Abstract
Objective
To evaluate the economic and clinical benefits of endometrial radiofrequency ablation (RFA) compared with other ablation techniques for the treatment of menorrhagia.
Methods
Using German health claims data, women meeting defined inclusion criteria for the intervention group (RFA) were selected. A comparable control group (other endometrial ablations) was established using propensity score matching. These two groups were compared during the quarter of treatment (QoT) and a follow-up of 2 years for the following outcomes: costs during QoT and during follow-up, repeated menorrhagia diagnoses during follow-up and necessary retreatments during follow-up.
Results
After performing propensity score matching, 50 cases could be allocated to the intervention group, while 38 were identified as control cases. Patients in the RFA group had 5% fewer repeat menorrhagia diagnoses (40% vs 45%; not significant) and 5% fewer treatments associated with recurrent menorrhagia (6% vs 11%; not significant) than cases in the control group. During the QoT, the RFA group incurred €578 additional costs (€2,068 vs €1,490; ns). However, during follow-up, the control group incurred €1,254 additional costs (€4,561 vs €5,815; ns), with medication, outpatient physician consultations, and hospitals costs being the main cost drivers. However, none of the results were statistically significant.
Conclusion
Although RFA was more cost-intensive in the QoT compared with other endometrial ablation techniques, an average total savings of €676 was generated during the follow-up period. While having evidence that RFA is clinically equivalent to other endometrial ablation procedures, we generated indications that RFA is non-inferior and favorable with regard to economic outcomes.
Keywords: menorrhagia, radiofrequency ablation, endometrial ablation, costs, Germany, health claims data
Introduction
The prevalence of heavy uterine bleeding is difficult to ascertain, as the amount and strength of bleeding can be perceived differently and subjectively graded by each patient.1 However, using one objective, clinical definition of menorrhagia, ie, menstrual blood loss of >80 mL/cycle and/or heavy menstrual bleeding for more than 7 days, it is assumed that approximately 10%–30% of all women worldwide are affected once in their lifetime.2,3
Since treatment alternatives exist, the actual treatment of choice depends on the anatomic characteristics of patients as well as on their preferences for preserving their uterus. Instead of undergoing hysterectomy, some women may prefer minimally invasive interventions with shorter hospital stays or outpatient treatment such as endometrial ablation.4
Various techniques for endometrial ablation can be used to destroy or remove the pathologic endometrium along with the superficial myometrium.1 The first-generation of endometrial ablation techniques involved a hysteroscopy-based removal of the endometrium under direct visualization by the surgeon, which meant that the outcome was largely dependent on the surgeon’s skills and experience.1 The procedures with second-generation devices, eg, radiofrequency ablation (RFA), are performed without direct visualization during the intervention, but are usually preceded and followed by a diagnostic hysteroscopy. These are safer and easier to learn, as they only require minor surgery and lead to shorter invasive interventions.1
There is much evidence on the advantages and disadvantages of first- or second-generation devices in terms of treatment outcomes. It has been reported that first-generation devices have a lower risk of equipment failure as well as nausea, vomiting, and uterine cramping.2,5 However, second-generation devices have been reported to offer advantages regarding procedure-associated complications, like fluid overload, perforation, cervical lacerations and hematometra, and shorter recovery times.2,5–8 A Cochrane review reported no difference in procedure-caused inability to work, requiring additional surgeries or subsequently performed hysterectomies; however, the results from other reviews reported that second-generation devices appeared to be more cost-effective.5,6
Current evidence suggests that RFA provides greater clinical benefits than other second-generation devices regarding amenorrhea rates and other clinical outcomes.6,7,9–11 However, so far, no economic benefits have been investigated. Therefore, within this analysis, we report the results extracted from German health claims data assessing the economic and clinical benefits of endometrial RFA compared to other, frequently used ablation techniques.
