 0.79.20
Assessment of frailty
The frailty status was assessed using the Edmonton Frailty Score (EFS) developed by Rolfson et al21 and been evaluated in a systematic review of de Vries et al.22 This instrument consists of nine domains and eleven items, each scoring 0 points (frailty absent or normal health), 1 point (minor errors or mild/moderate impairment), or 2 points (important errors or severely impaired). The domains include cognitive impairment, autoevaluation of general health status, functional dependency, presence of social support, drug treatments and adherence, nutrition and mood, presence of incontinence, and the “timed up and go (TUG)” test. The TUG tests the basic mobility skills of frail elderly persons. It consists of a measurement of the time in seconds for a person to rise from sitting from a standard arm chair, walk 3 m, turn, walk back to the chair, and sit down. A cutoff score of ≥20 seconds was shown to predict falls in community-dwelling frail elderly people.23
The total score from 0 to 3 points indicates no frailty; 4 or 5 points indicate prefrailty; 6 to 8 points indicates frailty; and 9 to 17 points indicates severe frailty. In order to dichotomize between frail and not frail, a score of 6 points or more was considered as frail. The eleven-item EFS has good psychometric properties and showed an acceptable Cronbach’s alpha of 0.62 in the present study.21 The inter-rater reliability between the PI and the research nurse was excellent (Kappa Cohen’s coefficient =0.85).
Other variables
Sociodemographic characteristics assessed included age, sex, marital status, and level of education (Table 1).
Table 1.
Sociodemographic characteristics of participants (n=114)
| Variables | |
|---|---|
| Age (years) | |
| Average (SD) | 83.2 (7.2) |
| Sex | |
| Female (%) | 74 (64.9) |
| Marital status | |
| Single (%) | 7 (6.1) |
| Married/partner (%) | 41 (36.0) |
| Divorced/separated (%) | 6 (5.3) |
| Widowed (%) | 60 (52.6) |
| Education | |
| Primary (%) | 13 (12.5) |
| Secondary (%) | 38 (36.5) |
| Vocational (%) | 33 (31.7) |
| University (%) | 20 (19.2) |
Abbreviation: SD, standard deviation.
Cognitive level was assessed using the MMSE by regrouping the seven domains of cognitive functioning.17,24 The eleven-item instrument measures orientation, memory, language, and psychomotor skills. The sum of the scores varies from 0 (severe cognitive impairment) to 30 (no cognitive impairment). A score of <24 points was considered as the cutoff point for cognitive impairment. The MMSE was mostly assessed by the PI, and the instrument presents good psychometric proprieties.25 An excellent inter-rater ratio was obtained between the PI and the research nurse with an intraclass correlation of 0.92.
Functional status was assessed using the Lawton Index of IADL, an assessment considered appropriate for older adults living at home.26,27 The IADL scale assesses independence versus dependence in more complex areas of daily living, such as using the telephone, shopping, preparing meals, cleaning and laundry, using public transport, management of medication and finances, and maintaining a home.28 A score of <16 indicates that the patient is independent, as documented elsewhere.28 Inter-rater reliability was excellent (Kappa Cohen’s coefficient =0.85). Finally, comorbidities were assessed using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). The CIRS-G scores diseases in 13 organ systems and grades each organ according to severity using explicit rules for classification. Data for CIRS-G scoring were compiled from comprehensive patient interview and chart records. A CIRS-G index of comorbidity of ≥2 implies the presence of moderate and severe illnesses.29 Inter-rater scores between the PI and the research nurse were very high (Pearson’s correlation =0.81). The number of prescribed medications was recorded during patient interviews and through patient records. Participants’ health status and average medication use are presented in Table 2.
Table 2.
