Abstract
Background and Objective
Although surveys in US showed that males are more prone to develop periodontitis, sex as a risk factor in periodontitis and its mechanism remain controversial. Animal models are ideal for investigating immunological mechanisms of sex dimorphism in periodontitis. This is because of the possibility to exclude the interference of gender-related risk factors, such as smoking and oral hygiene habits. Based on the human survey and reports on sex dimorphism in other diseases, our hypothesis is that sex is a risk factor in periodontitis.
Material and Methods
Different murine models (oral gavage model and ligature model) for periodontitis have been utilized to determine susceptibility to periodontitis in females and males. Periodontal bone levels were measured as the distance from the cementoenamel junction to the alveolar bone crest in young female or male mice (8–10 weeks of age). Differential expression of inflammatory mediators in the gingivae of female and male mice was determined by quantitative real-time PCR.
Results
In comparison with male mice, female mice displayed significantly (p < 0.05) increased periodontal bone loss, accompanied by elevated expression of pro-inflammatory cytokines (interleukin-1β, interleukin-6 and interleukin-17A) and higher number of oral bacteria.
Conclusion
Contrary to prevailing human results where periodontitis susceptibility is also influenced by confounding gender-related behaviors, in murine oral gavage model and ligature model, female mice appear to be more susceptible to periodontal bone loss than male mice. In ligature model, we observed significantly (p<0.05) higher CEJ-ABC, gingival pro-inflammatory cytokine production, and higher oral bacteria in female mice. Furthermore, our results imply that females develop periodontitis with a higher progression rate. Our study has therefore established that the models can be used to dissect the mechanisms underlying genuine gender-based differences in periodontal disease susceptibility and/or progression.
Introduction
Periodontitis is a chronic inflammatory disease which is driven by a dysbiotic microbial community and leads to loss of bone support of the dentition (1). This disease is the leading cause of tooth loss among adults (2), and estimated to afflict up to 90% of the worldwide population in its various forms (3). Periodontitis is also associated with systemic diseases, including heart disease, diabetes, lung disease, and pregnancy complications (3–5). National poll showed that periodontitis occurs more often in males in USA (6, 7). Whereas in some countries the survey produced similar results, i.e., that males are more prone to periodontitis than females (8), there are also reports from other areas showing that women develop more severe periodontitis than men (9, 10). The discrepancy might be due to the fact that the disease is influenced also by numerous environmental factors, including stress, smoking, alcohol consumption, personal dental hygiene, etc (11), some of which are closely associated with gender-related behaviors.
Animal models can therefore serve as useful tools for periodontal disease research by eliminating confounding variables present in humans. Among animal models, murine models are widely used due to the availability of high-quality immunological reagents and a large number of transgenic mice and mice with genetic depletions (12). An oral gavage model has widely been used as an experimental periodontitis model, in which periodontitis can been induced by oral inoculation with periodontitis-associated bacteria such as Porphyromonas gingivalis (P. gingivalis) (13). Another animal model to investigate periodontal disease is the ligature-induced periodontitis model, where a silk ligature is placed around molar teeth resulting in massive local accumulation of indigenous bacteria and induction of bone loss within a few days (14–16). In the current manuscript, we compared male and female mice for their susceptibility to periodontitis using both the oral gavage and the ligature-induced periodontitis models. Our hypothesis is that females may develop more severe periodontitis in animal models, while gender-related behaviors greatly affect the periodontitis progression and lead to inconsistent human survey reports (6, 7, 9, 10).
Material and Methods
Mice
C57/BL6 mice (8–10 weeks of age) were purchased from Jackson Lab. Animals were kept in animal facilities at University of Louisville. All handling and processing were in appliance with the established Federal and State policies and approved by Institutional Animal Care and Use Committee.
