Abstract
Overlying water, sediment, rhizosphere sediment and mangrove seedlings in the Futian mangrove forest were analyzed for heavy metals. The results showed that mangrove plant acidified sediment and increased organic matter contents. Except for chromium (Cr), nickel (Ni) and copper (Cu) in Aegiceras corniculatum sediment, heavy metals in all sediments were higher than in overlying water, rhizosphere sediment and mangrove root. Heavy metals in Avicennia marina sediments were higher than other sediments. The lower heavy metal biological concentration factors (BCFs) and translocation factors (TFs) indicated that mangrove plant adopted exclusion strategy. The geo-accumulation index, potential ecological risk index and risk assessment code (RAC) demonstrated that heavy metals have posed a considerable ecological risk, especially for cadmium (Cd). Heavy metals (Cr, Ni, Cu and Cd) mainly existed in the reducible fractions. These findings provide actual heavy metal accumulations in sediment-plant ecosystems in mangrove forest, being important in designing the long-term management and conservation policies for managers of mangrove forest.
Introduction
Heavy metal contaminants have gained attention increasingly since recent decades due to their persistence and biotoxicity in the water and soil environment [1–3]. Generally, the contaminants are released into ambient environments mainly via raw or insufficiently treated industrial wastewater effluents, vehicle emissions, mining and the combustion of coals [4, 5]. Located in estuaries or along coastlines, mangrove ecosystem contributes to metal pollution remediation by redistributing pollutants between the land and sea in their biogeochemical cycles. Generally, mangrove increases metal accumulation in sediments by modifying the soil acidity, redox potential, organic contents and salinity [6, 7], and subsequently reduces metal exposure to adjacent aquatic environment [8]. However, it is probable for metals being released and transported from sediments to water when soil physicochemical properties change, which may change their chemical speciation and cause potential ecological impacts and human health issues [9–12].
The systematical quantitative evaluation of heavy metal contamination contributes to the understanding of the potential ecological risk [13]. The most commonly cited assessment indices in environmental studies include geo-accumulation index (Igeo), potential ecological risk index (RI), risk assessment code (RAC), enrichment factors (EF) and mean probable effect level quotient (m-P-Q). These methods are widely used to evaluate the heavy metal pollution in farming soils [14], urban soils [15], mine soils [16], and lake sediments [17]. In order to update the comprehensive understanding on this problem, the integrated application of multi-assessment methods to evaluate the ecological risk is important [18–21].
Futian mangrove forest is the only mangrove located in the middle of Shenzhen, South China (adjacent to Mai Po Nature Reserve, Hong Kong) and has suffered serious heavy metal pollution since the early 1990s [22, 23]. Previous studies mainly focused on heavy metal accumulation in mangrove plants and sediments [22–25]. Up to now, no systematic and specialized research has focused on heavy metals in sediment-mangrove plant systems, which are important for understanding heavy metal geochemical cycling in mangrove ecosystem. Based on the discussion above, it was hypothesized that sediment accumulated most of the heavy metals in the whole mangrove system, thereby alleviating their toxicity. Consequently, this study was conducted to (1) determine the occurrence and distribution of heavy metals (Cr, Ni, Cu, As and Cd) in overlying water, sediments, rhizosphere sediments and mangrove seedlings; (2) evaluate heavy metal contamination degrees and potential ecological risk in sediment; (3) investigate the speciation of heavy metals in sediment.
Materials and Methods
2.1 Study area
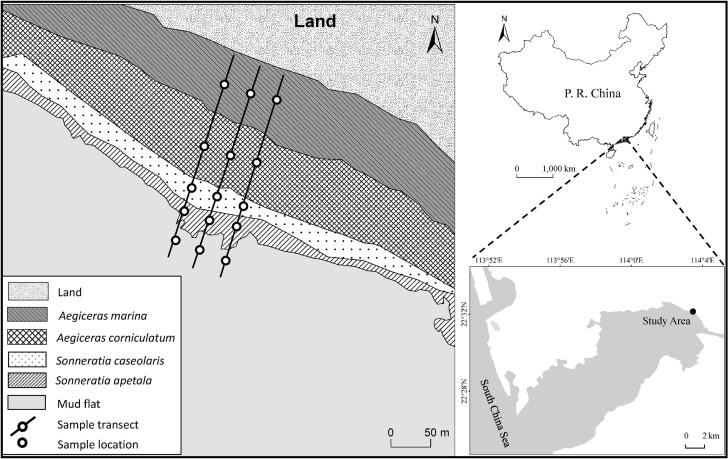
This study was carried out in the Futian National Nature Reserve (22°31.56′N, 114°00.40′E), located in the northeast coast (Fig 1) of Shenzhen Bay, with a length of 9 km along the coastline and a total area of 368 hectares. The Futian Nature Reserve is the smallest national reserve in China and the only one located in the hinterland of the modern metropolis, only 2.2 km away from the center of Shenzhen city. The mean annual temperature is 22.4°C and the mean annual precipitation is 1700–1900 mm mainly in April to September. The tides in Shenzhen Bay are semi-diurnal, with an average range of 1.9 m.
Fig 1. The location of study area.
2.2 Sample preparation
In November 2012, sediment, rhizosphere sediment, overlying water and seedlings samples were collected (Permit obtained from Neilingding-Futian National Nature Reserve of Guangdong, Futian National Nature Reserve; No specific permissions were required for the sample location, due to only some sediment, overlying water and mangrove seedlings were sampled; The land is protected and no endangered or protected species were sampled.). From land to sea, four mangrove species were distributed as follows (Fig 1): Avicennia marina (native) and Aegiceras corniculatum (native), Sonneratia caseolaris (introduced) and Sonneratia apetala (introduced). An adjacent unvegetated mud flat zone was chosen as a control (5 m from the forest edge), which had similar hydrological conditions and dissimilar biogeochemical cycles with mangroves [26–29]. Fifteen surficial sediment samples (0–20 cm, three duplicates at each site) were collected using acid-washed PVC pipes of length 80 cm and internal diameter 7.5 cm. After the holes were filled with water, fifteen overlying water samples were taken into acid-washed plastic jars. Twelve seedling samples (three duplicates for each species) with similar size and age were collected. The rhizosphere sediments, 5 mm-thick soil attached to the root surface, were gathered carefully with a plastic scraper. Seedling tissues (leaves, stems and roots) were oven-dried at 80°C until a constant weight was reached. After plants roots and stones were picked out from sediments and rhizosphere sediments, the sediment samples were also oven-dried at 80°C until a constant weight was reached. Then, the dry samples were ground into powder and passed through a 0.5-mm sieve. The remained sample on the sieve mainly contained little plants roots and stones, which were abandoned. The overlying water samples were filtered using 0.45μm filters and stored at 4°C for further analysis.
