Abstract
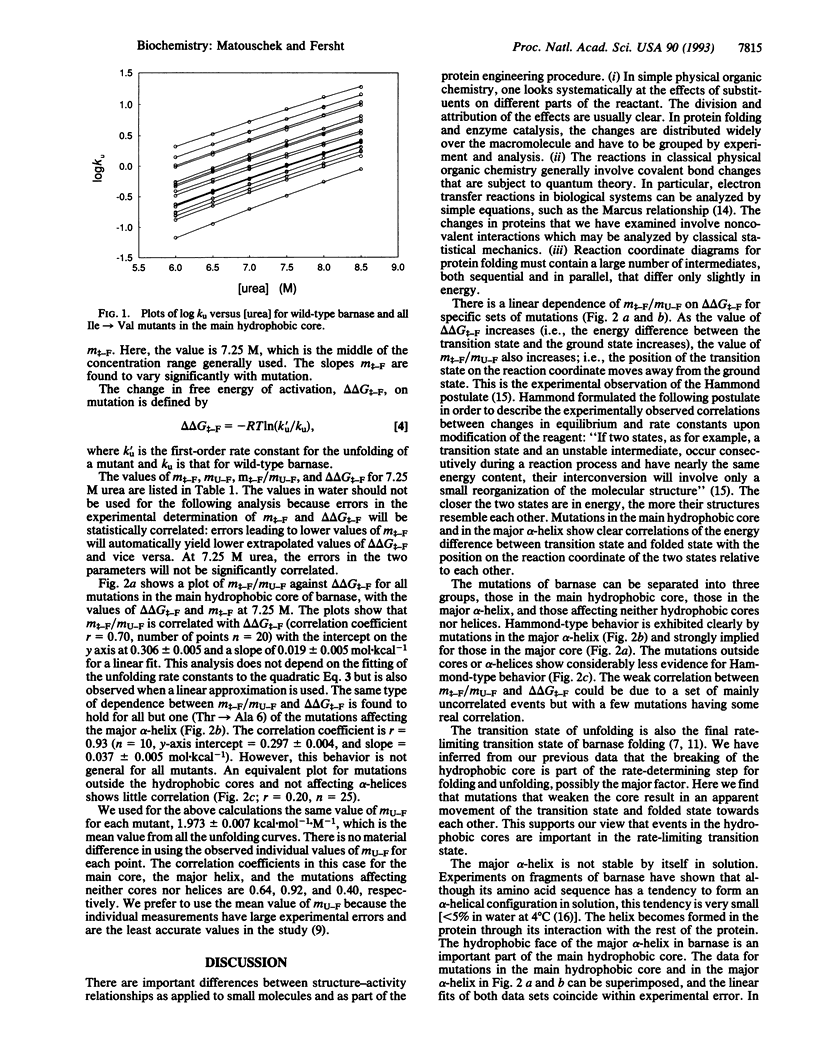
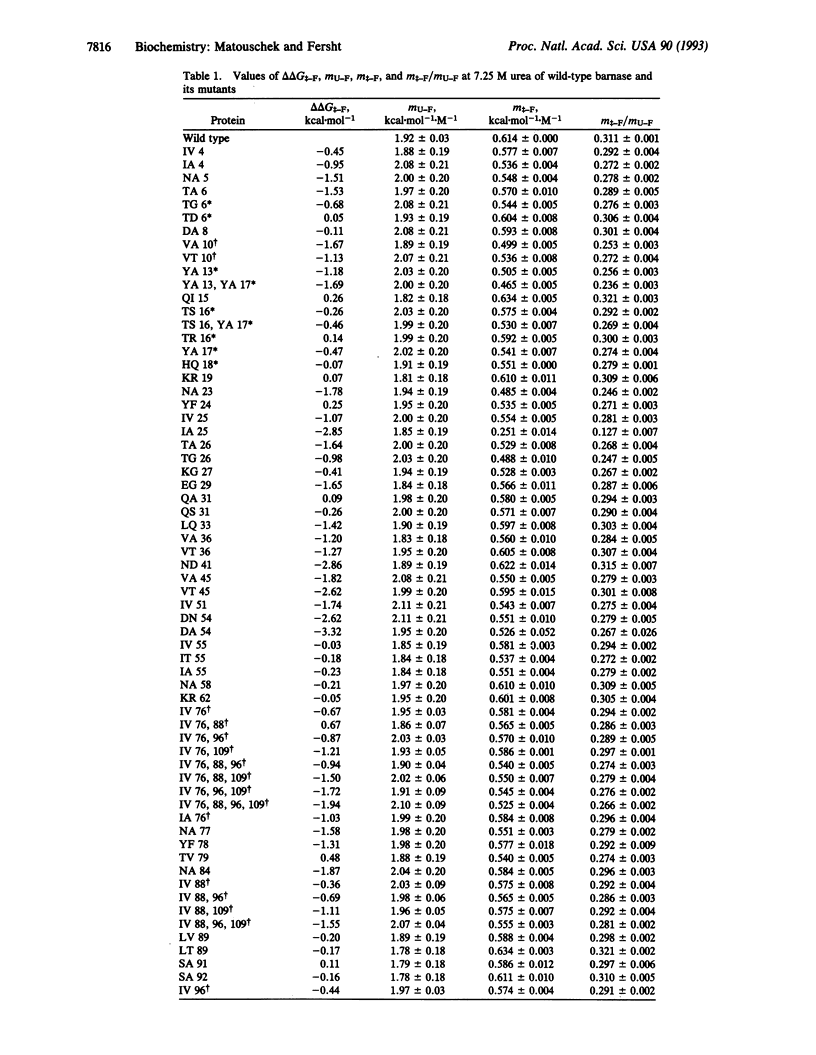
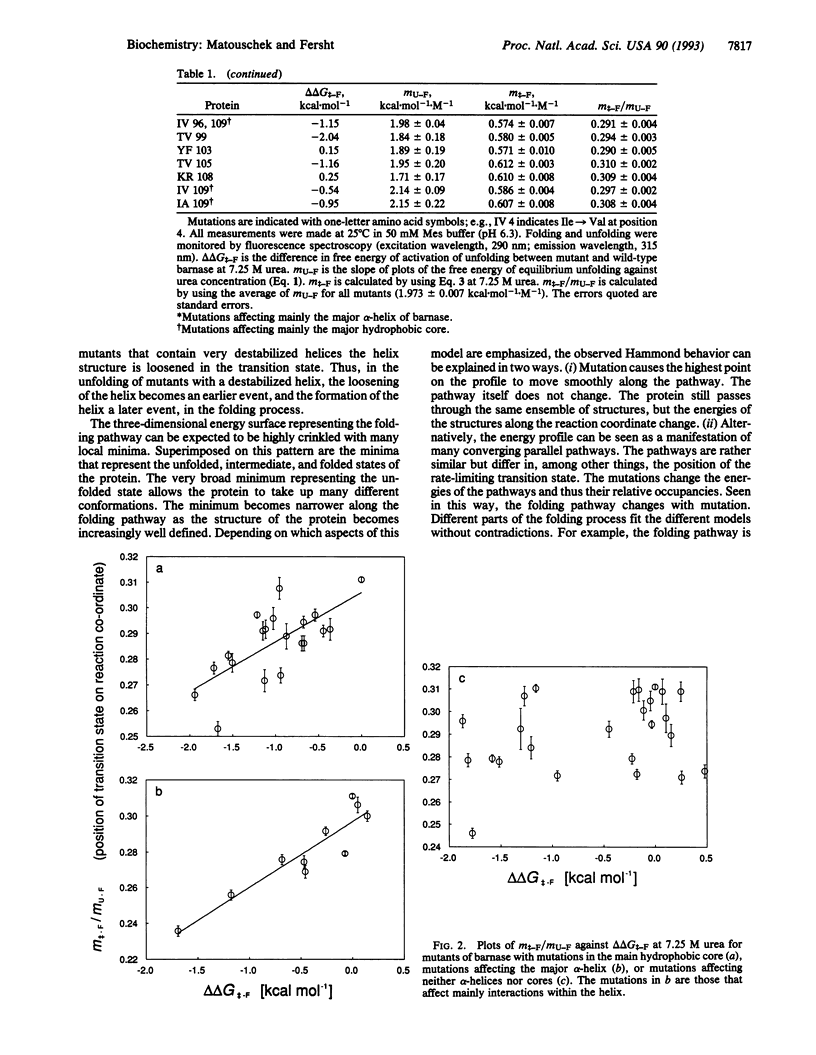
Transition states in protein folding may be analyzed by linear free-energy relationships (LFERs) analogous to the Brønsted equation for changes in reactivity with changes in structure. There is an additional source of LFERs in protein folding: the perturbation of the equilibrium and rate constants by denaturants. These LFERs give a measure of the position of the transition state along the reaction coordinate. The transition state for folding/unfolding of barnase has been analyzed by both types of LFERs: changing the structure by protein engineering and perturbation by denaturants. The combination has allowed the direct monitoring of Hammond postulate behavior of the transition state on the reaction pathway. Movement of the transition state has been found and analyzed to give further details of the order of events in protein folding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avis J. M., Fersht A. R. Use of binding energy in catalysis: optimization of rate in a multistep reaction. Biochemistry. 1993 May 25;32(20):5321–5326. doi: 10.1021/bi00071a006. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Toward a better understanding of protein folding pathways. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5082–5086. doi: 10.1073/pnas.85.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Leatherbarrow R. J., Wells T. N. Structure-activity relationships in engineered proteins: analysis of use of binding energy by linear free energy relationships. Biochemistry. 1987 Sep 22;26(19):6030–6038. doi: 10.1021/bi00393a013. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Bycroft M., Fersht A. R. Transient folding intermediates characterized by protein engineering. Nature. 1990 Aug 2;346(6283):440–445. doi: 10.1038/346440a0. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Fersht A. R. Mapping the transition state and pathway of protein folding by protein engineering. Nature. 1989 Jul 13;340(6229):122–126. doi: 10.1038/340122a0. [DOI] [PubMed] [Google Scholar]
- Sancho J., Neira J. L., Fersht A. R. An N-terminal fragment of barnase has residual helical structure similar to that in a refolding intermediate. J Mol Biol. 1992 Apr 5;224(3):749–758. doi: 10.1016/0022-2836(92)90559-3. [DOI] [PubMed] [Google Scholar]
- Serrano L., Kellis J. T., Jr, Cann P., Matouschek A., Fersht A. R. The folding of an enzyme. II. Substructure of barnase and the contribution of different interactions to protein stability. J Mol Biol. 1992 Apr 5;224(3):783–804. doi: 10.1016/0022-2836(92)90562-x. [DOI] [PubMed] [Google Scholar]