P. gingivalis-induced periodontitis model
Gingival inflammation and periodontal bone loss were induced in specific pathogen-free mice by oral inoculation with P. gingivalis, as described by Baker and our previous reports (13, 17, 18). Briefly, mice were orally inoculated five times at 2-day intervals with 109 CFU P. gingivalis (ATCC 33277) suspended in the vehicle of 2% carboxymethylcellulose in PBS. Sham-inoculated controls received the vehicle alone. The mice were euthanized 6 weeks after the first oral inoculation. At the termination of the experiments, alveolar bone loss and gingival inflammation were determined. Alveolar bone loss was determined under a dissecting microscope (×40 magnification) fitted with a video image marker measurement system (VIA-170K; Nikon Instruments, Melville, NY, USA) by measuring the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC). Fourteen predetermined points on the buccal surfaces of the maxillary molars were measured. To calculate bone loss, the mean CEJ-ABC distance of sham-infected mice was subtracted from the 14-site total CEJ-ABC distance for each mouse.
Ligature-induced periodontitis model
Age matched female and male mice had been randomly selected. Periodontal inflammation and bone loss was induced by tying a 5-0 non-absorbent silk ligature (Roboz Surgical Instrument Co., MD, USA) around the maxillary second molar, with the ligature placed in the gingival sulcus. The ligature had remained in the gingival sulcus through the experiments to facilitate the colonization of oral pathogens and induce inflammation. Age and gender matched mice were sham-ligated and served as controls. Alveolar bone loss was examined 7 days later. Six points were measured on the ligated second molar (three sites corresponding to mesial cusp, palatal groove, and distal cusp) and the affected adjacent regions (sites corresponding to distal cusp and distal groove of the first molar, and palatal cusp of the third molar).
Gingival inflammation
Gingival tissue was excised from around the maxillary molars for mRNA harvest. The expression of interested cytokines was determined by quantitative real-time PCR. Briefly, mRNA was extracted from gingival tissue, using the PerfectPure RNA kit (5 Prime; Fisher, Waltham, MA, USA). The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA) and quantitative real-time PCR was performed using the ABI 7500 System, according to the manufacturer’s protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for genes of interest or a housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) were purchased from Applied Biosystems. The following inventoried gene expression primers (Applied Biosystems) were used: IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), TNF (Mm00443258_m1), IL-17 (Mm00439618_m1), IL-10 (Mm00439614_m1), TGFβ (Mm01178820_m1). Gene expression has been normalized to that of GAPDH.
Histochemistry
Upper jaws (maxillae) with intact surrounding tissue were fixed in 4% paraformaldehyde, decalcified in Immunocal Solution (Decal Chemical Corp.) for 15 days, and embedded in OCT compound. Serial sections (7–8 µm thick) were cut and stained with primary FITC conjugated antibody to mouse IL-17A (LifeSpan BioSciences), or primary rabbit anti-mouse IL-1β or IL-6 (Abcam) and secondary Alexa647 or Alexa594 conjugated anti-rabbit IgG (Cell signaling). The specificity of staining was confirmed by using appropriate fluorescence-conjugated isotype controls. Images were captured using a fluorescence microscope (Nikon Elipse E800) and processed by Neurolucida.
Oral bacterial sampling
Oral cavity bacteria were sampled by holding sterile fine tip cotton swabs against the gum lines for 30 seconds. The swab extracts were then serially diluted and plated onto blood agar plates for growth and CFU determination.
Statistical Analysis
Data were evaluated by two-way ANOVA, and two-tailed t tests (InStat v3.06 program, GraphPad) when there are only two groups involved. Differences were considered significant at the p < 0.05 level.