Health status of participating delirious and nondelirious discharged older adults
| Variables | No delirium (n=20) | Delirium (n=94) | P-value |
|---|---|---|---|
| Sex | |||
| Male/female (n=114) | 32/62 | 8/12 | 0.612b |
| Age | |||
| Average (SD) | 83.2 (7.2) | 83.5 (7.2) | 0.875c |
| Edmonton Frailty Scale (EFS) | |||
| Average (SD) | 7.96 (2.6) | 10.05 (3.3) | <0.001c,* |
| Range | 1–17 | 1–17 | |
| Frail vs not fraild | |||
| Frail (%) | 71 (75.5) | 18 (90.0) | 0.235e |
| Not frail (%) | 23 (24.5) | 2 (10.0) | |
| Health status | |||
| Cognitive impairment, MMSE (SD) | 24.57 (4.3) | 19.20 (5.4) | <0.001c,* |
| Functional status, IADL (SD) | 20.59 (5.6) | 23.70 (6.0) | 0.029c,* |
| Comorbidities, CIRS-G (SD) | 13.68 (3.2) | 14.25 (2.5) | 0.458c |
| Average no daily medications (SD) | 6.30 (3.1) | 6.30 (2.6) | 0.997c |
Notes:
Pearson’s chi-squared test.
Student’s t-test.
Based on EFS, frail ≥6 points.
Fisher’s exact test.
Significance P≤0.05.
Abbreviations: SD, standard deviation; MMSE, Mini-Mental State Examination; IADL, Instrumental Activities of Daily Living; CIRS-G, Cumulative Illness Rating Scale for Geriatrics.
Data collection
After the participants had given their consent, data were collected during a single home visit within 48 hours of discharge from the hospital. During the home visits, the data collection procedure was standardized to avoid any bias by using an invariable sequence of questions. Data on delirium, frailty, cognition, health status, comorbidities, and medication treatment were collected immediately after consent of the patients at their place they live. It is important to mention that during hospitalization and at hospital discharge, no delirium of frailty assessment was done. A detailed description of the data collection procedure has been published elsewhere.16
Statistical analyses
Sociodemographic and baseline characteristics of subjects with and without frailty were compared using the Student’s t-test for continuous variables and a chi-squared distribution for categorical ones. The odds ratio (OR) of delirium/frailty was calculated using a contingency table in a joint frequency distribution. The association between delirium and frailty was assessed using linear regression, and this association was adjusted for age, cognitive state (MMSE), physical state (IADL), chronic conditions reported (CIRS-G), and polymedication. Collinearity was verified using a matrix correlation and tolerance values.30 All statistical tests were performed using the IBM-Statistical Package for the Social Sciences (IBM-SPSS®), Version 22.31 Statistical significance was set at P=0.05, and all tests were two tailed.
Results
Participant characteristics
No statistically significant differences were found between men and women with regard to being nondelirious or delirious (P=0.612) and their ages (P=0.875; Table 2). Similarly, there were no statistically significant differences with regard to being frail and nonfrail (P=0.560) and their ages (P=0.742; Table 3).
Table 3.
Health status among frail and nonfrail discharged older adults
| Variables | Not fraila (n=25) | Fraila (n=89) | P-value |
|---|---|---|---|
| Sex | |||
| Men/women | 10/15 | 30/59 | 0.560b |
| Age (SD) | |||
| Average (SD) | 82.80 (8.3) | 83.34 (6.9) | 0.742c |
| Health status | |||
| Cognitive impairment, MMSE (SD) | 26.28 (3.5) | 22.89 (5.0) | 0.002c,* |
| Functional status, IADL (SD) | 16.64 (4.7) | 22.39 (5.5) | <0.000c,* |
| Comorbidities, CIRS-G (SD) | 11.36 (3.6) | 14.46 (2.6) | <0.000c,* |
| Average number daily medications (SD) | 5.20 (2.4) | 6.61 (2.7) | 0.019c,* |
Notes:
Based on EFS, frail ≥6 points.
Pearson’s chi-squared test.
Student’s t-test.
Significance P≤0.05.
