Abstract
Aim
To detect cells expressing the stem cell marker ALDH1 (aldehyde dehydrogenase1) in the pulp of human permanent teeth and to investigate the expression of ALDH1 in isolated dental pulp cells.
Methodology
Pulp tissue was collected and processed for immunohistochemistry to detect ALDH1, STRO-1 and CD90 positive cells. In addition, cells were isolated and analyzed by flow cytometry for ALDH1 activity, and for the cell surface markers CD44, CD73, CD90, STRO-1 and CD45. Cells were also examined for multi-differentiation capacity. Within these cells, an ALDH1+ cell subpopulation was selected and evaluated for multi-differentiation capacity.
Results
The immunohistochemistry analyses showed that ALDH1, CD90 and STRO-1 positive cells were located mainly in the perivascular areas and nerve fibres of dental pulps. Cells on the fifth passage had high expression for CD44, CD73 and CD90, whereas moderate labeling was observed for STRO-1 and ALDH1 in flow cytometry analysis. On the same passages, cells were able to differentiate into osteogenic, adipogenic and chondrogenic lineages. The ALDH1+ cell subpopulation also demonstrated multi-lineage differentiation ability.
Conclusions
Dental pulp stem cells reside in the vicinity of blood vessels and nerve fibres, indicating the possible existence of more than one stem cell niche in dental pulps. Furthermore, ALDH1 was expressed by isolated dental pulp cells, which had mesenchymal stem cell characteristics. Thus, it can be suggested that ALDH1 may be used as a DPSC marker.
Keywords: aldehyde dehydrogenase 1, dental pulp, stem cells, stem cell niche
Introduction
Mesenchymal stem cells (MSCs) can be isolated from almost all tissues in humans, including the dental pulp (Da Silva Meireles et al. 2006). Although MSCs have been obtained from primary (SHEDs) and permanent teeth (DPSCs), there is a lack of information regarding the precise anatomical location of these cells. This is mainly attributed to the rarity of stem cells in the pulp, as well the absence of specific MSC markers that identify different stages of these cells during odontogenesis (Gronthos et al. 2000, 2002, Miura et al. 2003).
Niches are special microenvironments in tissues where stem cells are located. At these sites, complex molecular interactions occur, which maintain the essential properties of stem cells, including their undifferentiated state, self-renewal ability and capacity to give rise to different cell types, according to the organism’s needs (Fuchs et al. 2004, Scadden 2006). Some adult stem cell niches have already been described, but most of them remain unclear, including those that shelter dental pulp stem cells (Gronthos et al. 2002, Doetsch, 2003, Kiel et al. 2005).
MSCs are characterized by expressing a set of cell surface markers and not expressing others, although there is no specific marker to characterize these cells or to isolate a pure MSC population (Da Silva Meirelles et al. 2008). Different markers have been described and tested in order to define this population of cells. MSCs often express STRO-1, CD44, CD73, CD90 and CD105 and are negative for CD31, CD34, CD45, CD80, CD86 and HLA-DR (Musina et al. 2005, Dominici et al. 2006).
Aldehyde dehydrogenase 1 (ALDH1) is an intracellular enzyme that has been used to isolate and purify different populations of stem cells, such as hematopoietic, neural and cancer stem cells (Moreb 2008, Douville et al. 2009, Krishnamurthy et al. 2010). This marker has also been identified in SHEDs and DPSCs, by immunocytochemistry and immunofluorescence (Telles et al. 2008). ALDH1 activity is probably involved in the “stemness” of cells, where stem cell populations with high expression of this enzyme have a more undifferentiated profile (Moreb 2008). Hematopoietic stem cells selected based on the ALDH1 activity demonstrate enriched expression of primitive cell markers, as well as higher clonogenic progenitor function (Hess et al. 2006). The use of general metabolic markers, such as ALDH1 activity, is a promising technique to identify and isolate stem cells from multiple sources (Douville et al. 2009).
Thus, the identification of stem cell-specific markers for their prospective isolation is crucial for future tissue engineering or cell-based therapies to succeed. Likewise, the elucidation of mechanisms involving the regulation of stem cells depends on the development of in vitro models that reproduce as closely as possible the functional niche of these cells in vivo. The aim of this study was to detect cells expressing ALDH1 in the pulp of human permanent teeth and to investigate the expression of this marker in isolated dental pulp cells. Cells expressing the MSC markers CD90 and STRO-1, as well as ALDH1 in the pulp of human permanent molars was detected, in order to find the anatomical location of stem cells in this tissue. Furthermore, dental pulp cells from these teeth were isolated and evaluated for stem cell parameters. Within these cells, an ALDH1+ cell subpopulation was selected and the multi-differentiation capacity of these cells was evaluated.