Results and Discussion
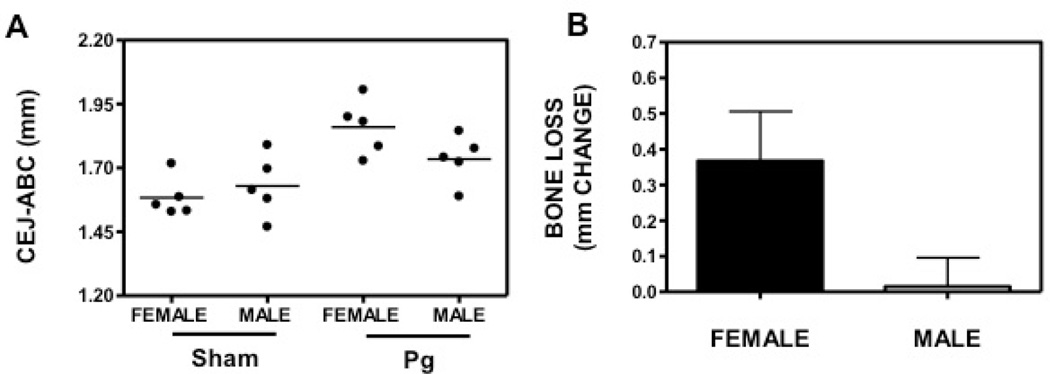
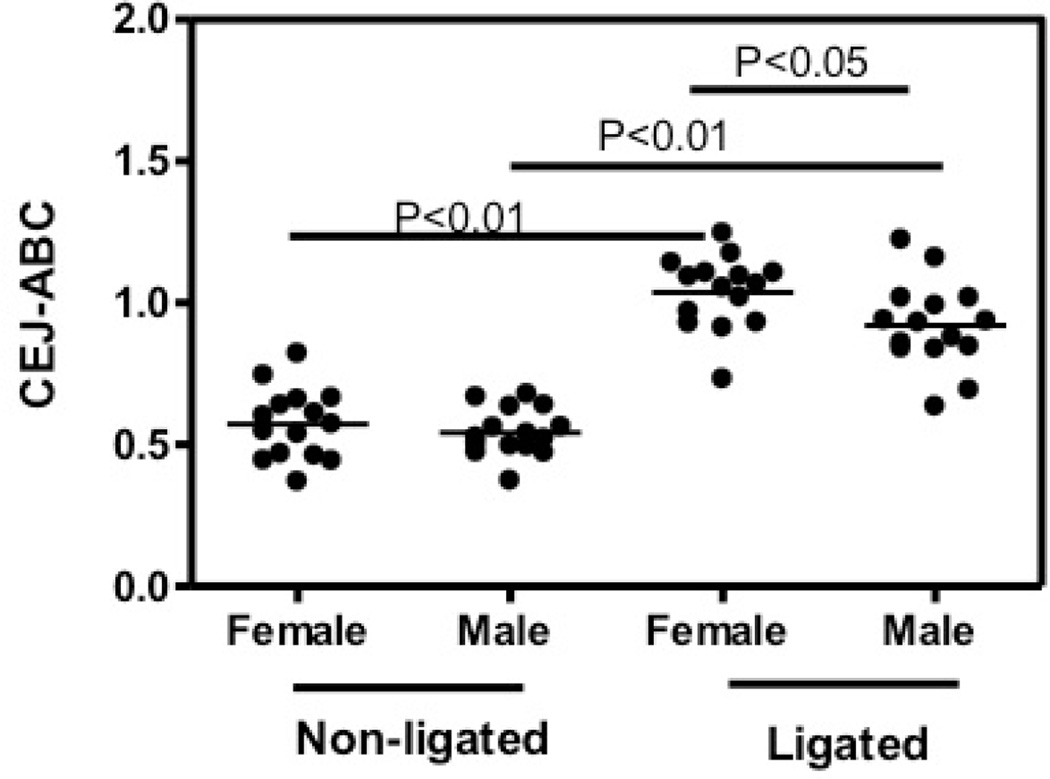
To identify the role of gender difference in periodontitis with exclusion of other behavior-related risk factors, we investigated the periodontal phenotype of female and male mice in a widely accepted oral gavage model to test bone loss in female and male mice (12, 13). Female or male mice were orally infected with 109 P. gingivalis for 5 times as described in Materials and Methods. Sham-infected mice were used as controls. At the termination of the experiment, we measured the CEJ-ABC distances of the female and male mice (Fig 1A). To calculate bone loss, CEJ-ABC readings of the controls were subtracted from those of experimental mice (Fig 1B). The results showed a trend that females developed more bone loss than male mice in the oral gavage model, although not significant. In addition to the alveolar bone loss, gingival inflammation was also determined since the inflammatory host response is responsible for bone loss. We tested the gingival expression of IL-6, which is elevated in periodontitis patients and was shown to drive periodontal disease and bone loss in animal models (19, 20). The result showed that although both female and male mice expressed higher level of IL-6 after P. gingivalis infection, indicating gingival inflammation induction, there was no difference in inflammation between female and male mice (Fig. 2). IL-17 is an important pro-inflammatory and bone-resorptive cytokine expressed by both innate and adaptive immune cells, such as CD4+ Th17 cells (21, 22). Our recent publication showed that IL-17 is driving bone loss in experimental murine periodontitis (14). On the other hand, anti-inflammatory cytokines, such as IL-10, have been shown to prevent or mitigate bone loss (23, 24). Similarly, there was no difference in IL-17 and IL-10 expression in female and male mice (Fig. 2). Lack of difference in gingival cytokine expression in oral gavage model might be because that the model is relatively mild. Oral pathogen infection causes inflammation at low level, failing to induce significant difference in gingival inflammation between females and males (Fig. 2). It is possible that while the difference in the