The primary outcome was the direct treatment costs in the quarter of treatment (QoT) and during a 2-year follow-up period; secondary outcomes were the number of recurrent menorrhagia diagnoses and the number of menorrhagia-associated treatments after the index therapy. Here, we had a closer look at subsequently performed hysterectomies, as the hysterectomy is currently the most frequent surgical treatment for women with menorrhagia.2,11 Due to the use of secondary, anonymized administrative data there was no need for informed consent or ethical approval.
Methods
Setting and data source
The analysis was based on anonymized billing data from a German health claims database, including approximately 4 million member records collected from over 80 German statutory health insurances (SHIs). The dataset included nearly 5% of the population covered by SHIs from January 1, 2008 to September 30, 2013. In Germany, health claims are submitted by health care providers working for the SHI. These billing data include the ICD-10-GM (German Modification of International Classification of Diseases) codes for the classification of diseases, the German version of the International Classification of Procedures in Medicine (ICPM) codes and the German Diagnosis Related Groups (G-DRG) codes for inpatient billing, as well as the German national drug codes (ATC codes) for outpatient drugs prescribed and delivered. Average costs are presented for six different categories: inpatient care (hospitalization), outpatient care, prescribed pharmaceuticals (outpatient setting), sick pay, remedies, medical aids, and total costs. While the category remedies includes orthopedic devices or other devices for preventive or compensatory use concerning disability, the category medical aids includes physiotherapy, massage, speech and language therapy, and occupational therapy. In outpatient settings, data are pooled and transferred to the SHI every 3 months, so the results of this analysis are presented per quarter. Privately insured patients in Germany are not included in this database.
The sample was created by random sampling data stratified by age and sex, so that the structure of the sample showed a similar distribution to that of the German population (information obtained from the Federal Statistical Office [DESTA-TIS]). There were no major differences in the prevalence of morbidity compared with the total distribution of morbidity for the German population covered by SHIs. Detailed information is published for each year by the German Federal Insurance Authority, which is responsible for implementing the risk-structure compensation scheme.12
Study design
The study design and outcomes were described à priori in a protocol. An observational and case-controlled health economic study (from a third-party payer’s perspective) was performed to compare endometrial RFA ablation with other ablation techniques.
We identified women who had undergone endometrial RFA (cases) and those who had undergone ablation with another technique (controls) between January 1, 2009 and September 30, 2011. We obtained data for these women for a further 2 years (up to September 30, 2013), since it has been reported that most menorrhagia-associated hysterectomies after failure of initial endometrial ablation are performed within 2 years.13,14
Cost data for medication, hospitalization, and sick pay were analyzed for the QoT and for 2-year follow-up period. The clinical outcome was the rate of relapse, defined as the rate of repeat diagnoses of menorrhagia after index treatment. We also recorded the number of women who underwent uterine treatments for recurring menorrhagia during follow-up.
Study groups
Eligible women were insured for the whole period, from 2008 to 2013, with a menorrhagia diagnosis (ICD-10-GM codes for menorrhagia: N92.0, N92.1 and N92.3-6) reported during January 1, 2009 to September 30, 2011. The ICD-10-GM code N92.2 was not included, as it covers heavy bleeding in women at the age of puberty who were not considered a relevant target group for the analysis. Women who met these first two criteria and who had a code for RFA (German ICPM code: 5-681.53) were included as cases.
In the first review of data for the controls, the number of endometrial laser ablations (German ICPM code: 5-681.51) was estimated to be insufficient to provide an adequate sample size. It was reported that transcervical hysteroscopic endometrial resection (TCER), rollerball resection and the balloon thermal ablation were equally effective.7,15,16 Therefore, the German ICPM codes for these ablation techniques (5-681.50, 5-681.52) were selected as specific inclusion criteria for controls (Table 1).
Table 1.
Relevant German ICPM codes for establishing the case and control groups
| Study group | German ICPM code | Intervention | |
|---|---|---|---|
| Intervention group | RFA | 5-681.53 | Radiofrequency ablation |
| Control group | Other endometrial ablations | 5-681.50 | Transcervical hysteroscopic endometrial resection (TCER)/rollerball resection |
| 5-681.52 | Balloon thermal ablation |
Abbreviations: RFA, radiofrequency ablation; ICPM, International Classification of Procedures in Medicine.