Abbreviations: SD, standard deviation; MMSE, Mini-Mental State Examination; IADL, Instrumental Activities of Daily Living; CIRS-G, Cumulative Illness Rating Scale for Geriatrics; EFS, Edmonton Frailty Scale.
Prevalence of delirium and frailty
Almost one-fifth of the sample (n=20) presented with delirium as measured by the CAM. Almost two-thirds of the frail older adults presented with three or more symptoms of delirium (Table 2). Three quarters of participants (n=89) presented with frailty, including 90% of the delirious participants (n=18). The mean frailty score for nondelirious participants was significantly lower than that for delirious participants (P<0.001; Table 2).
Measurements of IADL at hospital discharge showed statistically significant differences in dependency: nonfrail participants were less dependent than frail ones (P<0.001), and nondelirious participants were less dependent than delirious ones (P=0.029). Similar statistical differences were observed. With regard to cognitive status, nonfrail participants had a better cognitive status than frail ones (P=0.002), and nondelirious participants had a better cognitive status than delirious ones (P<0.001). Most of the participants had comorbidities and were being prescribed more than four medications. A significant difference was observed in the comorbidity rates and the average number of medications of frail and nonfrail participants (P=0.019), but the difference was not significant between nondelirious and delirious participants (P=0.997; Tables 2 and 3).
The high prevalence of frailty among delirious participants reflects the fact that in four of the eleven EFS items – namely cognitive impairment, medication adherence problems, incontinence, and the “timed up and go” test – the scores significantly increased in delirious participants compared to nondelirious participants (P≤0.001, P=0.018, P=0.004, and P=0.041, respectively; Table 4).
Table 4.
Distribution of EFS items in delirious and nondelirious discharged older adults
| Variables | Delirium | No delirium | P-value |
|---|---|---|---|
| Cognitive impairment | |||
| No errors | 3 (15.0%) | 19 (20.2%) | |
| Minor errors | 2 (10.0%) | 46 (48.9%) | |
| Major errors | 15 (75.0%) | 29 (30.9%) | 0.001* |
| General health | |||
| Number of hospitalizations | |||
| 0 | 0 (0.0%) | 18 (19.1%) | |
| 1–2 | 17 (85.0%) | 68 (72.3%) | |
| >3 | 3 (15.0%) | 8 (8.5%) | 0.086 |
| Autoevaluation of health | |||
| Excellent to good | 3 (15.0%) | 20 (21.3%) | |
| Acceptable | 9 (45.0%) | 59 (62.8%) | |
| Bad | 8 (40.0%) | 15 (16.0%) | 0.052 |
| Functional independence | |||
| IADL dependency score | |||
| 0–1 | 1 (5.0%) | 9 (9.6%) | |
| 2–4 | 4 (20.0%) | 40 (42.6%) | |
| 5–8 | 15 (75.0%) | 45 (47.9%) | 0.087 |
| Social support available | |||
| Always | 18 (90.0%) | 79 (84.0%) | |
| Mostly | 2 (10.0%) | 14 (14.9%) | |
| No support | 0 (0.0%) | 1 (1.1%) | 0.755 |
| Use of five or more medications | |||
| No | 7 (35.0%) | 33 (35.1%) | |
| Yes | 13 (65.0%) | 61 (64.9%) | 0.993 |
| Risks of nonadherence | |||
| No | 8 (40.0%) | 64 (68.1%) | |
| Yes | 12 (60.0%) | 30 (31.9%) | 0.018* |
| Nutrition, weight loss | |||
| No | 11 (55.0%) | 51 (54.3%) | |
| Yes | 9 (45.0%) | 43 (45.7%) | 0.952 |
| Mood, sad, or depressed | |||
| No | 13 (65.0%) | 57 (60.6%) | |
| Yes | 7 (35.0%) | 37 (39.4%) | 0.716 |
| Incontinence | |||
| No | 5 (25.0%) | 57 (60.6%) | |
| Yes | 15 (75.0%) | 37 (39.4%) | 0.004* |
| Up and go test | |||
| 0–10 seconds | 7 (35.0%) | 47 (50.0%) | |
| 11–20 seconds | 2 (10.0%) | 22 (23.4%) | |
| >20 seconds | 11 (55.0%) | 25 (26.6%) | 0.041* |
Notes: Pearson’s chi-squared test or Fisher’s exact test.