gingival inflammation is not detectable, this consistent tiny difference caused a noticeable trend in the irreversible bone loss (Fig. 1). Therefore, we utilized an intense ligature-induced periodontitis model to investigate the periodontal phenotype of female and male mice. Female or male C57BL/ 6 mice were ligated around 2nd molars. The controls are age and gender matched sham-ligated mice (non-ligated). We found that both female and male ligated mice showed significantly greater CEJ-ABC readings than the non-ligated controls (p<0.01), while female ligated mice showed even higher levels of CEJ-ABC reading in comparison to the male ligated group (p<0.05; Fig. 3). Both the female and male non-ligated control groups displayed similar bone heights. Our results indicated that female mice are more susceptible to bone loss in the ligature model.
Fig. 1.
Periodontal bone loss in P. gingivalis -infected female and male mice. The mm distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at 14 predetermined maxillary buccal sites and the readings were totaled for each mouse. The data are means ± SD (n = 5 mice). (A) CEJ-ABC reading of each mouse, represented by each dot. (B) Bone loss was calculated as total CEJ-ABC distance for each mouse subtracted with the mean CEJ-ABC distance of female and male sham-infected mice, respectively.
Fig. 2.
Relative expression of interested cytokines in the gingivae of P. gingivalis -infected female and male mice. Quantitative real-time PCR (qPCR) was used to determine gingival mRNA expression levels for the indicated molecules (normalized against GAPDH mRNA levels). The gingival tissues used were excised from female and male C57/BL6 mice. Results are shown as fold change relative to female and male sham-infected mice, respectively. Each data point represents the mean ± SD of 5 separate expression values, corresponding to qPCR analysis of individual mice.
Fig. 3.
Periodontal bone loss in ligated female and male mice. The mm distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at 6 most affected maxillary buccal sites and the readings were totaled for each mouse. The data are means ± SD (n = 5 mice). The CEJ-ABC reading of each mouse was represented by each dot. P-values between the groups were labeled.
