Abstract
In this study, calcium carbonate (CaCO3) nanoparticles with spherical structure were regulated by arginine and successfully synthesized via a facile co-precipitation method. The average particle size of as-prepared CaCO3 was about 900 nm. The properties of nanostructured CaCO3 particles were characterized by scanning electron microscope, Fourier transform infrared spectroscopy, X-ray diffraction and size distribution. After modified with polyethyleneimine (PEI), the ability of PEI-CaCO3 nanoparticles to carry GFP-marked p53 gene (pEGFP-C1-p53) into cancer cells to express P53 protein were studied. Meanwhile, the cytotoxicity, transfection efficiency, cells growth inhibition and the ability to induce apoptosis by expressed P53 protein were conducted to evaluate the performances of PEI-CaCO3 nanoparticles. The results show that prepared PEI-CaCO3 nanoparticles had good biocompatibility and low cytotoxicity in a certain concentration range. PEI-CaCO3 effectively transfected pEGFP-C1 gene into epithelial-like cancer cells. And with the expression of GFP-P53 fusion protein, pEGFP-C1-p53-gene-loaded PEI-CaCO3 particles significantly reduced the proliferation of cancer cells. These findings indicate that our PEI-modified CaCO3 nanoparticles are potential to be successfully used as carriers for gene therapy.
Keywords: calcium carbonate, gene therapy, nanoparticles, polyethyleneimine, p53
Introduction
Gene therapy is one of the potential ways in cancer treatments and has now been extensively studied [1–3]. The key point of gene therapy depends on efficient delivery of genetic reagents to targeted cells without significant toxicity and side effects [4]. Over last decades, the delivery carriers for gene therapy are under critical concern. An ideal gene delivery system would be biodegradable, non-toxic, non-immunogenic, specific targeting and should stimulate the gene expression with reliability [5, 6].
Numerous gene delivery systems, including viral and non-viral carriers, have been developed for improving gene transfection [4, 7–9]. Although viral carriers have higher efficiency, their potential immune response, high cost and difficult recombination have limited their clinical applications [10]. Because of easy preparation, reduction risk of immune response, non-viral carriers, such as cationic lipids [11], polymers [12], chitosan [13] and peptides [14] have attracted lots of attentions except lower gene transfection efficiency. Nevertheless, the safety of widely investigated non-viral carriers based on cationic lipids and polymers is still not satisfying [15].
Among different non-viral gene delivery systems, inorganic nanoparticles (NPs) such as silica [16], iron oxide [17], Au [18] and calcium-based materials have been recently exploited as the alternative non-viral carriers. They could be prepared and surface modified in different methods to own not only high transfection efficiency but also relative safety in vitro and in vivo [19].
Calcium carbonate (CaCO3) is one of the most abundant natural materials as well as hard tissues like bones and teeth [20]. Based on the previous studies and the recent reports about CaCO3 gene vectors, this gene or drug delivery system has many advantages for its excellent biocompatibility, low toxicity, non-immunogenicity and potential high loading capability [21–23]. When pure CaCO3 nanoparticles used as the gene vector, the efficiency of the vector to transfect pDNA into cells is in a quite low level, which hampered its utility for future clinical applications [21]. Polyethylenimine (PEI) has been demonstrated to be an efficient carrier or appropriate reagent for surface modification of particles, because of its ability to protect plasmids from degradation [22] and to improve of the gene release from cytolysosome [23] in cells.
In this study, nanostructured calcium carbonate particles with a rough surface were prepared and subsequently superficial modified with PEI. Then the PEI-CaCO3 NPs were used to deliver the tumor suppressor gene p53, which exists extensively in over 50% of different tumors and can induce the apoptosis of cancer cells by causing its DNA damage [24]. The physicochemical properties, cytotoxicity of CaCO3 NPs and the properties of PEI-CaCO3 NPs as a gene carrier were further investigated in vitro.