Materials and Methods
All the patients or their guardians signed a consent form prior to collecting the teeth, which was approved by the ethics committee of the Federal University of Bahia, registered under no 06/11. Samples were collected only after receiving informed consent from the patients.
Tissue Specimens
Nine sound third molars were collected from young adults (18–25 years old). To access the pulp chamber, grooves were prepared with a diamond bur at high speed under constant irrigation, taking care not to reach the pulp. Then, the teeth were cracked open and the pulp was removed.
Immunohistochemistry
ALDH1, CD90 and STRO-1expression was analyzed immunohistochemically on formalin-fixed, paraffin-embedded and 5-μm-thick sections of pulp tissue. Previously, the sections were stained with haematoxylin and eosin (H&E) for histological analysis. Antigen retrieval was performed with a citrate buffer solution, pH 6.0 (Dako, Carpinteria, CA, USA), in water a bath at 95°C for 40 min. Sequentially, sections were incubated with 0.3% hydrogen peroxide (Fischer Scientific, Pittsburgh, PA, USA) for 30 min, followed by a protein blocking agent (Background Sniper, Biocare Medical, Walnut Creek, CA, USA) for 10 min at room temperature. Primary antibodies against ALDH1, dilution 1:50 (Clone 44/ALDH1, BD Biosciences, Mountain View, CA, USA), CD90, dilution 1:50 (Clone EPR3132, Abcam, Cambridge, MA, USA), and STRO-1, dilution 1:100 (Clone STRO-1, Invitrogen, Grand Island, NY, USA) were incubated overnight in a humid chamber at 4 °C. Then, the sections were incubated with the MATCH 3 Mouse or Rabbit detection kit (horseradish-peroxidase-polymer - Biocare Medical). The tissues were revealed with 3′3-diaminobezidine (Dako) and counterstained with Gill’s hematoxylin. For negative controls, non-immune antibodies were used. Cells stained brown under an optical microscope were regarded as positive.
Isolation and culture of dental pulp cells
The dental pulp tissue was cut into pieces with a volume of 1 mm3, transferred to a 6-well plate containing Dulbecco’s modified Eagle medium - DMEM (Gibco, Invitrogen, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, 100 μg/mL of streptomycin and 10 μg/mL of amphotericin B (Gibco), and stored at 37° C and 5% CO2 for cell proliferation via the explant method and plastic adherence. The culture medium was changed every 3 or 4 days. When the culture reached approximately 90% confluence, cells were collected using 0.25% trypsin-EDTA (Gibco) and transferred to culture flasks until a new passage was necessary.
Flow cytometry analysis/FACS of ALDH1+ cells
A total of 5 × 105 cells per tube were incubated for 15 min at 4 °C with 5 μL of CD44, CD45, CD73 or CD90 (BD Biosciences, Mountain View, CA, USA), STRO-1 (Invitrogen) or IgG (BD Biosciences), conjugated with the fluorochromes FITC or APC (BD Biosciences). For detection of ALDH1 activity, the Aldefluor kit (Stem Cell Technologies, Durham, NC, USA) was employed according to the manufacture’s protocol. Briefly, a total of 1 × 106 cells were suspended with activated Aldefluor substrate (BODIPY-amino acetaldehyde - BAAA) or the negative control (diethylaminobenzaldehyde - DEAB) for 45 min at 37 °C. Data acquisition was performed on cells in the fifth passage, using the BD FACSAria II flow cytometer. The Aldefluor kit was also used to select the population with high ALDH1 enzymatic activity.
Cell differentiation in vitro
To evaluate the ability of isolated pulp cells to differentiate into osteogenic, adipogenic and chondrogenic lineages, cells of the fifth passage were seeded in the wells of a 12-well plate at a density of 5 × 103 cells/cm2. When cultures reached 60% confluence, the growth medium was replaced by a specific induction medium (STEMPRO™ Osteogenesis, Adipogenesis or Chondrogenesis Differentiation Kit, Invitrogen), which was replaced every 3 or 4 days. The cells were maintained in each of these three differentiation media for 2 to 4 weeks. For each experiment, a negative control consisting of the same cells maintained on conventional culture medium was used.