Propensity score analysis
As recommended in the guidelines by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for comparative effectiveness research using non-randomized studies in secondary data sources, the burden of morbidity of both groups was compared as described below.17–19
Variable selection
Comorbidity data for the cases and controls were collected for the year prior to treatment. We included age and binary dummy covariates for the comorbidities (ICD-10-GM codes, three digits) and prescribed drugs (ATC-codes, four digits) if at least five patients had the specific covariate. Using multivariate logistic regression, we estimated a propensity score for each subject with the predicted probability of exposure being conditional on 200 covariates.
Propensity score matching
The distribution of the estimated propensity scores for the cases and controls showed sufficient overlap for both groups, although the distribution for the controls was skewed to lower scores (Figure 1). The GenMatch matching algorithm with a caliper of 0.5 was used to minimize the observed covariate discrepancies.20 After matching, the distribution of the propensity score of both groups was similar (Figure 2) with the standardized absolute differences in mean age and health care costs showing no major differences.
Figure 1.
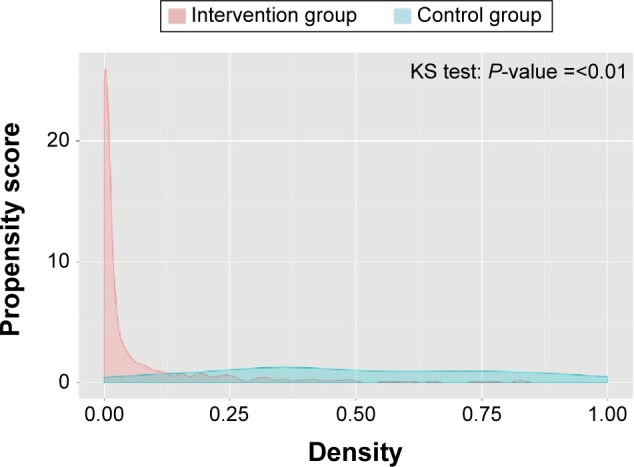
Distribution of propensity scores prior to matching.
Abbreviation: KS test, Kolmogorov–Smirnov test.
Figure 2.
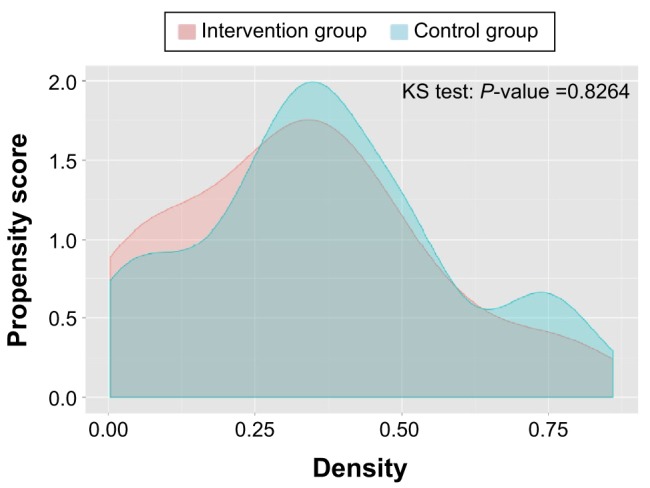
Distribution of propensity scores after matching.
Abbreviation: KS test, Kolmogorov–Smirnov test.
Statistics
The results were checked for any outliers by controlling descriptive statistics in detail. One control patient had over €120,000 in hospitalization costs in the follow-up period due to treatment for an oncologic disease. Since this was three times higher than the standard differences of the mean, we decided to trim the hospital costs of this one specific patient to the value of the next expensive control patient in accordance to Sekhon in order to limit the impact of the outlier.20 Average treatment costs were calculated for intervention and control groups. Student’s t-tests for differences in means were calculated. The means were reported with their 95% confidence intervals. Data were stored and analyzed using Microsoft Office Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) and SAS® (Version 9.2; SAS Institute Inc., Cary, NC, USA). The GenMatch algorithm was performed using R, the free statistical software.