Materials and Methods
Materials and cell culture
All chemicals were of analytical grade and used as received without further purification. Deionized water was used for all experiments. All experiments were conducted under ambient atmosphere. Calcium chloride (CaCl2), ethyl alcohol, sodium carbonate (Na2CO3), polyethyleneimine (PEI, Mw = 70 000) and arginine were purchased from Sigma-Aldrich (St Louis, MO). Plasmids were stored at −20°C. The rabbit anti-GAPDH antibody was purchased from Cell Signaling Technology. P53 antibody (Abcam, USA) reactions were visualized with an anti-rabbit IR secondary antibody (LICOR, USA).
Human cell lines, Hep3B, QSG-7701, H1299, 293a and Hela cells were obtained from American Type Culture Collection (Rockville, MD). All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) with 10% fetal bovine serum (FBS, Gibco) at 37°C in a 5% CO2 humidified incubator.
Preparation of calcium carbonate particles
We have formulated a simple and reproducible procedure for preparation of spherical CaCO3 nanoparticles. Briefly, 2% arginine solution and 60 mM Na2CO3 were mixed at the volume ratio of 1:1. Then the mixed solution was added into 60 mM CaCl2 dropwise under vigorous stirring for 15 min. The precipitates were separated from the solution by centrifugation, washed with ethanol and deionized water for several times and dried at 60°C. The obtained calcium carbonate nanoparticles in the form of white powder were denoted as ACa.
Characterization of ACa nanoparticles
The prepared ACa NPs were characterized using scanning electron microscope (SEM), Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD). For SEM observations, particle suspension in ethanol was dropped on silicon wafer, dried in air and then observed under SEM (SEM S-4800, Hitachi). X-ray powder diffraction (XRD) patterns were recorded with 2θ range from 20° to 70° on a D/Max 2200-PC diffractometer (Rigaku Corporation, Tokyo, Japan) with CuKα radiation (λ = 0.15418 nm) and graphite monochromator at ambient temperature. An FTIR spectroscopy was carried out with a Nexus spectrometer (Thermo Nicolet, Madison, WI) using the KBr disk method. Size distribution of as-prepared nanoparticles was measured by a Zetasizer Nano ZS (Malvern Instruments).
in vitro cell biocompatibility of ACa nanoparticles
QSG-7701 cells in 100 µl of complete medium (DMEM containing 10% FBS) were seeded in a 96-well plate (1 × 104 cells per well) and incubated at 37°C for 24 h under 5% CO2. Freshly prepared ACa NPs suspension at different concentrations of 0.1, 0.5, 1, 2, 3, 4, 5 and 6 mg/ml was added to each well with seeded cells and incubated at 37°C for 24 h, 48 h and 72 h. After determined intervals, 20 μl of 5 mg/ml MTT solution was added to each well and reacted at 37°C. After 4 h incubation, the supernatant was carefully removed with added 150 μl of DMSO to dissolve the formazan crystals. Cells viability was evaluated by measuring the absorbance at 490 nm through a microplate reader (Bio-Rad 550). The cell survival rate was expressed as Cell viability (%) = [OD490 (sample) − OD490 (blank)]/[OD490 (control) − OD490 (blank)] × 100%.
Loading plasmid with PEI-modified ACa nanoparticles
The as-prepared ACa NPs were preserved in a sterilized environment. Before loading plasmid, ACa NPs were firstly mixed with PEI solution and incubated at 37°C for 40 min. Then, a certain amount of plasmid was added to the PEI-ACa mixture at room temperature and incubated for 15–30 min to prepare PEI-ACa-pEGFP-C1 and PEI-ACa-pEGFP-C1-p53. The obtained PEI-ACa-plasmid particles were used for further study immediately after them prepared.
in vitro transfection mediated by PEI-ACa-DNA nanoparticles
Different cells in 1 ml of complete medium (DMEM containing 10% FBS) were seeded in a 24-well plate (5 × 104 cells per well) and incubated for 24 h; 50 μl of freshly prepared PEI-ACa-DNA particles containing 2.5 μg pEGFP-C1 was added to each well and incubated at 37°C. The gene expression was evaluated at 24 h, 48 h and 72 h by fluorescence microscope, respectively. The cells seeded on tissue culture plate were denoted as control.