2.3 Determinations
As for sediments and rhizosphere sediments, pH and conductivity were determined in deionized water extracts using a mass ratio of 1:5 (sediment to water). TOC in overlying water and sediment was determined by Analytikjena multi N/C 3100 (Germany). The total heavy metal concentrations were determined by subjecting the sediment samples to microwave digestion in a mixture of 9 ml of nitric acid (HNO3), 3 ml of hydrofluoric acid (HF) and 1 ml of hydrochloric acid (HCl). The four fractions of heavy metals were extracted using the improved BCR three step sequential extraction procedure proposed by Rauret et al. (1999) [30]. The four fractions were defined as water/acid soluble fraction (F1), reducible fraction (F2), oxidizable fraction (F3) and residual fraction (F4). Plant samples were subjected to microwave digestion in 9 ml HNO3 and 1 ml HCl [31, 32]. The total heavy metal concentrations in the whole plant-sediment system, and those in all extracted fractions in sediments were analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500).
The bioaccumulation indices were calculated using biological concentration factor (BCF) and translocation factor (TF) following the methods of Yoon et al. (2006) and Cui et al. (2007) [33, 34].
BCF = concentration in roots / concentration in sediments
TF = concentration in leaves / concentration in roots
2.4 Risk assessment methods
Geo-accumulation index (Igeo) was originally proposed (Müller 1969) to measure the temporal variation of heavy metals by comparing the present-day metal concentrations in aquatic sediments with the geochemical background (pre-civilized background values) [35]. Igeo is defined as: Igeo = log2 Cn/1.5Bn where Cn is the concentration of metal n measured in this study and Bn is the geochemical background value in the upper crust [36, 37]. The constant 1.5 was used to account for possible variation in background value due to lithogenic effect. Seven classes of Igeo were proposed [38]: Igeo≤0, uncontaminated (UC); 0<Igeo<1, uncontaminated to moderately contaminated (UMC); 1<Igeo<2, moderately contaminated (MC); 2<Igeo<3, moderately to heavily contaminated (MHC); 3<Igeo<4, heavily contaminated (HC); 4<Igeo<5, heavily to extremely contaminated (HEC); 5<Igeo, extremely contaminated (EC)
The potential ecological risk coefficient () and potential ecological risk index (RI) were calculated using the following formula [39]:
where -the contamination factor, -the concentration of heavy metals in the sediment, and —a reference value for heavy metals. -the metal toxic response factor. According to Hakanson (1980), the values for measured metal elements are as follows: Cr = 2, Ni = 5, Cu = 5, As = 10, Cd = 30. The degree of can be categorized as follows: < 40: low risk (LR), 40 ≤ < 80: moderate risk (MR), 80 ≤ < 160: considerable risk (CR), 160 ≤ < 320: high risk (HR) and ≥ 320: very high risk (VHR). RI was classified into four levels: RI < 150: low risk (LR), 150 ≤ RI < 300: moderate risk (MR), 300 ≤ RI < 600: considerable risk (CR) and RI ≥ 600: very high risk (VHR).
The risk assessment code (RAC) was first proposed by Perin et al. (1985) [40] and has been widely applied [2, 10, 41] to evaluate heavy metal pollution in sediments by applying a scale to the percentage of metals present in fraction F1 (water/acid-soluble fraction). A five-level risk classification has been categorized in terms of RAC: no risk (< 1%, NR), low risk (1%–10%, LR), medium risk (11%–30%, MR), high risk (31%–50%, HR), and very high risk (> 50%, VHR).
Results and Discussion
3.1 Physicochemical characteristics
There were no significant change of conductivity among four mangrove species and mud flat (Table 1). pH showed a descending trend from overlying water (pH 7.98–8.25) to rhizosphere sediments (pH 6.85–7.18) and sediments (pH 6.55–7.06) in all the five study zones. These results suggested that mangrove trees may lead to acidification of sediment, which is in agreement with the results of previous studies of mangroves [6, 42, 43] and other marine plants such as Spartina alterniflora [44, 45]. Except for A. corniculatum, the TOC levels decreased towards mud flat in sediment, implying that the sediments inside mangrove forest could retain organic matter. Additionally, the litter decay released nutrients into sediments, which contributes to the increase of organic matter in sediments (Table 1). Overall, mangrove plant improved the sediment fertility and promoted the physicochemical properties of the intertidal habitat, which would influence the biogeochemical processes of other elements (including heavy metals) in the sediment.
Table 1. The selected physicochemical properties in overlying water, sediments and rhizosphere sediment from four species and mud flat sites in the Futian mangrove forest, South China Sea.