Significance P≤0.05.
Abbreviations: EFS, Edmonton Frailty Scale; IADL, Instrumental Activities of Daily Living.
Association between frailty and delirium
The OR of delirium/frailty indicated that frailty was 1.19 times more present (statistically significant) among participants with delirium than among participants without delirium, with a 95% confidence interval (95% CI) of 1.00–1.43. Cognitive status (MMSE) seemed to be a stronger risk factor for delirium than frailty, with an OR of 4.31 (95% CI [1.51–12.28]). Neither IADL (OR 1.13; 95% CI [0.91–1.41]), age (OR 1.12; 95% CI [0.43–2.96]), and CIRS-G (OR 1.04; 95% CI (0.58–1.89]) nor polymedication (OR 1.03; 95% CI [0.34–3.14]) were significant risk factors for delirium.
Linear regression indicated that 32% of the variance in delirium was explained by frailty alone (R2=0.315; P<0.001), thus suggesting that frailty could be strongly associated.32 Being frail explained one-third of the presence of delirium in participants. In a multiple regression model, including age, cognitive and physical state, polymedication, and comorbidities, 64% of the variance in delirium could be explained by these variables (adjusted R2=0.635; P≤0.001; Table 5).
Table 5.
Simultaneous multiple regression analysis summary for age, polymedication, frailty, physical and cognitive impairment, and comorbidity
| Variables | B | SE B | Beta | t | P-value |
|---|---|---|---|---|---|
| Age | −0.036 | 0.015 | −0.143 | −2.354 | 0.020* |
| Polymedication | −0.029 | 0.044 | −0.042 | −0.658 | 0.512 |
| Cognitive impairment (MMSE) | −0.262 | 0.027 | −0.771 | −9.610 | <0.001* |
| Delirium (CAM) | −0.052 | 0.414 | −0.003 | −0.903 | 0.396 |
| Frailty (EFS) | 0.193 | 0.045 | 0.309 | 4.324 | <0.001* |
| Physical impairment (IADL) | −0.025 | 0.022 | −0.080 | −1.097 | 0.275 |
| Comorbidity | −0.033 | 0.040 | −0.056 | −0.816 | 0.417 |
| Constant | 11.472 | 1.776 | – |
Notes: Adjusted R2=0.64; F(6, 107) =33.81, P≤0.001.
Significant association of delirium.
Abbreviations: SE, standard error; MMSE, Mini-Mental State Examination; CAM, Confusion Assessment Method; EFS, Edmonton Frailty Scale; IADL, Instrumental Activities of Daily Living.