Identification p53 gene expression and evaluation the cell growth inhibition
H1299 cells in 100 µl of complete medium (DMEM containing 10% FBS) were seeded in a 96-well plate (1 × 104 cells per well) and incubated at 37°C for 24 h. The cells seeded on poly Styrene plate were denoted as control. Then the freshly prepared solution containing particular agents (PEI-ACa-pEGFP-C1-p53, PEI-ACa, free pEGFP-C1-p53) was added to each well and incubated at 37°C for 24 h, 48 h and 72 h.
At determined periods of cultivation, the H1299 cell extracts were subjected to SDS-PAGE and electroblotted onto the nitrocellulose membrane in a buffer containing 25 mM Tris-HCl (pH 8.3)/192 mM glycine/20% methanol. The membrane was blocked with blocking buffer and incubated with related antibodies overnight at 4°C. Then the membrane was washed and incubated with IRDye 700 anti-rabbit IgG (Lincoln, NA) for 1 h at room temperature. After washing, the membrane was analyzed by an Odyssey Infrared Imaging System (LI-COR, Inc.). The cell inhibition rate was also determined by MTT assay as mentioned in Loading plasmid with PEI modified ACa nanoparticles section.
Cancer cell apoptosis
In apoptosis testing, cells were seeded in a six-well plate and treated with PEI-ACa-pEGFP-C1-p53. Naked pEGFP-C1-p53 and untreated were took as the control groups. After 72 h, cells were stained with Hoechst 33342 (Molecular Probes, Eugene) at 1 mg/ml for 15 min. Then, cells were washed with PBS twice and imaged under fluorescence microscope. The cells seeded on poly Styrene plate were denoted as control.
Statistical analysis
All quantitative data were depicted as the mean ± standard deviation (n = 6). Tests of significance were performed using Student’s t-test. The value of P < 0.05 was considered statistically significant.
Results and Discussion
ACa nanoparticles synthesis and analysis
In this study, we prepared the CaCO3 NPs by the facile method with a large scale in laboratory. CaCO3 NPs with spherical structure were obtained and denoted as ACa.
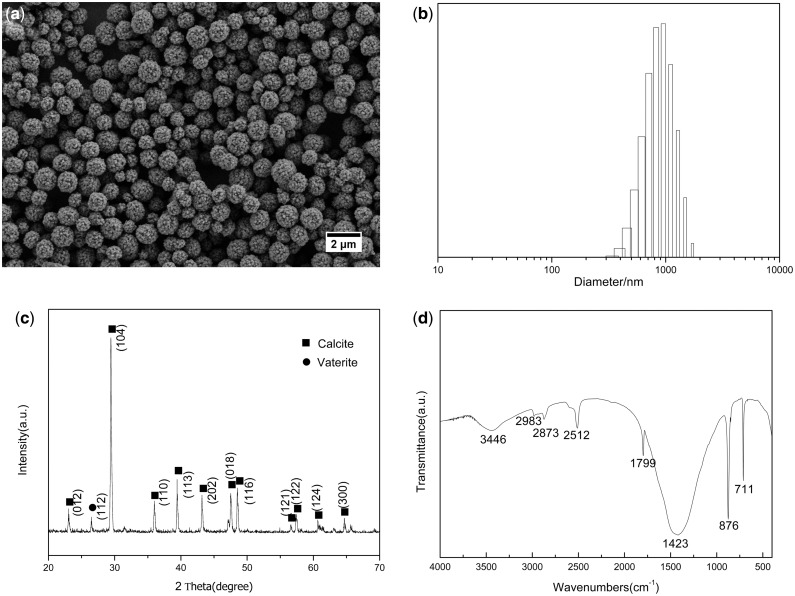
According to the literature [25, 26], the toxicity of the nanoscale material is closely related to size, structure and shape. As shown in SEM image (Fig. 1a), the ACa NPs presented uniform, spherical structure with average size of 900 nm (Fig. 1b). In addition, the surface of the particles was rough and consisted of many pores and channels in nanoscale, which facilitated loading NPs with bioactive molecules like proteins, drugs and gene. The crystal phases were characterized by XRD and FTIR. XRD pattern in Fig. 1c shows that the diffraction peaks were attributed to well-crystalline phase of calcite. The FTIR spectrum also demonstrated the typical absorption bands of calcite at 711 cm − 1 and 1423 cm − 1 as shown in Fig. 1d.