After the differentiation period, cultures were washed with deionized water and fixed in 4% paraformaldehyde for 1 h. The cells that underwent osteogenic differentiation were stained with Alizarin red (Sigma-Aldrich, St Louis, MO, USA), adipogenic differentiation with Oil Red (Sigma-Aldrich), and chondrogenic differentiation with Alcian blue (Sigma Sigma-Aldrich). The same protocol was employed to evaluate the ability of ALDH1+ cells to differentiate into the three referred cell lineages.
Results
Histological aspects and immunohistochemistry
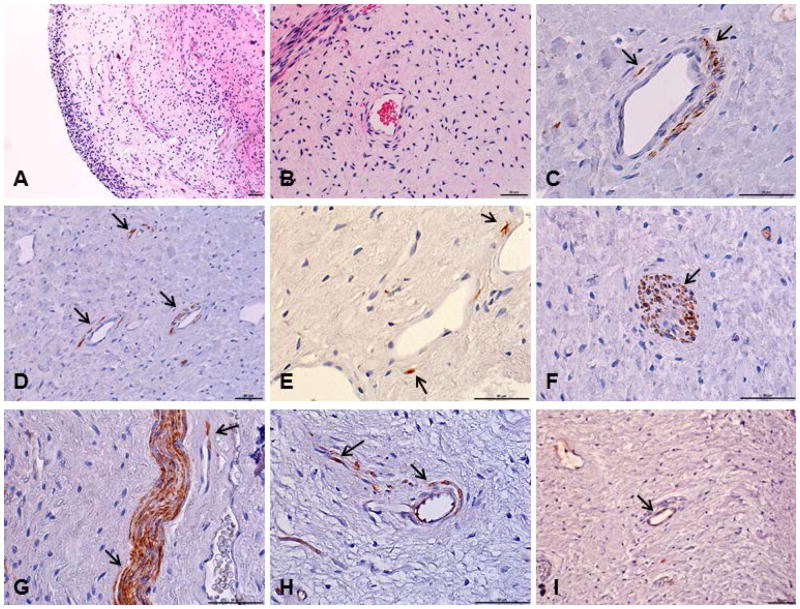
The histological analysis of the pulp revealed tissue with characteristics of vitality, with no evidence of inflammatory reaction, presence of odontoblastic layer, nerve fibres, blood vessels and high cellularity, within in a matrix of loose connective tissue (Figs. 1a, 1b). The immunohistochemistry was performed with ALDH1, CD90 and STRO-1 antibodies, for the localization of these proteins in the pulp, since these markers are often used to isolate and characterize different stem cell populations. Immunoreactivity to anti-ALDH1, anti-CD90 and anti-STRO-1 was detected mainly around blood vessels, but was also present in the nerve structures of the tissue. The ALDH1 and CD90 markers had a similar pattern of expression (Figs. 1c–1h), whereas less pronounced staining was observed for STRO-1 (Fig. 1i).
Figure 1.
Histological sections from dental pulp tissue stained with hematoxylin and eosin or submitted to immunohistochemical reaction. (a,b) Pulp tissue with no evidence of inflammatory reaction. (c,d,e,f) ALDH1 positive cells in human dental pulp. (g,h) CD90 positive cells. (i) STRO-1 positive cells. Stained cells were located mainly around the blood vessels and on the nerve structures of this tissue. Arrows indicate the immunoreactive cells. Scale bars: 50 μm.
Establishment of cell cultures
The isolation of dental pulp cells via the explant method was considered successful, since after 48 h of tissue culture, the cells started to migrate and proliferate on the plate (Fig. 2a). About two weeks after the beginning of tissue culture, the cells reached approximately 90% confluence on the plate and had a fibroblastoid form (Fig. 2b).
Figure 2.