Results
A total of 32,246/1,358,450 women with a continuous insurance status from 2008 to 2013 were identified with menorrhagia codes. Among the women with menorrhagia, 71 had treatment codes corresponding to RFA, 564 had codes for TCER/rollerball resection and 40 had codes for balloon thermal ablation. The final sample size for the analysis, after propensity score matching, was 88; 50 cases and 38 controls (36 had TCER/rollerball resection and two had balloon thermal ablation). Among the cases, 98% had previously undergone coagulation, endometrial ablation or resection (German ICPM code 5-681.-), compared with 58% of the controls.
Economic outcomes
By using propensity score matching, the average total costs prior to ablation were comparable for cases and controls (€1,100 vs €1,011). After adjustment of the costs for the patient whose costs were excessively high due to treatment of an oncologic disease, the different kinds of costs during the QoT were similar for cases and controls, except for hospital costs for cases, which were €766 higher than for controls with a higher overall cost of €578 (Table 2).
Table 2.
Average total direct costs (€) per patient during quarter of treatment and 2-year follow-up
| Medication | Outpatient physician consultations | Remedies/physio rehabilitation | Medical aids | Sick pay | Hospitalization | Total | |
|---|---|---|---|---|---|---|---|
| RFA in QoT | 61 | 310 | 2 | – | 3 | 1,692 | 2,068 |
| Other endometrial ablations in QoT | 85 | 476 | – | 3 | – | 926 | 1,490 |
| Follow-up after RFA | 495 | 1,355 | 95 | 34 | 557 | 2,025 | 4,561 |
| Follow-up after other endometrial ablations | 1,228 | 1,683 | 115 | 33 | 30 | 2,726 | 5,815 |
Abbreviations: RFA, radiofrequency ablation; QoT, quarter of treatment.
During the 2-year follow-up period, the overall costs for controls were €1,254 higher than that for cases (€4,561 vs €5,815). The main cost drivers were medication (€495 vs €1,228), outpatient physician consultations (€1,355 vs €1,683) and hospitalization (€2,025 vs €2,726) (Table 2). Taken together, the average total costs (QoT and follow-up) were €676 less for those women who underwent RFA compared with those who underwent other ablation techniques. However, none of the differences were statistically significant.
Repeated diagnosis and necessary retreatments
During the 2-year follow-up, 40% of the women treated with RFA had another diagnosis of menorrhagia, compared with 45% of the controls. Hence, 60% of the cases were successfully treated, since they did not have another diagnosis. Only 6% of the cases had codes for being surgically retreated for menorrhagia, compared with 11% of the controls. Regarding the frequency of hysterectomy after index endometrial ablation, we could not observe any difference between the two study groups (8% vs 8%). None of these differences were statistically significant.
Discussion
This study was performed following an analysis that reported the clinical and economic benefits of RFA compared with hysterectomy in women with menorrhagia; Kessel et al reported a total savings of €1,771 within 2 years after the first treatment when treating menorrhagia with RFA instead of hysterectomy.21
Generally, RFA is shown to be superior to other endometrial ablation techniques in terms of success rates. In our study, 60% of RFA treatments were successful, meaning that these women had no subsequent coding of uterine bleeding. In Kessel et al, the rate of women without repeat diagnoses after RFA was similar (57%), which stresses the internal validity of the database used.