Figure 1.
(a) SEM image, (b) Size distribution, (c) XRD pattern and (d) FTIR spectrum of ACa nanoparticles
Cell biocompatibility of ACa nanoparticles in vitro
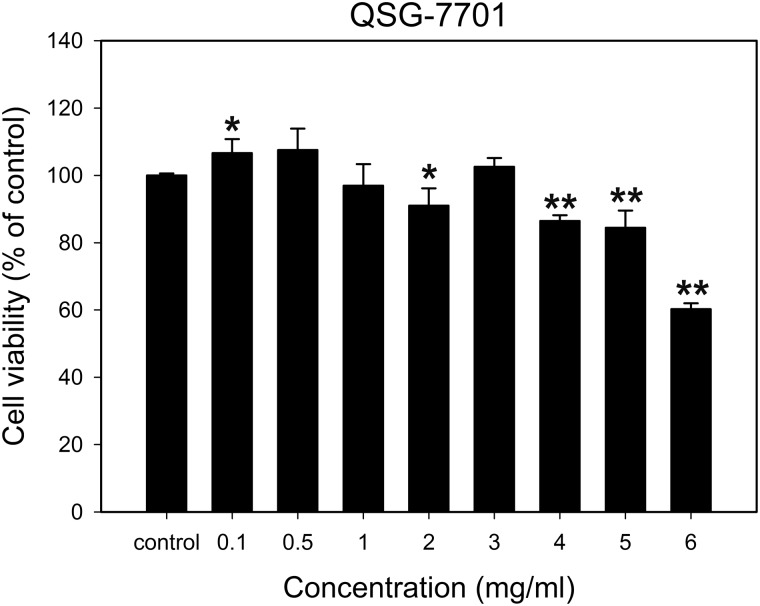
Biocompatibility is the key point for gene delivery system in the purpose of cancer gene therapy. To evaluate the cytotoxicity induced by as-prepared ACa NPs, an MTT assay was conducted to measure the viability of treated QSG-7701 cells. As shown in Fig. 2, it could be found that the cell viabilities were higher than 80% after being treated with ACa NPs with the range of particle concentration between 0.1 mg/ml to 5 mg/ml for 24 h, implying that ACa NPs did not have apparent cytotoxicity. However, when QSG-7701 cells encountered with 6 mg/ml ACa NPs, the cell viabilities decreased significantly to be around 60% (P < 0.01). CaCO3 is one of the fundamental inorganic components in mammalian hard tissues [27] and could be dissolved in low pH condition to release calcium and carbonate ions [28]. Both calcium and carbonate ions are naturally existed in mammalian body and safe to circulate or be reuse [29]. So the prepared spherical ACa NPs had good biocompatibility with only a little cytotoxicity in a certain range of concentration.
Figure 2.
Cell cytotoxicity of QSG-7701 cells treated with different concentrations of ACa NPs at 24 h. The percentage of cells viability was calculated relative to untreated cells and presented as the mean ± standard deviation (n = 6). *P < 0.05 and **P < 0.01
Transfection efficiency of PEI–ACa–pEGFP-C1 particles in vitro
The main purpose of carriers for gene delivery is to protect and deliver gene into nuclear of targeted cells avoiding intracellular degradation [30]. For most conditions, after gene-loaded non-viral carriers enter into lysosome, the degradations of both carriers and pDNA would occur, which leads to the low efficiency of gene transfection [27]. The prepared ACa NPs could adsorb gene to encapsulate gene because of its rough and porous structure. Based on the biodegradability of CaCO3 NPs in acid condition, CaCO3 NPs could degrade at low pH in the microenvironment of cancer cells or in liposome [28]. So the CaCO3 degradation could further lead to the release of DNA into the cytoplasm after being uptake by cancer cells. Ultimately, the released DNA would get into the nuclear for protein expression.