| From land to Seawater | |||||
|---|---|---|---|---|---|
| A. marine | A. corniculatum | S. caseolaris | S. apetala | Mud flat | |
| Overlying water | |||||
| pH | 8.21 | 7.98 | 8.14 | 8.12 | 8.25 |
| Conductivity (S·m-1) | 1.66 | 1.44 | 1.75 | 1.73 | 1.64 |
| TOC (mg·L-1) | 7.95 | 7.80 | 9.70 | 6.82 | 6.15 |
| Sediment | |||||
| pH | 6.55 | 6.86 | 6.93 | 6.43 | 7.06 |
| Conductivity (S·m-1) | 0.51 | 0.33 | 0.40 | 0.50 | 0.36 |
| TOC (mg·kg-1) | 530.50 | 252.50 | 485.00 | 451.00 | 424.00 |
| Rhizosphere sediment | |||||
| pH | 6.95 | 7.18 | 6.85 | 6.81 | |
| Conductivity (S·m-1) | 0.70 | 0.46 | 0.64 | 0.53 | |
| TOC (mg·kg-1) | 288.00 | 388.00 | 467.50 | 462.50 | |
3.2 Heavy metals distributions in overlying water-sediment-mangrove plant root
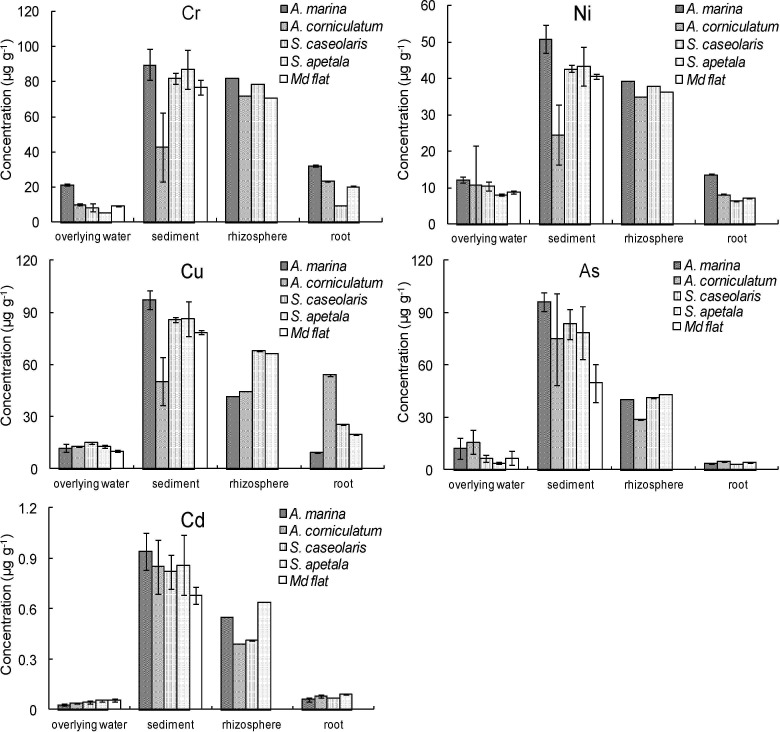
In the present study, the levels of Cr, Ni, Cu and Cd in all sampling sites basically followed the order: sediment > rhizosphere sediment > root > overlying water; while, the distribution of As was sediment > rhizosphere sediment > overlying water > root (Fig 2). The distribution of As in overlying water was higher than root, indicated that As (a kind of metalloid) was not as active as other metals, and could not be absorbed by mangrove root actively as that of other metals. Previously studies have shown that phosphorus competed with arsenic (As) in transfer and accumulation of As in plant [46, 47]. Thus, the large amount of phosphorus deposited in Futian mangrove sediment [48, 49] may compete with As transfer and absorption in mangrove plants, leading to lower As level in mangrove roots. The heavy metal contents in sediments were nearly four times higher than that in overlying water, and four to ten times higher than in mangrove root. Compared to sediment, the lower metal contents in rhizosphere sediment may be related to that mangrove plants passively took up heavy metals from the surrounding sediment [50, 51]. The heavy metals levels were highest in A. marina sediment, with the lowest levels of Cr, Ni and Cu occurred in A. corniculatum sediment. The levels of Cr, Ni and Cu in A. corniculatum zone were lower than other mangrove species and mud flat zone, very well be due to low level of TOC (Table 1), which could be explained, at least partly, by that TOC could regulate metal association in the sediments [52, 53]. The decomposition of organic matter may lead to acidification of sediment. However, no corresponding increased pH was detected for A. corniculatum (Table 1). In fact, other factors, such as particle size distributions, may also affect heavy metal accumulation [54, 55, 56]. Except for A. corniculatum, the basic distribution of heavy metal concentrations declined offshore, which may be related to that the landward area was close to the land-sourced pollutant emission outlets. Another possible reason may be due to that the distribution of A. marina was far away from seawater, and was less affected by tide dilution compared to other mangrove sediments and mud flat.
Fig 2. The distributions of heavy metals in overlying water-sediment-rhizosphere soil-root system in the Futian mangrove forest, South China.
The sample sites from land to seawater direction are: A. marina—A.corniculatum—S. caseolaris—S. apetala—Mud flat.
Generally, biological concentration factors (BCFs) and translocation factors (TFs) are widely used to estimate a plant’s abilities in accumulating metals from soils and transferring metals from roots to shoots, respectively [33]. If plants exhibit BCFs or TFs more than one, they are suitable for phytoremediation [57]. In the present study, BCFs and TFs were basically less than one (except for BCF of Cu in A. corniculatum, TF of Cu in A. marina and TF of Cd in A.corniculatum, Table 2). These results indicated that these mangrove species tend to restrict metal soil-root and root-shoot transformations, guaranteeing the conduction of various important metabolic activities including photosynthesis in the aboveground parts. Among the four mangrove species, A. corniculatum had the highest BCFs for all heavy metals measured except for Cd. A. marina and A. corniculatum had higher BCFs than S. caseolaris and S. apetala, which may be related to their location closer to the land, as well as their greater ability in absorbing metals from soils. In Table 2, higher TFs occurred in Sonneratia species (except for Cu and Cd), implying their higher sensitive to heavy metals stresses.
Table 2. The biological concentration factors (BCFs) and translocation factors (TFs) of heavy metals in selected mangrove species in Futian mangrove forest, South China Sea.
BCF = concentration in roots / concentration in sediment. TF = concentration in shoot / concentration in roots.
| Species | Cr | Ni | Cu | As | Cd |
|---|---|---|---|---|---|
| BCFs | |||||
| A. marina | 0.36 | 0.27 | 0.10 | 0.04 | 0.06 |
| A. corniculatum | 0.55 | 0.34 | 1.07 | 0.06 | 0.09 |
| S. caseolaris | 0.11 | 0.15 | 0.30 | 0.04 | 0.09 |
| S. apetala | 0.23 | 0.16 | 0.23 | 0.05 | 0.10 |
| TFs | |||||
| A. marina | 0.22 | 0.38 | 2.72 | 0.28 | 0.58 |
| A. corniculatum | 0.21 | 0.57 | 0.26 | 0.24 | 1.00 |
| S. caseolaris | 0.37 | 0.98 | 0.83 | 0.36 | 0.57 |
| S. apetala | 0.75 | 0.57 | 0.80 | 0.24 | 0.50 |
3.3 Heavy metal contamination assessment in sediment
The heavy metals concentrations, background values and marine sediment quality classification in the National Standard of China GB 18668–2002 are summarized for comparison (Table 3). In the present study, the mean Cr, Ni, Cu, As and Cd concentrations were as high as 1.95, 2.03, 7.99, 12.98 and 26.77 times the background values [58]. Class I sediment is suitable for mariculture, nature reserves, endangered species reserves, and leisure activities such as swimming, while Class II can be used for industry and tourism sites and Class III is only suitable for harbors. When compared with the Chinese government’s target values for marine sediment, Cr levels were under Class I standard for Marine Sediment Quality, while, the levels of Cu and Cd were above those of Class I, but lower than those of Class II. The levels of As exceed the Class II standard, but lower than Class III, indicating that As had some influence on the marine sediment quality to a certain extent.