The main aim of menorrhagia treatment is to stop heavy uterine bleeding, and, if possible, to induce amenorrhea. We were not able to assess whether women in this study remained completely free of uterine bleeding within the follow-up period, as light or moderate cases that were not reported to physicians were not included in the database. However, based on a previous study that reported an amenorrhea rate of 43%–56% 12 months after initial RFA, we can assume that the women included in our analysis remain free from bleeding over time.11 The higher success rate that we observed was also reported in another study that showed that RFA resulted in greater coverage of the endometrial surface and a lower failure rate compared with other endometrial ablation techniques.7
Bansi-Matharu et al observed that women who underwent RFA were less likely to have subsequent surgery compared with those who underwent first-generation ablation techniques (hazard ratio 0.69, 95% confidence interval 0.63–0.76, P<0.001), which was similar to our findings;22 fewer women (−5%) required subsequent treatment after RFA compared with other endometrial ablation techniques. Additionally, as reported in other studies, we did not observe any difference in the frequency of hysterectomy after RFA compared with other endometrial ablation techniques.5,13
Medication costs, in addition to those for hospitalization and outpatient consultations, contributed to the higher average costs associated with other endometrial ablation techniques. Although the average total costs for RFA were lower, costs for RFA were higher during QoT. The main cost driver was hospital costs; although, in Germany, all inpatient endometrial ablations are remunerated with the same German G-DRG code. One possible explanation for the systemically higher hospital costs during QoT following RFA is that patients stay longer in the hospital in order to generate higher case weights so that higher acquisition costs of RFA devices are better compensated. Although it is reported that RFA could require shorter operating times, there was no effect observed on the postoperative length of hospitalization, which could even be longer.16 It is of relevance that this analysis covered inpatient RFA cases only; generally, RFA is performed in the outpatient setting, where costs are even lower than reported for the inpatient setting. However, current reimbursement issues in the German outpatient setting made it impossible to detect outpatient cases in the database.
Despite the small sample sizes, it was possible to generate indications for favorable cost-effectiveness of RFA over other first- and second-generation endometrial ablation devices. The analysis demonstrates that the proven advantages of RFA regarding amenorrhea rates, shorter recovery rates, and other clinical outcomes actually can have an impact on economics by generating overall savings within a German patient population.
Limitations
Although health claims data offered many advantages, they also had some limitations, which should be considered when interpreting these results. First, only patients actually consulting a physician were reported in this German database. Patients who suffered from a condition but did not seek help from a physician contracted by an SHI were not included. As about 10% of the German population was not insured with an SHI, but with a private health insurance company, this 10% was not represented in the analysis.
Data were collected in these databases for billing purposes and not for research; therefore, the probability of inaccuracies, coding errors or up-codings could not be excluded, as they are generated during the daily routine.23 Currently, RFA is not widely offered in Germany. In the outpatient setting, RFA was not generally remunerated by the standard reimbursement schemes, but rather on a case-by-case approval system and, therefore, was not included in the German health claims database used. Hence, only inpatient RFAs were analyzed in this study, which led to a small sample size (N=88), which was insufficient to detect any statistically significant differences.
When collecting cases and controls, it was not differentiated whether a menorrhagia diagnosis was of a primary or secondary nature. Due to the uncertainty of what kind of primary diagnosis patients might have suffered from, it could not be excluded that results might be biased.
Another parameter that might have influenced the results of the RFA group was that it was not known whether patients were tested for or suffered from bleeding disorders. This question was not addressed in our analysis, as our study population covered predominantly women aged 30–50 years; most bleeding disorders are detected at an early age, thus, this issue seemed not to be of relevance for our purposes.
In our study, almost all cases underwent RFA subsequent to a prior coagulation/excision/ablation therapy (98%), whereas the controls underwent other endometrial ablation techniques more often without any prior treatment (58%). These figures were quite surprising, but could be explained by clinical inclusion and exclusion criteria for RFA therapy. To perform RFA, patients needed to be free of any septums, polyps or myoma. Hence, the high amount of prior treatments of diseased uterus tissue (German ICPM code 5-681.-) could be explained by the requirement of polyp- and myoma-free uteri and should not necessarily be seen as a second-line or repeated treatment (ablation) of menorrhagia, since a majority of these pretreatments may have occurred during the same session to prepare the patient for the RFA procedure. This may have been a bias for the results; although it is unclear to what extent this might have influenced the outcomes. In fact, the influence on the health economic outcomes was rather small due to the propensity score matching in which all cases showed similar amounts of expenditures prior to the actual index event.