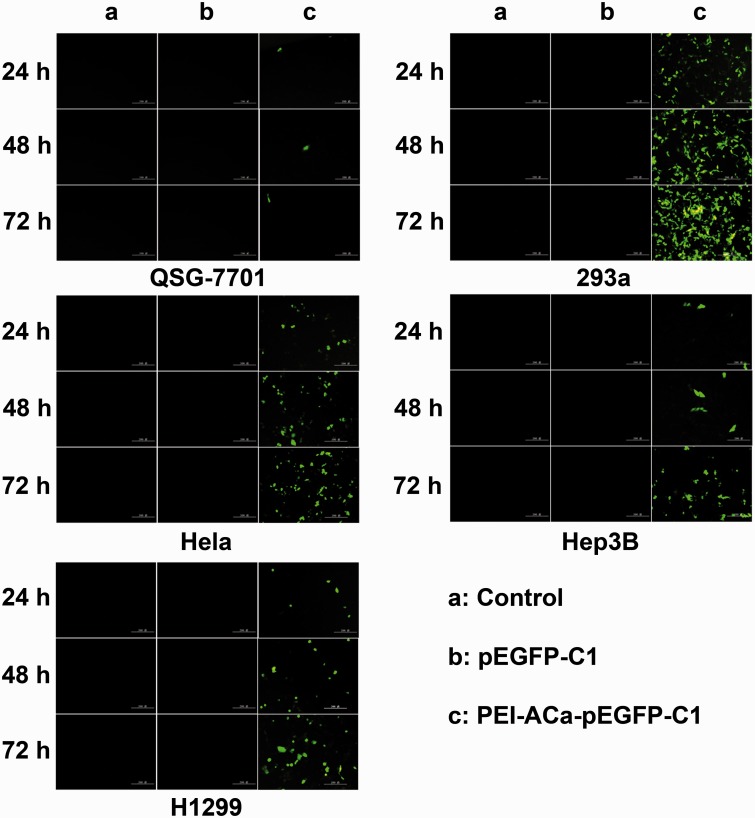
The in vitro GFP gene expression was performed using different cells lines for 24, 48 and 72 h. As shown in Fig. 3, naked pEGFP-C1 did not present any GFP expression because free gene could not penetrate the membrane of cells. For PEI–ACa–pEGFP-C1 particles, the fluorescence was lower in Hep3B, QSG-7701, H1299 and Hela cells except for 293a cells in the initial 24 h. However, the green florescence increased with time implying more GFP expression. As is well known, 293a is the typical cell line for the study of gene transfection. Compared with 293a cells, the GFP expression levels for others cells are relatively lower. In addition, the tests were also carried on MCF-7, V251, Bel-7404 and Huh-7 cells, and the transfection efficiency was still not as high as 293a (data not shown). We hypothesized that the epithelial-like cancer cells could facilitate the gene transfection, and more studies need to be investigated to prove this point.
Figure 3.