Table 3. Heavy metal concentrations in sediment, background level and guideline values of some different criteria (μg g-1 dw).
Note: TEL, threshold effect level, indicates concentrations below which adverse effects on biota are rarely observed. PEL, probable effects level, indicate concentrations above which adverse effects on biota are frequently observed.
| Cr | Ni | Cu | As | Cd | Reference | |
|---|---|---|---|---|---|---|
| Shenzhen Bay | 75.74 | 40.35 | 79.72 | 76.60 | 0.83 | This study |
| Background level | 38.80 | 19.83 | 9.98 | 5.90 | 0.03 | [58] |
| Class Ⅰ | 80.00 | — | 35.00 | 20.00 | 0.50 | [59] |
| ClassⅡ | 150.00 | — | 100.00 | 65.00 | 1.50 | [59] |
| Class Ⅲ | 270.00 | — | 200.00 | 93.00 | 5.00 | [59] |
| Threshold effect level (TEL) | 43.40 | 22.70 | 31.60 | 9.79 | 0.99 | [60] |
| Probable effect level (PEL) | 111 | 48.60 | 149 | 33 | 4.98 | [60] |
The threshold effect level (TEL) and probable effect level (PEL) for some substances with potential environmental risks were applied to facilitate the interpretation of sediment quality [60, 61]. The TEL represents concentrations below which adverse biological effects rarely occur, while the PEL is defined as the level above which adverse biological effects are expected to occur more often than not [45, 62, 63]. In the present study, mean As concentration was higher than its PEL value (Table 3), indicating that adverse biological effects may occur frequently. Levels of Cr, Ni and Cu were all higher than their TEL values, and below their corresponding PEL benchmarks, suggesting that adverse biological effects caused by Cr, Ni and Cu may be observed occasionally. In addition, Cd level was lower than its TEL and PEL values, indicating no adverse biological effects.
In fact, heavy metals always occur in sediments as complex mixtures. To determine the possible biological effects of combined metals, mean PEL quotients (m-P-Q) for the five heavy metals were calculated using the following formula: m-P-Q = ∑ Cx/PELx)/n. where Cx is the sediment concentration of component x, PELx is the PEL for compound x and n is the number of components. Three classes of toxicity probability for biota were defined as follows [45, 62]: m-P-Q <0.1 (8% probability of being toxic); 0.11–1.5 (21% probability of being toxic); 1.51–2.3 (49% probability of being toxic); >2.3 (73% probability of being toxic). In the present study, the combination of five studied metals has a 21% probability of being toxic, with the mean PEL quotients as follows: 1.12 (A. marina), 0.73 (A. corniculatum), 0.98 (S. caseolaris), 0.96 (S. apetala) and 0.74 (Mud flat). Similar results were also observed in intertidal Bohai Bay and the coastal Shandong Peninsula (Yellow Sea), where the mean PEL quotients of studied heavy metals have a 21% probability of being toxic [45, 64, 65].
In the present study, background values were selected as the reference for assessment of heavy metal pollution [36, 37]. The Igeo results showed that the sediments in study area ranged from uncontaminated to heavily contaminated quality for all heavy metals (Table 4). The Igeo values of heavy metals in A. marina sediment were higher than other sediments,which may be related to land-sourced heavy metal pollutant emission outlets. Another possible explanation may be that the distribution of A. marina was far away from seawater, and was less affected by tide dilution compared to other mangrove sediments and mud flat. Similar results have also been reported in sediments from mangrove forest and adjacent mud flat in Zhangjiang Estuary [27], where total heavy metal concentrations were forest > forest edge > mudflat. Cu was at moderately contaminated level with uncontaminated to moderately contaminated quality for A. corniculatum sediment. Except for uncontaminated quality of Cr and Ni in A. corniculatum sediment, Cr and Ni ranged from uncontaminated to moderately contaminated quality for other sediments. Cd showed moderately to heavily contaminated quality with Igeo ranging from 2.21 to 2.68. Concentrations of As remained at heavily contaminated levels in the study sites, except for moderately to heavily contaminated quality in mud flat. Considering the five metals, the accumulation expressed a descending trend toward sea (except for Cr, Ni and Cu in A. corniculatum sediment), implying that the location was of great influence in metal accumulation.
Table 4. Potential ecological risk assessments of Cr, Ni, Cu, As and Cd in the sediment of Futian mangrove forest, South China Sea.