- Serrano L., Matouschek A., Fersht A. R. The folding of an enzyme. III. Structure of the transition state for unfolding of barnase analysed by a protein engineering procedure. J Mol Biol. 1992 Apr 5;224(3):805–818. doi: 10.1016/0022-2836(92)90563-y. [DOI] [PubMed] [Google Scholar]
- Serrano L., Matouschek A., Fersht A. R. The folding of an enzyme. VI. The folding pathway of barnase: comparison with theoretical models. J Mol Biol. 1992 Apr 5;224(3):847–859. doi: 10.1016/0022-2836(92)90566-3. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Toney M. D., Kirsch J. F. Tyrosine 70 fine-tunes the catalytic efficiency of aspartate aminotransferase. Biochemistry. 1991 Jul 30;30(30):7456–7461. doi: 10.1021/bi00244a013. [DOI] [PubMed] [Google Scholar]
- Wells T. N., Fersht A. R. Protection of an unstable reaction intermediate examined with linear free energy relationships in tyrosyl-tRNA synthetase. Biochemistry. 1989 Nov 14;28(23):9201–9209. doi: 10.1021/bi00449a036. [DOI] [PubMed] [Google Scholar]
- Wells T. N., Fersht A. R. Use of binding energy in catalysis analyzed by mutagenesis of the tyrosyl-tRNA synthetase. Biochemistry. 1986 Apr 22;25(8):1881–1886. doi: 10.1021/bi00356a007. [DOI] [PubMed] [Google Scholar]