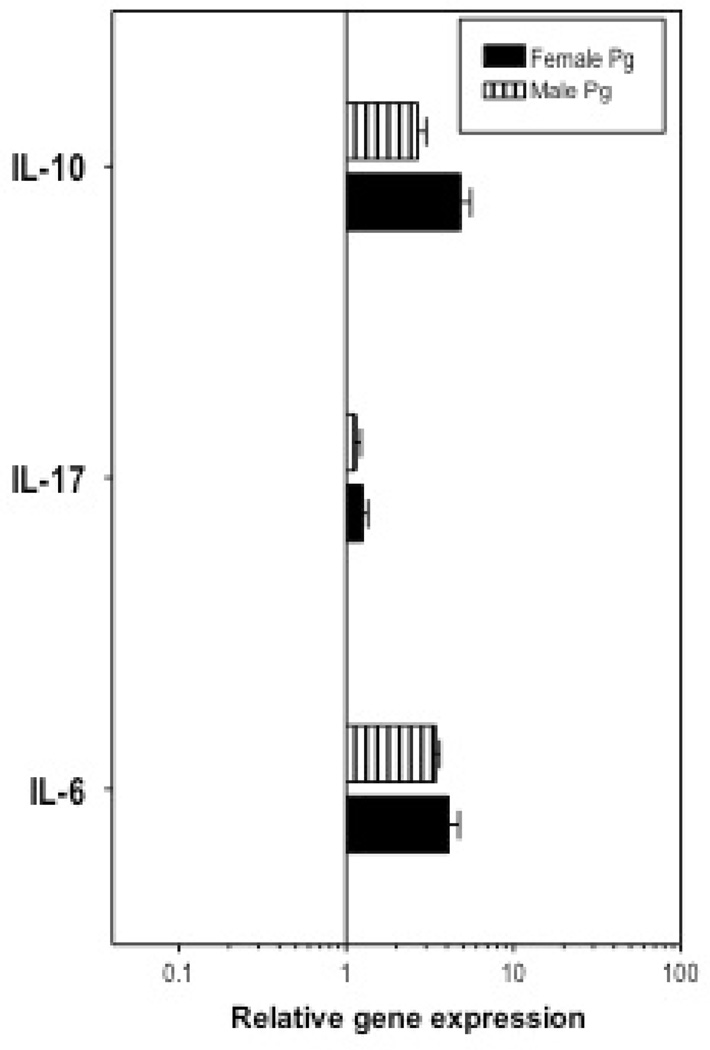
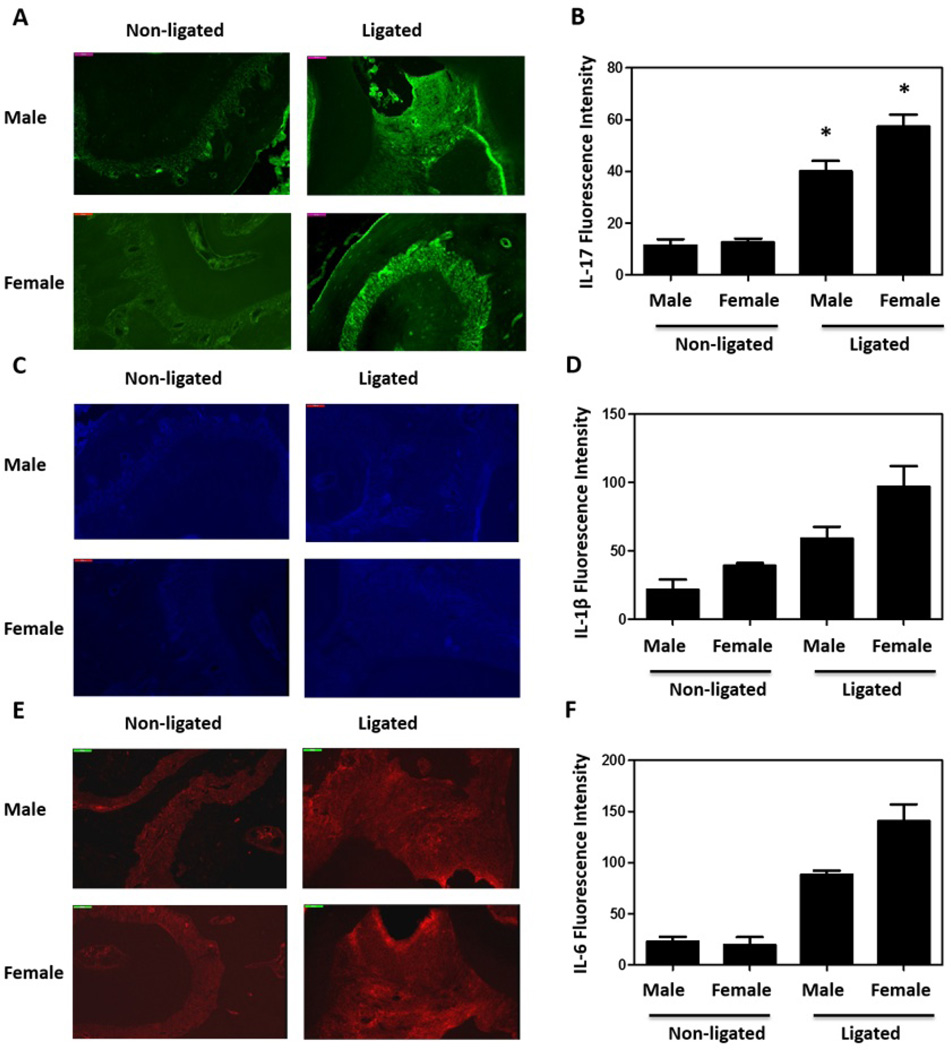
Analysis of the periodontal inflammatory response by real-time quantitative PCR showed significant differences in certain periodontitis-related pro-inflammatory cytokines between female and male mice. Besides IL-6 and IL-17, IL-1β and TNFα are also expressed in periodontitis patients at high level and effect on periodontal disease progression (2, 19, 20, 25, 26). Consistent with our bone loss observation, we detected significantly higher upregulation in the expression of IL-1β, IL-6, and IL-17 in females (p<0.05; Fig. 4). Although not significant, TNFα expression was also upregulated in female in higher level. On the other hand, the expression of anti-inflammatory cytokines IL-10 and TGFβ did not show difference in female and male gingival tissues (Fig. 4), implying that more gingival inflammation in females caused more severe periodontitis. Furthermore, histochemistry experiments were performed to confirm the higher expression of pro-inflammatory cytokines at protein level. Because IL-17 expression exhibits greater change than other cytokines (Fig. 4), we stained IL-17 in gingival tissue (Fig. 5A) and found that the IL-17 expression is indeed higher in female mice after ligation (Fig. 5B), confirming our real-time PCR results. We also stained the gingival tissue samples with IL-1β (Fig. 5C) and IL-6 (Fig. 5E). After ligation, there are more IL-1β and IL-6 in gingival tissues, while we did not detect significantly higher IL-1β (Fig. 5D) and IL-6 (Fig. 5F) in female mice than male ones. It is possibly due to the limited sample size in the histochemistry experiments (3 mice each group), and less difference in IL-1β and IL-6 between females and males than IL-17, implied by our real-time PCR results (Fig. 4).
Fig. 4.
Relative expression of interested cytokines in the gingivae of ligated female and male mice. Quantitative real-time PCR (qPCR) was used to determine gingival mRNA expression levels for the indicated molecules (normalized against GAPDH mRNA levels). The gingivae used were excised from female and male C57/BL6 mice. Results are shown as fold change relative to female and male sham-ligated mice, respectively. Each data point represents the mean ± SD of 5 separate expression values, corresponding to qPCR analysis of individual mice. Asterisks indicate statistically significant (p < 0.05) differences between female and male mice.
Fig. 5.
Expression of interested cytokines in the gingivae of ligated female and male mice. Histochemistry was used to confirm gingival IL-17 expression (A,B). (A) Representative fluorescent confocal images of IL-17 were shown (Green); and (B) the fluorescence intensities of these and additional representative images from independent mice (3 per group) were quantified using Neurolucida. Asterisks indicate statistically significant (p < 0.05) differences between ligated female and male mice. Representative fluorescent confocal images of IL-1β (C) (Blue) and IL-6 (E) (Red) were also shown; and the fluorescence intensities of IL-1β (D) and IL-6 (F) from independent mice (3 per group) were quantified.