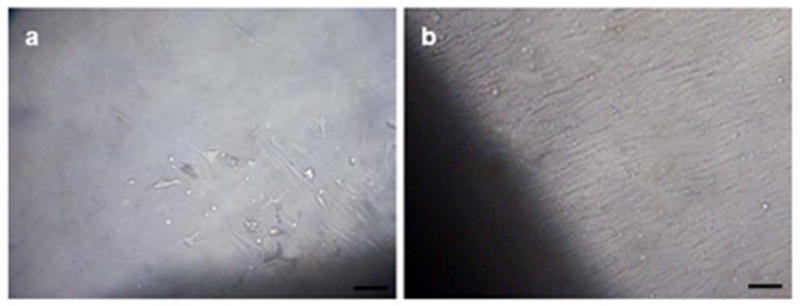
Behavior of the cell culture after proliferation from dental pulp tissue (explants). Images (a) after 48 hours and (b) two weeks of tissue culture. Scale bars: 50 μm.
Immunophenotyping/FACS of ALDH1+ cells
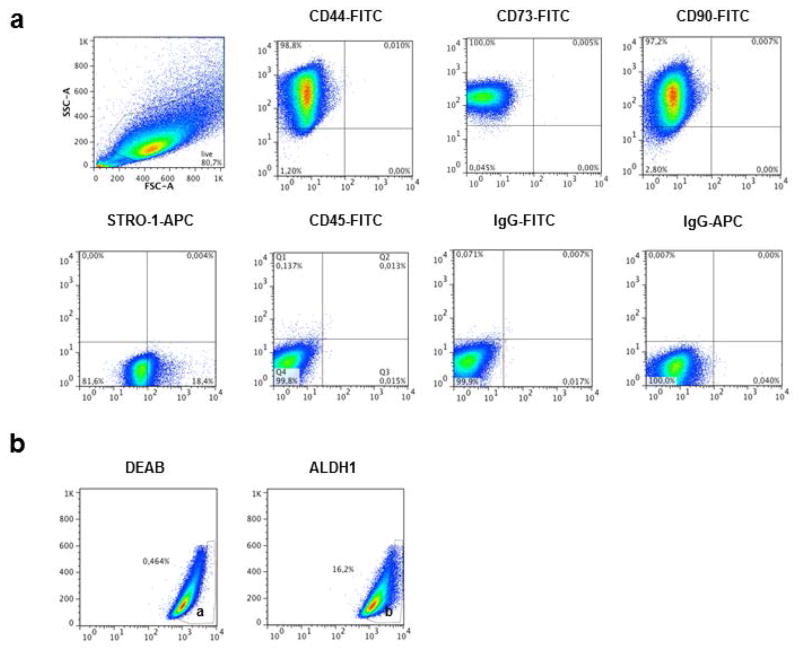
The results from flow cytometry analysis confirmed the characteristics of MSCs of the isolated pulp cells (Fig. 3a). More than 90% of the cells in culture were positive for CD44, CD73 and CD90, whereas moderate labeling was observed for STRO-1 (18.4%) and ALDH1 (16.2%). The cells had negative expression for CD45 and for the controls. Isolated pulp cells were gated for positivity to ALDH1 using DEAB (ALDH1 inhibitor) as reference (Fig. 3b).
Figure 3.
(a) Immunophenotypic profile of cells isolated from the pulp of human permanent teeth. Flow cytometry graphics show the expression of chosen markers (CD44, CD73, CD90, STRO-1, CD45) compared with controls (IgG-FITC, IgG-APC). (b) Representative flow cytometric analysis of ALDH1 activity on dental pulp cells. (a) Cells incubated with Aldefluor substrate and the specific inhibitor of ALDH, DEAB, are used to stablish the baseline fluorescence of these cells and to define the ALDHBr region. (b) Incubation of these cellls with Aldefluor substrate in the absence of the inhibitor induces a shift in fluorescence defining the ALDHBr population. This gate was used to select the ALDH1+ cell population.
Multi-differentiation capacity
The multi-lineage differentiation potential of the isolated cells was confirmed using standard immunocytochemical staining (Fig. 4a). After 4 weeks of induction, Oil Red O staining demonstrated the presence of lipid droplets in the cultures submitted to the adipogenic differentiation. The osteogenic differentiation was observed by the calcium deposits stained with Alizarin red, and the chondrogenic differentiation could be seen by the Alcian blue staining of the glycosaminoglycans. Both osteogenic and chondrogenic differentiated cells were visualized after 2 weeks of induction. The ALDH1+ cells were also able to differentiate into the three cell lineages tested (Fig. 4b).
Figure 4.