No collinearity was found between frailty scores, CAM scores (T-B Kendall =−0.130; variance inflation factor <10), IADL scores (r=0.44; variance inflation factor <10), and MMSE scores (r=0.45; variance inflation factor <10).33
Discussion
Our results indicate that frailty is strongly associated with delirium in older patients as one-fifth of the participants presented delirium at hospital discharge.2 Nine of ten delirious participants were frail, whereas only three quarters of the nondelirious participants were frail. These findings are consistent with those of other recent studies indicating an elevated prevalence of frailty among discharged older adults with delirium.34,35 However, our study results do not allow to explain this association, and it has recently been mentioned by Teale and Young that the underlying interaction between these two geriatric syndromes is poorly understood.5
Almost all the participants had some functional impairment, and a third of participants presented a significant cognitive impairment at discharge, which corroborates results from other recent studies.36 This highlighted an urgent need for health care owing to a high number of hospitalized older patients presenting several symptoms of frailty. In addition, as already documented elsewhere, discharged older patients face a risk of delirium due to the presence of multifactorial delirium risk factors.37 Some studies have observed a strong relationship between delirium and physical decline, as manifested by an elevated number of hospital-related falls. However, the present study was unable to confirm this relationship, as hospital records concerning adverse events during hospitalization, such as falls and other deleterious events, were incomplete.38 As in other studies, our results showed no association between age and frailty within the cohort.8
In the present study, frailty alone is associated with delirium in more than one-third of cases. In a more complex model – including age, physical and cognitive impairment, polymedication, and polymorbidity – the association of delirium increased, having an impact in up to two-thirds of cases. Thus, cognitive screening alone was more strongly related to delirium than frailty, but cognitive and frailty screening together was even better. These findings confirm that comprehensive geriatric assessment (CGA) and screening for frailty increase early detection of delirium and delirium risks in discharged older adults. Above all, hospitalization itself should be considered as an important delirium and frailty risk factor. Interdisciplinary preventive care strategies focusing on limiting physical and cognitive decline during hospitalization may be effective in averting delirium and frailty and other poor posthospitalization outcomes.39 Health care reforms focusing on building more effective and efficient care models for older inpatients are urgently needed to develop “senior-friendly” hospitals, including specialized geriatric care units.
Although the delirium rate among our participants was consistent with other studies, the prevalence of frailty was less easy to compare.35 The challenges of detecting delirium in patients after hospital discharge are well described, in contrast to frailty where prevalence is more dependent on the measurement scale.40 In the present study, delirium detection and frailty assessment were combined within clinical assessment and routinely collected data; they could be determined for all patients, regardless of their cognitive or functional abilities, thus increasing their potential utility in the clinical setting. The presence of both frailty and delirium confer a particularly poor prognosis, such as dependency, institutionalization, or even premature death.15,41 However, as delirium and frailty are both viewed as modifiable geriatric syndromes and given the current trend for moving health care out of the hospital into the home, this raises important questions about patient care management.7 Nevertheless, there are links between delirium and frailty.42,43 Although frailty is a chronic condition and delirium is a more acute condition, the unavoidable question of a common pathophysiological mechanism persists. Frailty and delirium may be different clinical expressions of a shared vulnerability to stress in older adults, and future research should determine whether this vulnerability is age related, pathological, genetic, environmental, or most likely, a combination of all of these factors.44 Besides the fact that both syndromes represent significant sources of morbidity and mortality for older adults, both of them are multifactorial, with risk factors and potentially causative mechanisms (eg, inflammation, atherosclerosis, and poor nutrition) that overlap.7 Although there are proven measures for preventing delirium, evidence regarding interventions to improve outcomes following delirium remains uncertain.2,39 Similarly, while complex interventions such as education, optimized nutrition, and exercise have been suggested for delaying or preventing frailty, there is as yet no evidence that such interventions can mitigate these outcomes in frail older inpatients.45,46 Whether multicomponent, nonpharmacological, interdisciplinary interventions are effective in preventing frailty and delirium in hospitalized older adult patients should be the focus of further inquiry. Further research on a larger group of discharged older adults is also required.
Finally, this study has both strengths and limitations. The first strength was the comprehensive geriatric assessment approach used, involving patient records, clinical and CAM assessment after discharge, and optimized delirium detection and frailty assessment. Another strength was that all the participants were accurately screened for their health status and frailty indicators, and they were discriminated by the presence of delirious or nondelirious symptoms.