Fluorescence images of different cell lines treated with pEGFP-C1-p53 or PEI-ACa-pEGFP-C1-p53. The images were taken at 24 h, 48 h and 72 h, respectively. Scale bars represent 200 μm
The expression of p53 gene and the inhibition of cell growth
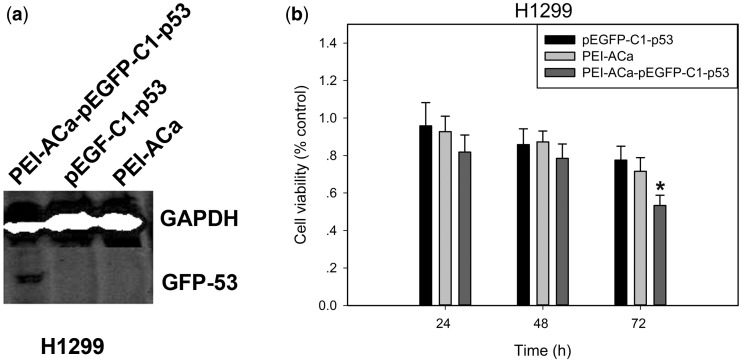
To investigate the expression of p53 gene loaded within PEI-ACa NPs, western blotting analysis was performed in H1299 cells. As shown in Fig. 4a, there was a specific band at 90 kDa (GFP-P53 protein) in PEI-ACa-pEGFP-C1-p53 group, while the control group and naked pEGFP-C1-p53 group did not show this band.
Figure 4.
(a) GFP-P53 protein expression from different groups identified by western blotting. (b) Growth inhibition of H1299 cells treated with pEGFP-C1-p53, PEI-ACa and PEI-ACa- pEGFP-C1-p53 at 24 h, 48 h and 72 h. The percentage of cells viability was calculated relative to untreated cells and presented as the mean ± standard deviation (n = 6). *P < 0.05
Then, the cancer cells viability was analyzed by MTT assay in vitro. As shown in Fig. 4b, treatment with reduced tumor cells H1299 proliferation in a time-dependent manner compared with PEI-ACa and pEGFP-C1-p53. No such reduction was observed between pEGFP-C1-p53 and PEI-ACa. These results indicate that our PEI-ACa-pEGFP-C1-p53 particles specifically reduce proliferation of H1299 cells by controlling the expression of P53 protein in vitro.
Cell apoptosis of PEI–ACa–pEGFP-C1 particles in vitro
Cell apoptosis, which is a process of programmed cell death, could occur in multi-cellular organisms [31]. The apoptosis is controlled by diverse range of cancer suppressor genes including p53 gene. And when DNA has been suffered from damage, the p53 gene could activate DNA repair proteins to induce the growth inhibition of cells and cell apoptosis [32]. Hence, delivering p53 gene into cancer cells could inhibit cell proliferation and stimulate the apoptosis.
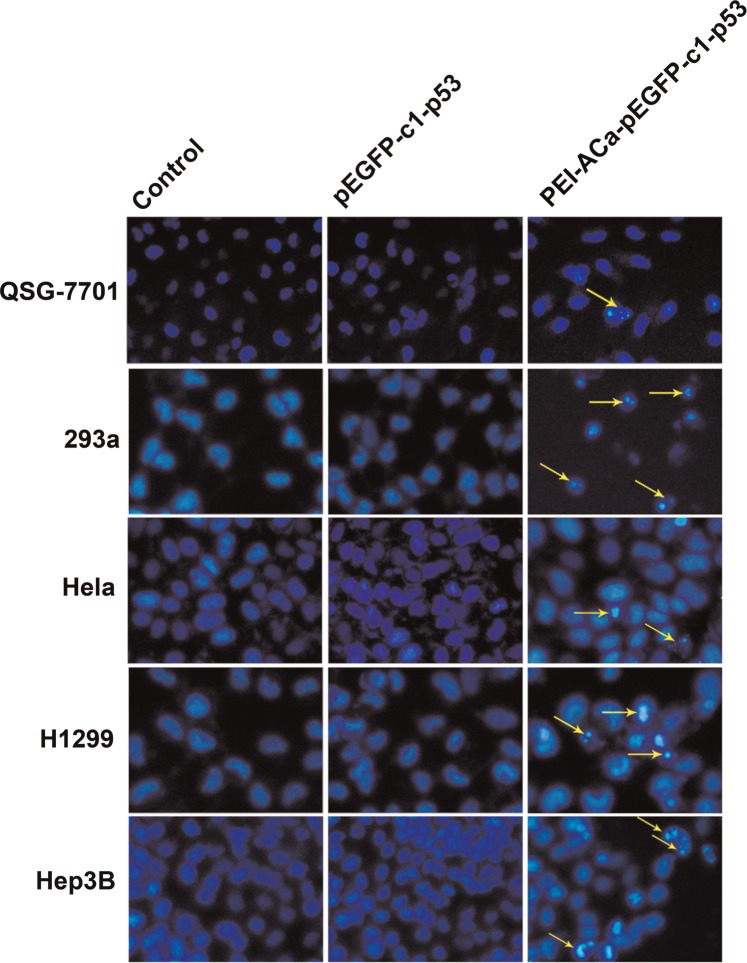
To further verify the successful delivery of the gene into the cells, we studied the cell apoptosis by Hoechst 33342 staining. The nuclear morphology of cells was visualized by a fluorescence microscope after stained with Hoechst 33342 dye for 72 h in Fig. 5. The arrows pointed out the apoptotic cells. No significant cell apoptosis was observed between control and naked pEGFP-C1. The results showed that PEI-ACa-pEGFP-C1-p53 apparently caused tumor cell nuclear fragmentation, chromatin condensation and DNA fragmentation.
Figure 5.
Apoptosis images of different cells transfected by pEGFP-C1-p53 and PEI-ACa- pEGFP-C1-p53. The nuclear of cells was stained with Hoechst 33342 nuclear stain at 72 h and observed under fluorescence microscope. The arrows indicated the apoptosis cells
Conclusion