| Heavy metals | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Cu | As | Cd | ||||
| Geo-accumulation index (Igeo) | ||||||||
| A.marina | 0.77/UMC | 0.76/UMC | 1.37/MC | 3.68/HC | 2.68/MHC | |||
| A.corniculatum | -0.30/UC | -0.30/UC | 0.43/UMC | 3.32/HC | 2.53/MHC | |||
| S. caseolaris | 0.65/UMC | 0.51/UMC | 1.20/MC | 3.48/HC | 2.48/MHC | |||
| S. apetala | 0.73/UMC | 0.53/UMC | 1.20/MC | 3.48/HC | 2.48/MHC | |||
| Mud flat | 0.55/UMC | 0.43/UMC | 1.07/MC | 2.73/MHC | 2.21/MHC | |||
| Potential ecological risk index | RI | |||||||
| A.marina | 4.63/LR | 12.80/LR | 48.71/MR | 163.42/HR | 940.00/VHR | 1169.56/VHR | ||
| A.corniculatum | 2.21/LR | 6.16/LR | 25.30/LR | 126.88/CR | 850.00/VHR | 1010.55/VHR | ||
| S. caseolaris | 4.23/LR | 10.76/LR | 43.06/MR | 141.49/CR | 820.00/VHR | 1019.54/VHR | ||
| S. apetala | 4.49/LR | 10.93/LR | 43.27/MR | 133.02/CR | 860.00/VHR | 1051.71/VHR | ||
| Mud flat | 3.96/LR | 10.22/LR | 39.36/LR | 84.34/CR | 680.00/VHR | 817.88/VHR | ||
| Risk assessment code (RAC) | ||||||||
| A.marina | 0.45/NR | 8.83/LR | 1.86/LR | 2.46/LR | 12.32/MR | |||
| A.corniculatum | 0.32/NR | 6.03/LR | 1.95/LR | 1.85/LR | 8.50/LR | |||
| S. caseolaris | 0.39/NR | 7.66/LR | 2.63/LR | 1.47/LR | 15.55/MR | |||
| S. apetala | 0.26/NR | 5.44/LR | 1.84/LR | 1.30/LR | 11.35/MR | |||
| Mud flat | 0.44/NR | 7.95/LR | 2.83/LR | 1.53/LR | 16.08/MR | |||
Similar to distribution of Igeo values in sediment, the values of each heavy metal in A. marina sediment were higher than other sediment, indicating higher heavy metal contamination (Table 4). In all sediments, Cr and Ni showed low ecological risk with values lower than 40. Except for low ecological risk of Cu in A. corniculatum and mud flat, moderate risks of Cu were detected in other sediments. A. marina sediment demonstrated high risk of As, with considerable risk of As shown in other sediments. Cd showed rather higher values (from 680 to 940), representing very high ecological risk. To quantify the overall potential ecological risks of observed metals, comprehensive potential ecological risk RI is calculated as the sum of all five risk factors. RI represented the sensitivity of the biological community to the toxic metal elements and illustrated the potential ecological risk caused by the overall contamination [66]. In the present study, the RI values in all sediments were higher than 600, indicating very high ecological risk. Furthermore, the A. marina sediment showed the highest risk compared to other sediments.
3.4 Heavy metal speciation in sediment
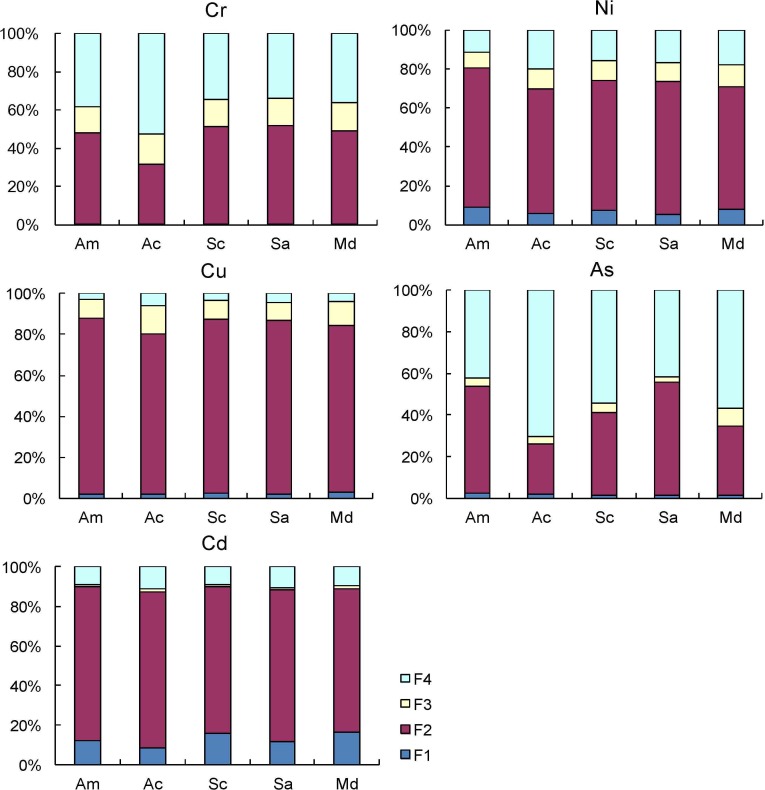
The toxicity and bioavailability of heavy metals were not only related to their total contents, but also to their speciation [67]. The reducible fraction referred to metal associated with Fe and Mn oxides was reducible, which might be released when subjected to more reducing conditions. The oxidizable fraction referred to metal bound to organic matter and might be released in oxidizing conditions. The water/acid soluble fraction referred to metal that was exchangeable and bioavailable, which was adsorbed on sediment surface by relatively weak electrostatics interactions and sensitive to changes in pH [68, 69]. In the present study, as for Cr, Ni, Cu and Cd, the dominant phase was in the reducible fraction (Fig 3), indicating their strong association with Fe/Mn oxides, from which release of heavy metals into the water column can be expected under prevailing environmental conditions [9, 70]. Beside for reducible fraction, another main phase of As in sediment was residual fractions bound in mineral lattice. The residual fraction of As showed that the release of As is unlikely under environmental conditions, indicating relatively less mobility and bioavailability and less harmful to the environment.
Fig 3. The speciation distributions of heavy metals in sediment in Futian mangrove forest, South China.
F1, water/acid-soluble fraction; F2, reducible fraction; F3, oxidizable fraction; F4, residual fraction; Total, total metal concentration. The sample sites from land to sea are: A. marina—A.corniculatum—S. caseolaris—S. apetala—Mud flat. Am, Avicennia marina; Ac, Aegiceras corniculatum; Sc, Sonneratia caseolaris; Sa, Sonneratia apetala; Md, Mud flat.