In addition to the relatively small sample group, it seems important to mention the limiting factor that data were collected at a single hospital site, and thus generalization of these results needs caution. Furthermore, comprehensive geriatric assessment data from the emergency department was incomplete, and therefore we could not make a precise interpretation of participants’ mental status at hospital admission. Another limitation of the study concerns the correctional nature of the data. Using the EFS did impose some methodological limits to the study, as the most widely used screening tool to assess frailty is that proposed by Fried et al.47 This requires the presence of at least three of the five following criteria: weight loss, exhaustion, weak grip strength, slow walking speed, and low physical activity. However, exhausted older inpatients, once discharged, are often unable to complete these performance-based tests, and thus many of the participants in the present study could not have been assessed using such physical testing.
The syndromes of delirium and frailty are highly associated in discharged older adults. However, currently, detecting their delirium remains problematic for several reasons. On the other hand, assessing frailty, either through systematic CGA or by using specific frailty-detection tools, gives health care professionals the opportunity to improve the effectiveness of primary prevention strategies for delirium, by promptly ascertaining which discharged older adults are at a high risk of presenting with that syndrome.
Acknowledgments
We thank the patients and their families for their participation in this study. This work would not have been possible without the input of Ms Ariane Kapps, a Master of Science student who helped to develop the intervention, as well as that of all the participating nurses of the Home Health Care Service of Valais. The authors also thank Ms Sylvana Gerber for her contribution to successful data collection.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465–470. doi: 10.1503/cmaj.080519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721–727. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 5.Teale EA, Young JR. Multicomponent delirium prevention interventions: not as effective as NICE suggest? Age and Ageing. 2015;44(6):915–917. doi: 10.1093/ageing/afv120. [DOI] [PubMed] [Google Scholar]
- 6.McAvay GJ, Van Ness PH, Bogardus ST, Jr, et al. Older adults discharged from the hospital with delirium: 1-year outcomes. J Am Geriatr Soc. 2006;54(8):1245–1250. doi: 10.1111/j.1532-5415.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 7.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santangelo A, Testai M, Maugeri D. Delirium is marker of frailty? Study in a population over 90-year old recovered in a Sicilian RSA. Eur Psychiatry. 2010;25:588. [Google Scholar]
- 9.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(suppl 2):S262–S268. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abellan Van Kan G, Vellas B. Is the mini nutritional assessment an appropriate tool to assess frailty in older adults? J Nutr Health Aging. 2011;15(3):159–161. doi: 10.1007/s12603-011-0031-7. [DOI] [PubMed] [Google Scholar]
- 11.Buurman BM, Hoogerduijn JG, van Gemert EA, de Haan RJ, Schuurmans MJ, de Rooij SE. Clinical characteristics and outcomes of hospitalized older patients with distinct risk profiles for functional decline: a prospective cohort study. PLoS One. 2012;7(1):e29621. doi: 10.1371/journal.pone.0029621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clegg A, Young J. The frailty syndrome. Clin Med. 2011;11(1):72–75. doi: 10.7861/clinmedicine.11-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112(5):1199–1201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pol RA, van Leeuwen BL, Visser L, Izaks GJ, van den Dungen JJAM, Tielliu IFJ. Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: a prospective cohort study. Eur J Vasc Endovasc Surg. 2011;42(6):824–830. doi: 10.1016/j.ejvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Leung JM, Tsai TL, Sands LP. Preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112(5):1199–1201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verloo H, Goulet C, Morin D, von Gunten A. Effect estimation of an innovative nursing intervention to improve delirium among home-dwelling older adults: a randomized controlled pilot trial. Dement Geriatr Cogn Dis Extra. 2015;5(1):176–190. doi: 10.1159/000375444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Laplante J, Cole MG, McCusker J, Singh S, Ouimet MA. Confusion assessment method. Validation of a French-language version. Perspect Infirm. 2005;3(1):12–20. [PubMed] [Google Scholar]