The controls consisted of women who had undergone different endometrial ablation techniques (TCER, rollerball resection, balloon thermal ablation), which may vary in their effectiveness and outcomes. Unfortunately, it was not possible to assess each endometrial ablation technique individually, as the sample sizes were not sufficiently large in the database. A few balloon thermal ablation cases were included, as the initial idea of the analysis was to compare RFA to many endometrial ablation techniques; the small sample size was not a reason to exclude these cases post hoc.
Regarding average total cost savings, it would have been desirable to detect at what point in time costs assimilated, but as the database only covered 6 years in total, a follow-up of 2 years was the maximum period we could have chosen.
Conclusion
In conclusion, the main findings of our analysis were that inpatient RFA was associated with fewer recurrences and lower rates of subsequent uterine surgical treatments, suggesting that the long-term success rate of RFA was more favorable compared to that for other ablation techniques. Although the costs were higher for RFA initially, follow-up costs for women who had undergone RFA were lower, leading to an average lower cost of €676 per patient over 2 years. The average total cost, including QoT and follow-up, was also lower for women who had undergone RFA. None of the results were of statistical significance.
RFA is a treatment option that shows beneficial clinical results and indications for favorable cost-effectiveness compared with other ablation techniques, but further research with larger sample sizes is necessary to generate results that are more robust. However, the presented results will already be of interest to several stakeholder groups, like clinicians, health care providers, and third-party payers, striving for the best and most cost-effective long-term treatment of menorrhagia. Furthermore German SHIs are able to use our results as an external benchmark to compare with their own results.
Acknowledgments
The authors would like to thank M Jochmann (Tagesklinik Hoyerswerda), G Kreuz (Tagesklinik Hoyerswerda), T Muscheid (Kplus Gruppe), A Nugent (Tagesklinik Altonaer Straße), and S Kessel for excellent medical and methodological advice while designing the study and analyzing the results. The statistical analysis was performed by the Health Risk Institute GmbH in coordination with Elsevier Health Analytics. This study was funded by Hologic Deutschland GmbH, manufacturer of the NovaSure® endometrial ablation device.
Footnotes
Disclosure
All authors have served as consultants to Hologic Deutschland GmbH. R Soeder and B Neukirch did not receive any consultancy fee for their role as advisors. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the paper apart from those disclosed.
References
- 1.National Collaborating Centre for Women’s and Children’s Health . Heavy menstrual bleeding – clinical guideline. London: RCOG Press; 2007. [Google Scholar]
- 2.Duckitt K. Managing perimenopausal menorrhagia. Maturitas. 2010;66(3):251–256. doi: 10.1016/j.maturitas.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Pollock W, Jamieson W. Next-generation NovaSure® device for endometrial ablation: assessing ease-of-use among physicians. Int J Womens Health. 2012;4:109–113. doi: 10.2147/IJWH.S30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrel-releasing intrauterine device, or hysterectomy. Fertil Steril. 2004;82(1):160–166. doi: 10.1016/j.fertnstert.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Lethaby A, Penninx J, Hickey M, Garry R, Marjoribanks J. Endometrial resection and ablation techniques for heavy menstrual bleeding (review) Cochrane Database Syst Rev. 2013;8:CD001501. doi: 10.1002/14651858.CD001501.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Kroft J, Liu G. First- versus second-generation endometrial ablation devices for treatment of menorrhagia: a systematic review, meta-analysis and appraisal of economic evaluations. J Obstet Gynaecol Can. 2013;35(11):1010–1019. doi: 10.1016/S1701-2163(15)30789-1. [DOI] [PubMed] [Google Scholar]
- 7.Daniels JP, Middleton LJ, Champaneria R, et al. Second generation endometrial ablation techniques for heavy menstrual bleeding: network meta-analysis. BMJ. 2012;344:e2564. doi: 10.1136/bmj.e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels JP. The long-term outcomes of endometrial ablation in the treatment of heavy menstrual bleeding. Curr Opin Obstet Gynecol. 2013;25(4):320–326. doi: 10.1097/GCO.0b013e3283630e9c. [DOI] [PubMed] [Google Scholar]
- 9.Bongers MY, Bourdrez P, Mol BW, Heintz AP, Brölmann HA. Randomised controlled trial of bipolar radio-frequency endometrial ablation and balloon endometrial ablation. BJOG. 2004;111(10):1095–1102. doi: 10.1111/j.1471-0528.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- 10.Clark TJ, Samuel N, Malick S, Middleton LJ, Daniels J, Gupta JK. Bipolar radiofrequency compared with thermal balloon endometrial ablation in the office: a randomized controlled trial. Obstet Gynecol. 2011;117(1):109–118. doi: 10.1097/AOG.0b013e3182020401. [DOI] [PubMed] [Google Scholar]
- 11.Gimpelson RJ. Ten-year literature review of global endometrial ablation with the NovaSure® device. Int J Womens Health. 2014;6:269–280. doi: 10.2147/IJWH.S56364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The German Federal Insurance Authority Risikogruppenanteile [Risk Group shares] [Accessed November 11, 2015]. Available from: http://www.bundesversicherungsamt.de/risikostrukturausgleich/info-dateien-und-auswertungen/risikogruppenanteile.html.