Generally, heavy metals in the water/acid soluble fraction (F1) are bound to carbonates in the weakest strength and could be absorbed by the biota directly [10]. So the percentage of metals in this fraction might indicate the potential risk to the biota. Except for Cu, the RAC values of heavy metals in A.marina sediment were higher than other sediments (Table 4). Furthermore, the RAC values of Cr in all sediments were lower than one percent, indicating that there was no Cr risk for biota in this mangrove ecosystem. Though As was heavily contaminated according to Igeo and Eri criteria based on total concentration, the lower As risk was detected based on water/acid soluble fraction of As. These results indicated that most of As was not biologically toxic. The RAC method has been reported in previous studies [10, 41, 71], and similar different Igeo and RAC results have been reported by Kumar et al. (2012) who found that Mn and Zn had higher risk potential than Fe, Cu, and As based on RAC method; while strongly to moderately contaminated Fe and moderate to uncontaminated other heavy metals were shown by Igeo values. For Ni, Cu and As, whose RAC ranged between one and ten percent, the potential risk remained at low level. Generally, there was medium risk when some metal’s F1 proportion was in a range of 11–30%. In this study, only Cd showed medium risk except for Cd in A. corniculatum sediment. Compared to previous studies about RAC values of Cd in sewage sludge [2, 13, 72], the RAC levels of Cd were relatively higher, suggesting that heavy Cd contamination has occurred in Shenzhen Bay. Overall, heavy metals introduced by anthropogenic activities has posed a considerable ecological risk to the biota in terms of the speciation (especially for Cd), and deserved enough attention.
Conclusions
This is the first study to reveal heavy metals in sediment-mangrove plant system in mangrove forest, South China. In the present study, mangrove modified the physicochemical properties of sediments by acidifying soils and increasing organic matter contents. The low BCFs and TFs of heavy metals indicated that mangrove species adopted the exclusion strategy to cope with heavy metal stress. As was toxicologically important due to its concentrations being higher than its TEL and PEL. The sediment had 21% probability of toxicity based on mean PEL quotient. The Igeo values showed that both Cd and As remained at nearly heavily contaminated level in the study sites. and RI results suggested that Cd and As showed rather considerable and very high risk to the surroundings. In terms of the speciation, the majority of each metal element appeared in the reducible fraction. According to the RAC values, heavy metals have posed a considerable ecological risk to the biota, especially for Cd.
Acknowledgments
This work was financially supported by the Program of Science and Technology of Shenzhen (JCYJ20120829170028566, JCYJ20130402164725017, JCYJ20140903101847739), and the Program of National Natural Science Foundation of China (31400446), and the Program of Assessment and Restoration of Mangrove Geiwei of Shenzhen Bay. We thank Dr. Hualin Xu from the Neilingding-Futian National Nature Reserve of Guangdong, Futian National Nature Reserve for critically reading and revising the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
Funding provided by Program of Science and Technology of Shenzhen JCYJ20120829170028566, Ruili Li; Program of Science and Technology of ShenzhenJCYJ20140903101847739, Ruili Li; Program of Science and Technology of Shenzhen JCYJ20130402164725017, Ruili Li; and Program of National Natural Science Foundation of China (31400446), Ruili Li.
References
- 1.Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30: 685–700. [DOI] [PubMed] [Google Scholar]
- 2.Huang HJ, Yuan XZ, Zeng GM, Zhu HN, Li H, Liu ZF, et al. (2011) Quantitative evaluation of heavy metals’ pollution hazards in liquefaction residues of sewage sludge. Bioresource Technol 102: 10346–10351. [DOI] [PubMed] [Google Scholar]
- 3.Na GS, Fang XD, Cai YQ, Ge LK, Zong HM, Yuan XT, et al. (2013) Occurrence, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar Pollut Bull 69: 233–237. 10.1016/j.marpolbul.2012.12.028 [DOI] [PubMed] [Google Scholar]
- 4.Li C, Lu FY, Zhang Y, Liu TW, Hou W (2008) Spatial distribution characteristics of heavy metals in street dust in Shenyang city. Ecol Environ 17: 560–564 (in Chinese with English Abstract). [Google Scholar]
- 5.Sun YB, Zhou QX, Xie XK, Liu R (2010) Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang. China. J Hazard Mater 174: 455–462. 10.1016/j.jhazmat.2009.09.074 [DOI] [PubMed] [Google Scholar]
- 6.Zhou YW, Zhao B, Peng YS, Chen GZ (2010) Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Mar Pollut Bull 60: 1319–1324. 10.1016/j.marpolbul.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Sekomo CB, Nkuranga E, Rousseau DPL, Lens PNL (2011) Fate of Heavy Metals in an Urban Natural Wetland: The Nyabugogo Swamp (Rwanda). Water Air Soil Poll 214: 321–333. [Google Scholar]
- 8.Nath B, Birch G, Chaudhuri P (2013) Trace metal biogeochemistry in mangrove ecosystems: A comparative assessment of acidified (by acid sulfate soils) and non-acidified sites. Sci Total Environ 463: 667–674. 10.1016/j.scitotenv.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Yu RL, Hu GR, Wang LJ (2010) Speciation and ecological risk of heavy metals in intertidal sediments of Quanzhou Bay, China. Environ Monit Assess 163: 241–252. 10.1007/s10661-009-0830-z [DOI] [PubMed] [Google Scholar]
- 10.Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study: Mahanadi basin, Indian. J Hazard Mater 186: 1837–1846. 10.1016/j.jhazmat.2010.12.081 [DOI] [PubMed] [Google Scholar]
- 11.Morgan B, Rate AW, Burton ED (2012) Trace element reactivity in FeS-rich estuarine sediments: Influence of formation environment and acid sulfate soil drainage. Sci Total Environ 438: 463–476. 10.1016/j.scitotenv.2012.08.088 [DOI] [PubMed] [Google Scholar]