- 19.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hair JKJ, Black WC, Babin BJ, Anderson RE. Multivariate Data Analysis: A Global Perspective. 7th ed. Boston: Pearson Education Inc; 2010. [Google Scholar]
- 21.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;6:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed up & go: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 24.Derouesné C, Poitreneau J, Hugonot L, Kalafat M, Dubois B, Laurent B. Le mini-mental state examination (MMSE): un outil pratique pour l’évaluation de l’état cognitif des patients par le clinicien, version française consensuelle. [Mini mental state Exam: a practical tool for clinicians to assess the cognitive state of patients, French consensual version] La Presse Médicale. 1999;28(21):1141–1148. French. [PubMed] [Google Scholar]
- 25.Naugle RI, Kawczak K. Limitations of the mini-mental state examination. Cleve Clin J Med. 1989;56(3):277–281. doi: 10.3949/ccjm.56.3.277. [DOI] [PubMed] [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 27.Buurman BM, van Munster BC, Korevaar JC, de Haan RJ, de Rooij SE. Variability in measuring (instrumental) activities of daily living functioning and functional decline in hospitalized older medical patients: a systematic review. J Clin Epidemiol. 2011;64(6):619–627. doi: 10.1016/j.jclinepi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, Dartigues JF. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40(11):1129–1134. doi: 10.1111/j.1532-5415.1992.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 30.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th ed. Boston: Pearson Education Inc; 2013. [Google Scholar]
- 31.IBM-SPSS . Statistical Package for Social Sciences. Somer, NY: IBM Corporation; 2011. [Google Scholar]
- 32.Cohen J. Statistical Power and Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NY: Lawrence ERdbaum Associates; 1988. [Google Scholar]
- 33.Mason CH, Perreault WD., Jr Collinearity, power, and interpretation of multiple regression analysis. J Market Res. 1991;28(3):268–280. [Google Scholar]
- 34.Eeles EMP, Peel NM, White SV, O’Mahony MS, Bayer AJ, Bhat R. Frailty and illness severity in relation to delirium in older inpatients. Australas J Ageing. 2013;32:28. [Google Scholar]
- 35.Joosten E, Demuynck M, Detroyer E, Milisen K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6-month mortality in hospitalized older patients. BMC Geriatr. 2014;14:1. doi: 10.1186/1471-2318-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoogerduijn JG, Buurman BM, Korevaar JC, Grobbee DE, de Rooij SE, Schuurmans MJ. The prediction of functional decline in older hospitalised patients. Age Ageing. 2012;41(3):381–387. doi: 10.1093/ageing/afs015. [DOI] [PubMed] [Google Scholar]
- 37.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. J Am Med Assoc. 2011;306(11):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 38.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. J Am Med Assoc. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 39.Cameron I, Fairhall N, Gill L, Lockwood K, Langron C, Aggar C. Developing interventions of frailty. Adv Geriatr. 2015;2015:845356. [Google Scholar]
- 40.Clegg A, Westby M, Young JB. Under-reporting of delirium in the NHS. Age Ageing. 2011;40(2):283–286. doi: 10.1093/ageing/afq157. [DOI] [PubMed] [Google Scholar]
- 41.Eeles EMP, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012;41(3):412–416. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- 42.Andrew MK. Le capital social et la santé des personnes âgées. [Social capital and healthy aging] Retraite et Société. 2005;46:132–145. French. [Google Scholar]
- 43.Agostini JV, Zhang Y, Inouye SK. Use of a computer-based reminder to improve sedative-hypnotic prescribing in older hospitalized patients. J Am Geriatr Soc. 2007;55(1):43–48. doi: 10.1111/j.1532-5415.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 44.Bergman H, Ferrucci L, Guralnik JM, Hogan DB, Hummel S, Karunananthan S. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oo MT, Tencheva A, Khalid N, Chan YP, Ho SF. Assessing frailty in the acute medical admission of elderly patients. J R Coll Physicians Edinb. 2013;43(4):301–308. doi: 10.4997/JRCPE.2013.404. [DOI] [PubMed] [Google Scholar]
- 46.Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, age UK and Royal College of General Practitioners report. Age Ageing. 2014;43(6):744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 47.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]