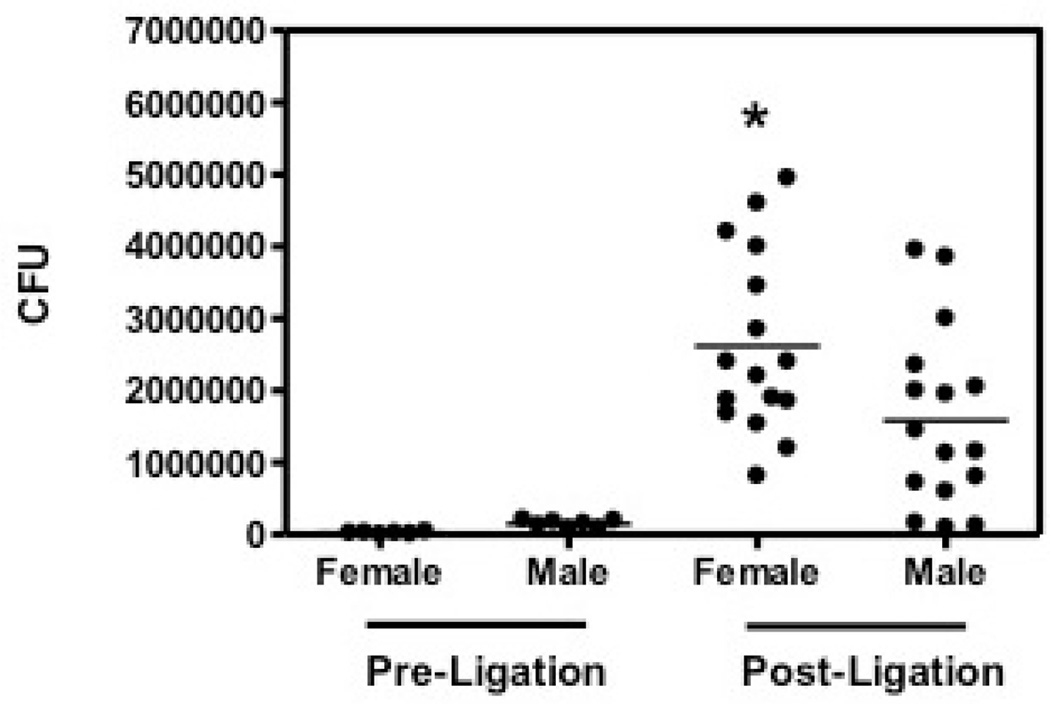
Recent research has led to a new model of periodontal disease pathogenesis, according to which periodontitis is initiated by a synergistic and dysbiotic microbial community rather than by select ‘periopathogens’ (27, 28). The number of the bacteria can also indicate the severity of the disease. Anaerobic colony forming units (CFUs) within the oral cavities of male or female mice were examined (Fig. 6). Before the ligation, oral bacteria of the males and females were determined for the baseline. The oral bacterial counts of pre-ligation mice were essentially similar. Seven days after ligation, we found that female mice had a much greater bacterial burden than that of their counterpart male mice, which suggested the involvement of bacteria in this bone-loss model (p<0.05; Fig. 6). Alternatively, the higher bacterial counts may reflect the increased inflammation experienced by female mice, since periodontal bacteria obtain essential nutrients from inflammatory tissue breakdown (e.g., degraded collagen peptides and heme-containing compounds) (29). In summary, female mice showed higher alveolar bone loss, gingival inflammation, and a greater oral bacterial burden, which indicated that female mice are more prone to more severe periodontitis, at least in ligature-induced periodontitis animal model.
Fig. 6.