- 12.Li LZ, Wu HF, van Gestel CAM, Peijnenburg WJGM, Allen HE (2014) Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ Pollut 188: 144–152. 10.1016/j.envpol.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Yuan HZ, Shen J, Liu EF, Wang JJ, Meng XH (2011) Assessment of nutrients and heavy metals enrichment in surface sediments from Taihu Lake, a eutrophic shallow lake in China. Environ Geochem Hlth 33: 67–81. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Nayek S, Saha RN, Satpati S (2008) Assessment of heavy metal accumulation in macrophyte, agricultural soil, and crop plants adjacent to discharge zone of sponge iron factory. Environ Geol 55: 731–739. [Google Scholar]
- 15.Zhu XF, Ji HB, Chen Y, Qiao MM, Tang L (2013) Assessment and sources of heavy metals in surface sediments of Miyun Reservoir, Beijing. Environ Monit Assess 185: 6049–6062. 10.1007/s10661-012-3005-2 [DOI] [PubMed] [Google Scholar]
- 16.Huang XX, Zhu Y, Ji HB (2013) Distribution, speciation, and risk assessment of selected metals in the gold and iron mine soils of the catchment area of Miyun Reservoir, Beijing, China. Environ Monit Assess 185: 8525–8545. 10.1007/s10661-013-3193-4 [DOI] [PubMed] [Google Scholar]
- 17.Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SMM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa—Ethiopian Rift Valley Lake. Environ Sci Pollut Res 20: 8663–8671. [DOI] [PubMed] [Google Scholar]
- 18.Rath P, Panda UC, Bhatta D, Sahu KC (2009) Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments-a case study: Brahmani Nandira rivers, India. J Hazard Mater 163: 632–644. 10.1016/j.jhazmat.2008.07.048 [DOI] [PubMed] [Google Scholar]
- 19.Panda UC, Rath P, Bramha SN, Sahu KC (2010) Application of factor analysis in geochemical speciation of heavy metals in the sediments of a lake system-Chilika (India): a case study. J Coastal Res 26: 860–868. [Google Scholar]
- 20.Wu QH, Tam NFY, Leung JYS, Zhou XZ, Fu J, Yao B, et al. (2014) Ecological risk and pollution history of heavy metals in Nansha mangrove, South China. Ecotox Environ Safe 104: 143–151. [DOI] [PubMed] [Google Scholar]
- 21.Sakan S, Devic G, Relic D, Andelkovic I, Sakan N, Dordevic D (2015) Risk assessment of trace element contamination in river sediments in Serbia using pollution indices and statistical methods: a pilot study. Environ Earth Sci 73: 6625–6638. [Google Scholar]
- 22.Xie HW, Wen B, Guo Y, Shi YZ, Wu YH (2010) Community characteristics and distribution of metal elements in mangroves in Futian of Shenzhen, China. Guihaia 30: 64–69 (in Chinese with English abstract). [Google Scholar]
- 23.He B, Li RL, Chai MW, Qiu GY (2014) Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environ Geochem Hlth 36: 467–476. [DOI] [PubMed] [Google Scholar]
- 24.Tam NFY, Li SH, Lan CY, Chen GZ, Li MS, Wong YS (1995) Nutrients and heavy metal contamination of plants and sediments in Futian mangrove forest. Hydrobiologia 295: 149–158. [Google Scholar]
- 25.Zhang J, Wang H, Chen G, Li M (2001) Transportation, accumulation and circulation of heavy metals in mangrove in Futian, Shenzhen. Guangzhou Environ Sci 16: 36–39 (in Chinese with English abstract). [Google Scholar]
- 26.Clark MW, McConchie D, Lewis DW, Saenger P (1998) Redox stratification and heavy metal partitioning in Avicennia-dominated mangrove sediments: a geochemical model. Chem Geol 149: 147–171 [Google Scholar]
- 27.Liu JC, Yan CL, Kate LS, Zhang RF, Lu ZH (2010) The distribution of acid-volatile sulfide and simultaneously extracted metals in sediments from a mangrove forest and adjacent mudflat in Zhangjiang Estuary, China. Mar Pollut Bull 60: 1209–1216. 10.1016/j.marpolbul.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 28.Fernandes LL, Nayak GN (2012) Heavy metals contamination in mudflat and mangrove sediments. Chem Ecol 28: 435–455. [Google Scholar]
- 29.Zhang RF, Yan CL, Liu JC (2013) Effect of mangroves on the horizontal and vertical distributions of rare earth elements in sediments of the Zhangjiang Estuary in Fujian Province, Southeastern China. J Coastal Res 29: 1341–1350. [Google Scholar]
- 30.Rauret G, Lopez-Sanchez JF, Sahuquillo A (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monitor 1: 57–61. [DOI] [PubMed] [Google Scholar]
- 31.US EPA (United States Environmental Protection Agency).Method 3052: microwave assisted acid digestion of siliceous and organically based matrices SW-846.DC:Washington; 1996.
- 32.Man YB, Sun XL, Zhao YG, Lopez BNL, Chung SS, Wu SC, Cheung KC, Wong MH (2010) Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environ Int 36: 570–576. [DOI] [PubMed] [Google Scholar]
- 33.Yoon J, Cao XD, Zhou QX, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Envir 368: 456–464. [DOI] [PubMed] [Google Scholar]
- 34.Cui S, Zhou Q, Chao L (2007) Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environ Geol 51: 1043–1048. [Google Scholar]
- 35.Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2: 108–118. [Google Scholar]
- 36.Martin JM, Meybeck M (1979) Elemental mass balance of material carried by major world rivers. Mar Chem 7: 173–206. [Google Scholar]
- 37.Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution Oxford: Blackwell. [Google Scholar]
- 38.Müller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chemiker Zeitung 105: 157–164. [Google Scholar]
- 39.Hakanson L (1980) Ecological risk index for aquatic pollution control-A sedimentological approach. Water Res 14: 975–1001. [Google Scholar]
- 40.Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Orio AA (1985). Heavy metal speciation in the sediments of Northern Adriatic sea: A new approach for environmental toxicity determination. Proceedings of the International Conference “Heavy Metals in the Environment”; Sep 454–6; Athens, Greece: CEP Consultants.