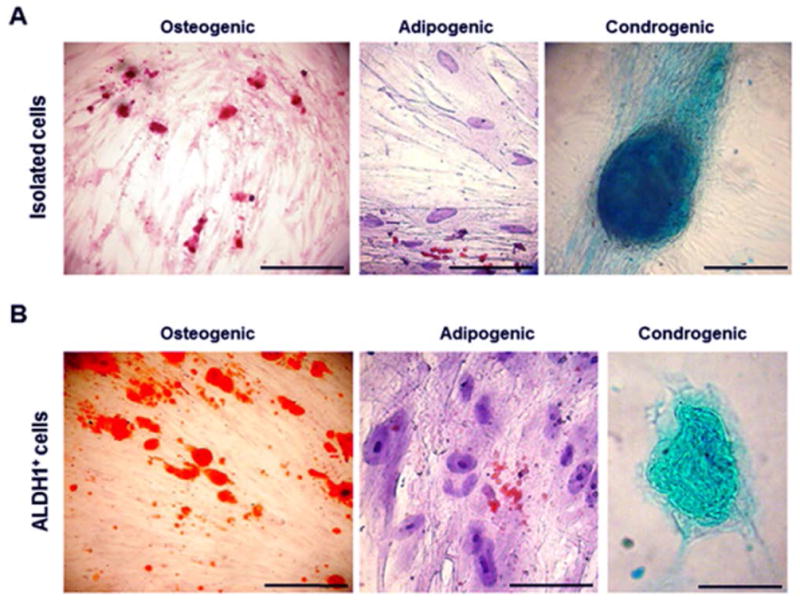
(a) Multiple lineage differentiation properties of the isolated dental pulp cells, (b) as well as of the ALDH1+ cell population derived from these cells. Osteogenic differentiation visualized by calcium deposits stained with Alizarin Red. Adipogenic differentiation visualized by Oil Red staining showing lipid vacuoles. Chondrogenic differentiation visualized by Alcian Blue staing of glycosaminoglycans. Scale bars: 50 μm.
Discussion
Cells with positive expression for ALDH1, CD90 and STRO-1 were detected in the vicinity of blood vessels, as well as along the nerve structures of this tissue. These results support previous reports, where positive cells for the markers STRO-1, the best known MSC marker, CD146, a vascular smooth muscle marker, and the pericyte-associated antigen 3G5 were located in the blood vessel walls of human dental pulp tissue, and also in the perineurium of nerve bundles (Shi & Gronthos 2003). Cells expressing another group of markers (STRO-1, CD90, CD105 and CD146) were also located together with the vascular and nerve fibres of this tissue (Alongi et al. 2010).
Location of MSCs at perivascular sites throughout the body would provide these cells with easy access to all tissues in the organism. In case of an injury, MSCs are released by the rupture of blood vessels, migrate to the affected site and differentiate into the required cell type, promoting tissue repair (Kolf et al. 2007). In the affected area, MSCs are capable of secreting immunomodulatory molecules, minimizing the extent of tissue damage and decreasing the inflammatory response, allowing tissue regeneration (Aggarwal & Pittenger 2005, Da Silva Meirelles et al. 2008). Similarly, the secretion of trophic factors by MSCs in the damaged area can inhibit apoptosis, stimulate angiogenesis and stimulate the mitosis of tissue-intrinsic progenitors (Caplan 2008).
Both endothelial cells and pericytes such as the smooth muscle cells surrounding blood vessels can constitute the MSC niche, and contribute to a perivascular location of these cells (Scadden 2006). Recent studies that have associated the location of MSCs with blood vessels have suggested a strong correlation between MSCs and pericytes (Caplan 2008). Typical pericyte markers, such as CD146, have also been expressed in MSCs from several tissues, including dental pulp, indicating a possible association between the two cell populations (Shi & Gronthos 2003, Caplan 2008, Crisan et al. 2008). However, the ability of pericytes to act as MSCs remains controversial. In an ultrastructural analysis of human dental pulp microvasculature, pericytes were thought to originate from endothelium and migrate away from vessel walls to undergo transition to a fibroblastic phenotype, during normal tissue turnover (Carlile et al. 2000). In an in vivo study, two days after intentional damage to the dental pulp of mice, only 15% of the newly differentiated odontoblasts were derived from pericytes, suggesting that MSCs of the same tissue may have a distinct origin (Feng et al. 2011).