Oral cavity bacteria in ligated female and male mice. Oral bacteria were sampled before ligation (Pre-ligation) and at the termination of the experiment (Post-ligation). The number of cultivable anaerobic bacteria from each mouse was represented by each dot. Asterisks indicate statistically significant (p < 0.05) differences in oral bacteria between female and male mice 7 days after ligation, before the termination of the experiment.
Sixty mice have been used in our ligation model, which is much smaller than the sample size of human survey (6, 7). Although our results showed significantly more severe inflammation and bone loss in female mice, animal models using other species and other strains, as well as more human survey strictly excluding other risk factors, are needed to further confirm sex as risk factor for periodontitis. Interestingly, our animal model-based results that females tend to develop more severe periodontitis stands in contrast to most human surveys and studies (Fig. 1 and Fig. 3). This can be explained that most of the confounding gender-related behavioral risk factors have been excluded. Another possible explanation is that females are at greater risk for periodontitis progression once the disease is initiated (7). Indeed, there is one study showed that females are more associated with the progression of severe periodontitis (10).
Therefore, with their limitations, animal models are still appropriate for investigating the role of gender per se in host susceptibility to periodontitis, keeping in mind that gender-related behavioral or environmental factors can also contribute to periodontitis in humans. Our present work in the ligature model has therefore set the stage for a model that can be used to dissect the mechanisms underlying genuine gender-based differences in periodontal disease susceptibility and/or progression.
ACKNOELEDGEMENT
This work was supported by University of Louisville School of Dentistry Dean Research Program, University of Louisville Research Initiation Grant (S.L.), and NIH DE017138 (G.H.).
Footnotes
The authors report no conflicts of interest related to this work.
References
- 1.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 3.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 6.Burt B. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 7.Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: a systematic review. J Periodontol. 2010;81:1379–1389. doi: 10.1902/jop.2010.100044. [DOI] [PubMed] [Google Scholar]
- 8.Hermann P, Gera I, Borbely J, Fejerdy P, Madlena M. Periodontal health of an adult population in Hungary: findings of a national survey. J Clin Periodontol. 2009;36:449–457. doi: 10.1111/j.1600-051X.2009.01395.x. [DOI] [PubMed] [Google Scholar]
- 9.Farina R, Tomasi C, Trombelli L. The bleeding site: a multi-level analysis of associated factors. J Clin Periodontol. 2013;40:735–742. doi: 10.1111/jcpe.12118. [DOI] [PubMed] [Google Scholar]
- 10.Norderyd O, Hugoson A, Grusovin G. Risk of severe periodontal disease in a Swedish adult population. A longitudinal study. J Clin Periodontol. 1999;26:608–615. doi: 10.1034/j.1600-051x.1999.260908.x. [DOI] [PubMed] [Google Scholar]
- 11.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 12.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe T, Hosur KB, Hajishengallis E, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013 doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S, Krauss JL, Domon H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce DL, Nishiyama S, Liang S, et al. Host adhesive activities and virulence of novel fimbrial proteins of Porphyromonas gingivalis. Infect Immun. 2009;77:3294–3301. doi: 10.1128/IAI.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossomando EF, Kennedy JE, Hadjimichael J. Tumour necrosis factor alpha in gingival crevicular fluid as a possible indicator of periodontal disease in humans. Arch Oral Biol. 1990;35:431–434. doi: 10.1016/0003-9969(90)90205-o. [DOI] [PubMed] [Google Scholar]
- 20.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4+ T Cells and the Proinflammatory Cytokines Gamma Interferon and Interleukin-6 Contribute to Alveolar Bone Loss in Mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 22.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 23.Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003;82:632–635. doi: 10.1177/154405910308200812. [DOI] [PubMed] [Google Scholar]
- 24.Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10: late onset. J Periodontal Res. 2004;39:194–198. doi: 10.1111/j.1600-0765.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 25.Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison AC. Measurement of interleukin-1 alpha and-1 beta in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J Periodontal Res. 1990;25:156–163. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 26.Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL-1beta profiles in periodontal disease. J Clin Periodontol. 2002;29:48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 27.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]