A simple method had been developed to synthesis the spherical ACa NPs by regulated with arginine and the prepared nanoparticles show good biocompatibility. PEI-modified ACa (PEI-ACa) effectively transfected pEGFP-C1 to the specific epithelial-like cancer cells. And PEI-ACa could successfully deliver pEGFP-C1-p53 gene into the cells which expresses the GFP-P53 fusion protein and induce cell apoptosis. Our study provides useful insights for formulating efficient and safe gene delivery carriers.
Funding
This work was supported by National Natural Science Foundation of China (51272236, 51502265), Program for 521 Excellent Talents and Science Foundation of Zhejiang Sci-Tech University (13042158-Y) and Zhejiang Provincial Top Key Discipline of Biology.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Liu TC, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther 2008; 15:877–84. [DOI] [PubMed] [Google Scholar]
- 2.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525–41. [DOI] [PubMed] [Google Scholar]
- 3.Kwon OJ, Kang E, Choi JW. et al. Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J Control Release 2013; 169:257–65. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Li W, Ma N. et al. Non-viral gene delivery methods. Curr Pharm Biotechnol 2013; 14:46–60. [PubMed] [Google Scholar]
- 5.Jin L, Zeng X, Liu M. et al. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics 2014; 4:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra S. Human gene therapy: a brief overview of the genetic revolution. J Assoc Physicians India 2013; 6:127–33. [PubMed] [Google Scholar]
- 7.Pezzoli D, Candiani G. Non-viral gene delivery strategies for gene therapy: a “ménage à trois” among nucleic acids, materials, and the biological environment. J Nanopart Res 2013; 15:1–27. [Google Scholar]
- 8.Karuppannan AK, Qiang J, Chang CC. et al. A novel baculovirus vector shows efficient gene delivery of modified porcine reproductive and respiratory syndrome virus antigens and elicits specific immune response. Vaccine 2013; 31:5471–8. [DOI] [PubMed] [Google Scholar]
- 9.Huang S, Kamihira M. Development of hybrid viral vectors for gene therapy. Biotechnol Adv 2013; 31:208–23. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Hung M-C. Cancer Gene Therapy by Viral and Non-Viral Vectors. John Wiley & Sons, 2014. [Google Scholar]
- 11.Zhi D, Zhang S, Cui S. et al. The headgroup evolution of cationic lipids for gene delivery. Bioconjug Chem 2013; 24:487–519. [DOI] [PubMed] [Google Scholar]
- 12.Aied A, Greiser U, Pandit A. et al. Polymer gene delivery: overcoming the obstacles. Drug Discov Today 2013; 18:1090–8. [DOI] [PubMed] [Google Scholar]
- 13.Raftery R, O'Brien FJ, Cryan S-A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013; 18:5611–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhakamy NA, Nigatu AS, Berkland CJ. et al. Noncovalently associated cell-penetrating peptides for gene delivery applications. Ther Deliv 2013; 4:741–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. Int J Pharm 2014; 459:70–83. [DOI] [PubMed] [Google Scholar]