Regarding the immunostaining of the nerve structures in the dental pulp, earlier studies have shown that the expression of STRO-1 was restricted to the perineurium surrounding the nerve bundles (Shi & Gronthos 2003, Yoshida et al. 2012). Here, ALDH1, CD90 and STRO-1 positive cells were found along the nerve bundles. The reactivity of these markers commonly used to identify MSCs in the nerve structures of the pulp can be explained by the embryological origin of these cells. Although MSCs are considered to have mesoderm origin, one study demonstrated that embryonic MSCs might originate from the neuroepithelium as well as from the neural crest (Takashima et al. 2007). On the other hand, the presence of capillaries in the endoneurum could explain the reactivity of the nerve structures to these markers, since clear and evident immunostaining using these markers was seen around the blood vessels.
It can be speculated that DPSCs may reside in more than one anatomical site in the same tissue, since positive cells for the stem cell markers CD90, STRO-1 and ALDH1 were identified around blood vessels and also in the nerve fibres of the pulp. The presence of more than one stem cell niche in one tissue could be due to the existence of a hierarchy among the stem/progenitor cell populations (Sloan & Waddington 2009). In fact, it has been previously established that other stem cell populations, such as hematopoietic stem cells (HCSs), reside in distinct anatomical sites within bone marrow (Kiel et al. 2005, Moore 2006).
The identification of a definitive marker allowing the prospective isolation of MSCs from fresh tissue would be of the utmost importance (Da Silva Meirelles et al. 2008). This study used a combination of immunohistochemistry, flow cytometry, FACS sorting and multilineage differentiation induction as a strategy to assess where stem cells are located in the pulp of permanent teeth, as well as to evaluate ALDH1 as a potential marker to identify and select dental pulp stem cells.
The cytosolic enzyme ALDH1 is responsible for oxidizing a variety of intracellular aldehydes to carboxylic acids, that is considered a detoxifying enzyme (Douville et al. 2009). Assays based on the ALDH1 activity have been described to isolate both murine and human stem cells from various tissues and organs (Krishnamurthy et al. 2010, Roehrich et al. 2013, Zheng et al. 2014). Functional properties of tissue-resident stem cells for their prospective isolation, regardless of cell-surface marker expression, have been intensely tested, since the cell surface epitopes can vary with the metabolic state of the cell, the experimental conditions used, and the successive passages of the in vitro cultured cells (Roehrich et al. 2013).
In this study, cells were isolated from the dental pulp of permanent teeth via the explant method. The isolated cells demonstrated parameters usually observed for MSCs, such as positive expression for certain markers (CD44, CD73, CD90 and STRO-1), and absence of others (CD45), which is in accordance with previous studies where this isolation method was employed for harvesting dental pulp stem cells (Huang et al. 2006, Martens et al. 2012, Hilkens et al. 2013). The isolated cells were able to differentiate into the osteogenic, adipogenic and chondrogenic cell lineages, confirming the multi-differentiation potential of these cells, which is one of the minimal criteria for defining MSCs (Dominici et al. 2006). In this study, the expression of ALDH1 activity in the isolated pulp cells was considered moderate (16.2%), even though there are no references in the literature regarding expression of the activity of this enzyme in DPSCs. Lower levels of the ALDH1 activity were found in previous studies where stem cells from different origins were evaluated (Ma et al. 2008, Huang et al. 2009, Jiang et al. 2009). Since it is speculated that ALDH1 is involved in the stemness of stem cells, and that the population of MSCs accounts for less than 1% of the total cells of a given tissue or organ, the results from flow cytometry analysis along with those found through the immunohistochemistry assay are coherent with the existing data in the literature (Sloan & Waddington 2009, Li et al. 2014).
In order to infer that the ALDH1+ cells observed in the pulp through the immunohistochemistry assay were stem cells, a population of cells based on the ALDH1 activity were sorted by FACS and tested for multi-differentiation potential. Clear and evident differentiation of the cells into the osteogenic, adipogenic and chondrogenic cell lineages were visualized through a standard immunocytochemical staining technique. Furthermore, in this study surface markers commonly employed to characterize DPSCs, such as STRO-1 and CD90, also showed positive expression in the pulp of human permanent teeth. The immunostaining with these markers had the same pattern as that observed for ALDH1, i.e. in the perivascular areas and in the nerve fibres of the dental pulp.