- 41.Kumar A, Ramanathan AL, Prabha S, Ranjan RK, Ranjan S, Singh G (2012) Metal speciation studies in the aquifer sediments of Semria Ojhapatti, Bhojpur District, Bihar. Environ Monit Assess 184: 3027–3042. 10.1007/s10661-011-2168-6 [DOI] [PubMed] [Google Scholar]
- 42.Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110: 195–205. [DOI] [PubMed] [Google Scholar]
- 43.Lu HL, Yan CL, Liu JC (2007) Low-molecular-weight organic acids exuded by mangrove roots and their effect on cadmium species change in the rhizosphere. Environ Exp Bot 61: 159–166. [Google Scholar]
- 44.Zhou HX, Liu JE, Zhou J, Qin P (2008) Effect of an alien species Spartina alterniflora Loisel on biogeochemical processes of intertidal ecosystem in the Jiangsu coastal region, China. Pedosphere 18: 77–85. [Google Scholar]
- 45.Chai MW, Shi FC, Li RL, Shen XX (2014) Heavy metal contamination and ecological risk in Spartina alterniflora marsh in intertidal sediments of Bohai Bay, China. Mar Pollut Bull 84: 115–124. 10.1016/j.marpolbul.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 46.Poynton CY, Huang JW, Blaylock MJ, Kochian LV, Elless MP (2004) Mechanism of arsenic hyperaccumulation in Pteris species: root As influx and translocation. Planta 219: 1080–1088. [DOI] [PubMed] [Google Scholar]
- 47.Rathinasabapathi B, Raman SB, Kertulis G, Ma L (2006) Arsenic-resistant proteobacterium from the phyllosphere of the arsenic-hyperaccumulating fern (Pteris vittata L.) reduces arsenate to arsenite. Can J Microbiol 52: 695–700. [DOI] [PubMed] [Google Scholar]
- 48.Li CH, Wang Q, Zhang WJ, Yu H, Wang B, Shi XH (2013) Study on the nutritional status of mangrove forest soils in Shenzhen Bay. Guangdong Agri Sci 40: 53–56. [Google Scholar]
- 49.Mei XQ, Yang Y, Tam NFY, Wang YW, Li L (2014) Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res 50: 147–159. 10.1016/j.watres.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 50.Dahmani-Muller H, van Oort F, Gélie B, Balabane M (2000) Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ Pollut 109: 231–238. [DOI] [PubMed] [Google Scholar]
- 51.Greger M, Landberg T (2008) Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 312: 195–205. [Google Scholar]
- 52.Perry CT, Berkeley A (2009) Intertidal substrate modification as a result of mangrove planting: impacts of introduced mangrove species on sediment microfacies characteristics. Estuar Coast Shelf S 81: 225–237. [Google Scholar]
- 53.De Jonge M, Teuchies J, Meire P, Blust R, Bervoets L (2012) The impact of increased oxygen conditions on metal-contaminated sediments. Part I: effects on redox status, sediment geochemistry and metal bioavailability. Water Res 46: 2205–2214. 10.1016/j.watres.2012.01.052 [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro AP, Figueiredo AMG, Santos JO, Dantan E, Cotrim MEB, Figueira RCL, et al. (2013) Combined SEM/AVS and attenuation of concentration models for the assessment of bioavailability and mobility of metals in sediments of Sepetiba Bay (SE Brazil). Mari Pollut Bull 68: 55–63. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang W, Gao XL (2014) Assessment of heavy metal impact on sediment quality of the Xiaoqinghe estuary in the coastal Laizhou Bay, Bohai Sea: Inconsistency between two commonly used criteria. Mari Pollut Bull 83: 352–357. [DOI] [PubMed] [Google Scholar]
- 56.Younis AM, El-Zokm GM, Okbah MA (2014) Spatial variation of acid-volatile sulfide and simultaneously extracted metals in Egyptian Mediterranean Sea lagoon sediments. Environ Monit Assess 186: 3567–3579. 10.1007/s10661-014-3639-3 [DOI] [PubMed] [Google Scholar]
- 57.Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil—rhizosphere plant system, fundamentals and potential application of phytoremediation. J Biotechnol 99: 259–278. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Zheng CJ (1988) Environmental Background Values Data Sheet. China Environmental Science Press, China. [Google Scholar]
- 59.SEPA (State Environmental Protection Administration of China), (2002). Marine Sediment Quality (GB 18668–2002). Standards Press of China, Beijing. [Google Scholar]
- 60.MacDonald DD, Ingersol CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Con Tox 39: 20–31. [DOI] [PubMed] [Google Scholar]
- 61.MacDonald DD, Carr RS, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5: 253–278. 10.1007/BF00118995 [DOI] [PubMed] [Google Scholar]
- 62.Long ER, Jay Field L, MacDonald DD (1998) Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ Toxicol Chem 17: 714–727. [Google Scholar]
- 63.Yang Y, Chen F, Zhang L, Liu J, Wu S, Kang M (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf. Mar Pollut Bull 64: 1947–1955. 10.1016/j.marpolbul.2012.04.024 [DOI] [PubMed] [Google Scholar]
- 64.Gao XL, Li PM (2012) Concentration and fractionation of trace metals in surface sediments of intertidal Bohai Bay, China. Mar Pollut Bull 64: 1529–1536. 10.1016/j.marpolbul.2012.04.026 [DOI] [PubMed] [Google Scholar]
- 65.Li GG, Hu BQ, Bi JQ, Leng QN, Xiao CQ, Yang ZC (2013) Heavy metals distribution and contamination in surface sediments of the coastal Shandong Peninsula (Yellow Sea). Mar Pollut Bull 76: 420–426. 10.1016/j.marpolbul.2013.08.032 [DOI] [PubMed] [Google Scholar]
- 66.Shi G, Chen Z, Bi C, Li Y, Teng J, Wang L, et al. (2010) Comprehensive assessment of toxic metals in urban and suburban street deposited sediments (SDSs) in the biggest metropolitan area of China. Environ Pollut 158: 694–703. 10.1016/j.envpol.2009.10.020 [DOI] [PubMed] [Google Scholar]
- 67.Lian YW, Xu JS, Lin P, Meguro S, Kawachi S (1999) Five heavy metals in propagules of ten mangrove species of China. J Wood Sci 45: 343–347. [Google Scholar]
- 68.Thomas RP, Ure AM, Davidson CM, Littlejohn D (1994) Three-stage sequential extraction procedure for the determination of metals in river sediments. Anal Chim Acta 286: 423–429. [Google Scholar]
- 69.Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21: 1335–1346. [Google Scholar]
- 70.Guo TZ, DeLaune RD, Patrick WH (1997) The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environ Int 23: 305–316. [Google Scholar]
- 71.Abdel-Satar AM, Goher ME (2015) Heavy metals fractionation and risk assessment in surface sediments of Qarun and Wadi El-Rayan lakes, Egypt. Environ Monit Assess 187: 346 10.1007/s10661-015-4592-5 [DOI] [PubMed] [Google Scholar]
- 72.Zhai Y, Chen H, Xu B, Xiang B, Chen Z, Li C, et al. (2014) Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresource Technol 159: 72–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.