- 16.Hartono SB, Phuoc NT, Yu M. et al. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J Mater Chem B 2014; 2:718–26. [DOI] [PubMed] [Google Scholar]
- 17.Hu SH, Hsieh TY, Chiang CS. et al. Surfactant‐free, lipo‐polymersomes stabilized by iron oxide nanoparticles/polymer interlayer for synergistically targeted and magnetically guided gene delivery. Adv Healthc Mater 2014; 3:273–82. [DOI] [PubMed] [Google Scholar]
- 18.Yoo SM, Kang M, Kang T. et al. Electrotriggered, spatioselective, quantitative gene delivery into a single cell nucleus by Au nanowire nanoinjector. Nano Lett 2013; 13:2431–5. [DOI] [PubMed] [Google Scholar]
- 19.Tian H, Chen J, Chen X. Nanoparticles for gene delivery. Small 2013; 9:2034–44. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Wang F, Zhang J. et al. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids. Res Chem Intermed 2013; 39:2407–15. [Google Scholar]
- 21.Zhao D, Wang C-Q, Zhuo R-X. et al. Modification of nanostructured calcium carbonate for efficient gene delivery. Colloids Surf B Biointerfaces 2014; 118:111–6. [DOI] [PubMed] [Google Scholar]
- 22.Dehshahri A, Alhashemi SH, Jamshidzadeh A. et al. Comparison of the effectiveness of polyethylenimine, polyamidoamine and chitosan in transferring plasmid encoding interleukin-12 gene into hepatocytes. Macromol Res 2013; 21:1322–30. [Google Scholar]
- 23.Bandyopadhyay P, Kren BT, Ma X. et al. Enhanced gene transfer into HuH-7 cells and primary rat hepatocytes using targeted liposomes and polyethylenimine. Biotechniques 1998; 25:282–92. [DOI] [PubMed] [Google Scholar]
- 24.Asharani P, Xinyi N, Hande MP. et al. DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine (Lond) 2010; 5:51–64. [DOI] [PubMed] [Google Scholar]
- 25.Auffan M, Rose J, Bottero JY. et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 2009; 4:634–41. [DOI] [PubMed] [Google Scholar]
- 26.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006; 6:662–8. [DOI] [PubMed] [Google Scholar]
- 27.Zheng N, Yin L, Song Z. et al. Maximizing gene delivery efficiencies of cationic helical polypeptides via balanced membrane penetration and cellular targeting. Biomaterials 2014; 35:1302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Zhao D, Li F. et al. Co-delivery of genes and drugs with nanostructured calcium carbonate for cancer therapy. RSC Adv 2012; 2:1820–6. [Google Scholar]
- 29.Kong XD, Xu SJ, Wang XM. et al. Calcium carbonate microparticles used as a gene vector for delivering p53 gene into cancer cells. J Biomed Mater Res A 2012; 100A:2312–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T. Gene delivery for cancer therapy. Curr Drug Deliv 2014; 11:233–42. [DOI] [PubMed] [Google Scholar]
- 31.Peter ME. Programmed cell death: apoptosis meets necrosis. Nature 2011; 471:310–2. [DOI] [PubMed] [Google Scholar]
- 32.Mellert H, Espinosa JM. Tumor suppression by p53: is apoptosis important or not? Cell Rep 2013; 3:1335–6. [DOI] [PubMed] [Google Scholar]