Since there is no specific marker to characterize MSCs, but rather a group of markers used most often, it seems reasonable that certain metabolic parameters could be similar between stem cells from different origins. It is possible that cells from distinct tissues and organs use similar strategies to maintain their stem cell state and to regulate proliferation and differentiation. In this context, there are several indications that the activity of ALDH1 is a common feature among stem cells (Cai et al. 2004). This is the first study that has evaluated the expression of ALDH1 in the pulp tissue.
Conclusion
The results support the hypothesis that DPSCs originate from cells present within the blood vessel walls and suggest the possible existence of more than one stem cell niche in the dental pulp. Moreover, ALDH1 was expressed by isolated dental pulp cells, which have mesenchymal stem cell characteristics. Thus, it can be suggested that ALDH1 can be used as a DPSC marker. Further studies should be performed to better understand the biological role and influence of ALDH1 positive cells on dental pulp.
Acknowledgments
This work was funded by grant BOL 0541/2010 from FAPESB. Funded in part by grant R01-DE21410 from the NIH/NIDCR. The authors thank the Flow Cytometry Platform from Gonçalo Moniz Research Center, FIOCRUZ-BA, and the Program for Technological Development in Tools for Health - PDTIS for use of its facilities.
Footnotes
The authors deny any conflicts of interest related to this study.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Alongi DJ, Takayoshi Y, Song Y, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regenerative Medicine. 2010;5:617–31. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Weiss ML, Rao MS. In search of “stemness”. Experimental Hematology. 2004;32:585–98. doi: 10.1016/j.exphem.2004.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–30. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Carlile MJ, Sturrock MG, Chisholm DM, et al. The presence of pericytes and transitional cells in the vasculature of the human dental pulp: an ultrastructural study. Histochemical Journal. 2000;32:239–45. doi: 10.1023/a:1004055118334. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;11:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Da Silva Meirelles L, Caplan AI, Nardi NB. In search of in vivo identity of mesenchymal stem cells. Stem cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Current Opinion in Genetics & Development. 2003;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Douville J, Beaulieu R, Baliki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells and Development. 2009;83:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proceedings of the National Academy of Sciences U S A. 2011;108:6503–8. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Gehron Robey P, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. Journal of Dental Research. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–9. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens P, Gervois P, Fanton Y, et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell and Tissue Research. 2013;353:65–78. doi: 10.1007/s00441-013-1630-x. [DOI] [PubMed] [Google Scholar]
- Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Research. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. Journal of Endodontics. 2006;32:1066–73. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Molecular Cancer Research. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish haematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Research & Therapy. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Research. 2010;70:9969–78. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xiang Y, Xiang L, et al. ALDH maintains the stemness of lung adenoma stem cells by suppressing the Notch/CDK2/CCNE pathway. PLOS ONE. 2014;9:e92669. doi: 10.1371/journal.pone.0092669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan KW, Lee TK, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Molecular Cancer Research. 2008;6:1146–53. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- Martens W, Wolfs E, Struys T, et al. Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs. 2012;196:490–500. doi: 10.1159/000338654. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Current Stem Cell Research & Therapy. 2008;3:237–46. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bulletin of Experimental Biology and Medicine. 2005;139:504–9. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- Roehrich ME, Spicher A, Milano G, et al. Characterization of cardiac-resident progenitor cells expressing high aldehyde dehydrogenase activity. Biomed Research International. 2013;2013:503047. doi: 10.1155/2013/503047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of Bone and Mineral Research. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Waddington RJ. Dental pulp stem cells: what, where, how? International Journal of Paediatric Dentistry. 2009;19:61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–88. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Telles PDS, Tarquinio S, Botero T, et al. ALDH and CD44 expression in dental pulp stem cells. In: 86th General Session & Exibition of the IADR, Toronto, ON, CA. Journal of Dental Research. 2008;87 (Spec Iss B):0327. [Google Scholar]
- Yoshiba N, Yoshiba K, Ohkura N, et al. Immunohistochemical analysis of two stem cell markers of α-smooth muscle actin and STRO-1 during wound healing of human dental pulp. Histochemistry and Cell Biology. 2012;138:583–92. doi: 10.1007/s00418-012-0978-4. [DOI] [PubMed] [Google Scholar]
- Zheng R, Wang J, Wu Q, et al. Expression of ALDH1 and TGFβ2 in benign and malignant breast tumors and their prognostic implications. International Journal of Clinical and Experimental Pathology. 2014;7:4173–83. [PMC free article] [PubMed] [Google Scholar]