- 13.Fürst SN, Philipsen T, Joergensen JC. Ten-year follow-up of endometrial ablation. Acta Obstet Gynecol Scand. 2007;86(3):334–338. doi: 10.1080/00016340601089701. [DOI] [PubMed] [Google Scholar]
- 14.Comino R, Torrejon R. Hysterectomy after endometrial ablation-resection. J Am Assoc Gynecol Laparosc. 2004;11(4):495–499. doi: 10.1016/s1074-3804(05)60082-5. [DOI] [PubMed] [Google Scholar]
- 15.Van Zon-Rabelink IA, Vleugels MP, Merkus HM, de Graaf R. Efficacy and satisfaction rate comparing endometrial ablation by rollerball electrocoagulation to uterine balloon thermal ablation in randomised controlled trial. Eur J Obstet Gynaecol Reprod Biol. 2004;114(1):97–103. doi: 10.1016/j.ejogrb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Garside R, Stein K, Wyatt K, Round A, Price A. The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling. Health Technol Assess. 2004;8(3):iii, 1–155. doi: 10.3310/hta8030. [DOI] [PubMed] [Google Scholar]
- 17.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources. Report of the ISPOR Retrospective Database Analysis Task Force–Part I. Value Health. 2009;12(8):1044–1052. doi: 10.1111/j.1524-4733.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 18.Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design on nonrandomized studies of treatment effects using secondary data sources. Report of the ISPOR Retrospective Database Analysis Task Force–Part II. Value Health. 2009;12(8):1053–1061. doi: 10.1111/j.1524-4733.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NL, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources. Report of the ISPOR Retrospective Database Analysis Task Force–Part III. Value Health. 2009;12(8):1062–1073. doi: 10.1111/j.1524-4733.2009.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Sekhon J. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. Journal of Statistical Software. 2011;42(7):1–52. [Google Scholar]
- 21.Kessel S, Hucke J, Goergen C, Soeder R, Roemer T. Economic and clinical benefits of radiofrequency ablation versus hysterectomy in patients suffering from menorrhagia: a retrospective analysis with German health claims data. Expert Rev Med Devices. 2015;12(3):365–372. doi: 10.1586/17434440.2015.1015988. [DOI] [PubMed] [Google Scholar]
- 22.Bansi-Matharu L, Gurol-Urganci I, Mahmood TA, Templeton A, van der Meulen JH, Cromwell DA. Rates of subsequent surgery following endometrial ablation among English women with menorrhagia: population-based cohort study. BJOG. 2013;120(12):1500–1507. doi: 10.1111/1471-0528.12319. [DOI] [PubMed] [Google Scholar]
- 23.Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies – report of the ISPOR Task Force on retrospective databases. Value Health. 2003;2(6):90–97. doi: 10.1046/j.1524-4733.2003.00242.x. [DOI] [PubMed] [Google Scholar]


