Abstract
Background
Infections caused by Salmonella enterica, a Gram-negative facultative anaerobic bacteria belonging to the family of Enterobacteriaceae, are major threats to the health of humans and animals. The recent availability of complete genome data of pathogenic strains of the S. enterica gives new avenues for the identification of drug targets and drug candidates. We have used the genomic and metabolic pathway data to identify pathways and proteins essential to the pathogen and absent from the host.
Methods
We took the whole proteome sequence data of 42 strains of S. enterica and Homo sapiens along with KEGG-annotated metabolic pathway data, clustered proteins sequences using CD-HIT, identified essential genes using DEG database and discarded S. enterica homologs of human proteins in unique metabolic pathways (UMPs) and characterized hypothetical proteins with SVM-prot and InterProScan. Through this core proteomic analysis we have identified enzymes essential to the pathogen.
Results
The identification of 73 enzymes common in 42 strains of S. enterica is the real strength of the current study. We proposed all 73 unexplored enzymes as potential drug targets against the infections caused by the S. enterica. The study is comprehensive around S. enterica and simultaneously considered every possible pathogenic strain of S. enterica. This comprehensiveness turned the current study significant since, to the best of our knowledge it is the first subtractive core proteomic analysis of the unique metabolic pathways applied to any pathogen for the identification of drug targets. We applied extensive computational methods to shortlist few potential drug targets considering the druggability criteria e.g. Non-homologous to the human host, essential to the pathogen and playing significant role in essential metabolic pathways of the pathogen (i.e. S. enterica). In the current study, the subtractive proteomics through a novel approach was applied i.e. by considering only proteins of the unique metabolic pathways of the pathogens and mining the proteomic data of all completely sequenced strains of the pathogen, thus improving the quality and application of the results. We believe that the sharing of the knowledge from this study would eventually lead to bring about novel and unique therapeutic regimens against the infections caused by the S. enterica.
Introduction
Salmonella enterica is a Gram-negative facultative anaerobic intracellular bacterium. According to the classification scheme of Kauffmann-White [1], more than 2500 serological variants (or serovars) were categorized in six subspecies [2, 3]. Most of the serovars have a broad range of hosts while some have adapted to specific hosts. The mechanism of adaptation is currently unclear [4]. Typically, S. enterica serovars infect the host through the mouth, leading to the three major symptoms: enterocolitis, bacteremia and enteric fever, or asymptomatic chronic carriage [5]. Human pathogens include serovar Typhi, Paratyphi, Typhimurium, Sendai, Choleraesuis, Dublin and many others [3].
Pathogenesis of Salmonella enterica initiates with its entry in the host organism. Salmonella is usually acquired from the environment by contact with a carrier host or by oral intake of contaminated food or water. After ingestion, Salmonella survives the low pH of the stomach, eventually leading to entry of the intestine where it uses a type III secretion system to deliver effecter proteins essential for intestinal invasion [6]. Hereafter, bacterial progression within the host is different in Non-Typhoidal Salmonella and Typhoidal Salmonella. Non-typhoidal Salmonella serovars induce a localized inflammation which, in immunocompetent persons, results in enterocolitis with the infiltration of polymorphonuclear leukocytes (PMNs) into the sub-mucosal epithelium [7]. In Typhoidal Salmonella, intestinal inflammation is moderate, largely consisting of macrophage infiltration [8] and the bacteria is distributed and reaches the blood either directly or via the mesenteric lymph nodes or are transported within leukocytes, causing bacteremia [9]. Both types of Salmonella grow and persist in systemic tissues where they adapt to the intracellular environment. The pathogen can escape from host cells using secretion systems [10].
A genome is the set of genes in a single functional organism, whereas the pangenome of a prokaryote is the set of non-redundant genes which includes a core genome containing genes present in all strains; dispensable genes that are absent from one or more strains, but not all; and genes that are unique to each strain [11]. Recently, microbial pangenomics has attracted the scientific community which was inspired by the accessibility to sequenced data of whole-genomes of the strains of particular species [12–15]. Simultaneously, research on pan-proteomics was also initiated to study the effects of similarities and differences at the protein level among the strains of specie [16–18]. As of October 13, 2015, there were only 45 target genes reported in DrugBank Database for S. enterica, which covers only 1.6% of its core genome size i.e. 2,800 [19]. Since the pathogen has developed resistance against conventional drugs, so there is a dire need to find new therapeutic drug targets.
In the present study, we took the whole proteome sequence data of 42 strains of 19 serovars of S. enterica and KEGG-annotated metabolic pathway data of Homo sapiens, identified and discarded S. enterica homologs of human proteins in unique metabolic pathways (UMPs) and identified enzymes essential to the pathogen using DEG database. We compared our results to a previous study [20] where they searched for new antimicrobial targets by focusing on different metabolic enzymes of a single serovar and comparing the results with other serovars at the genome level. In a more recent report, the pangenomic analyses of 22 complete and 23 draft genome sequences was performed [19]. However, to the best of our knowledge the current study is the first subtractive core proteomic analysis of the unique metabolic pathways applied to any pathogen for the identification of drug targets primarily essential enzymes.
Methodology
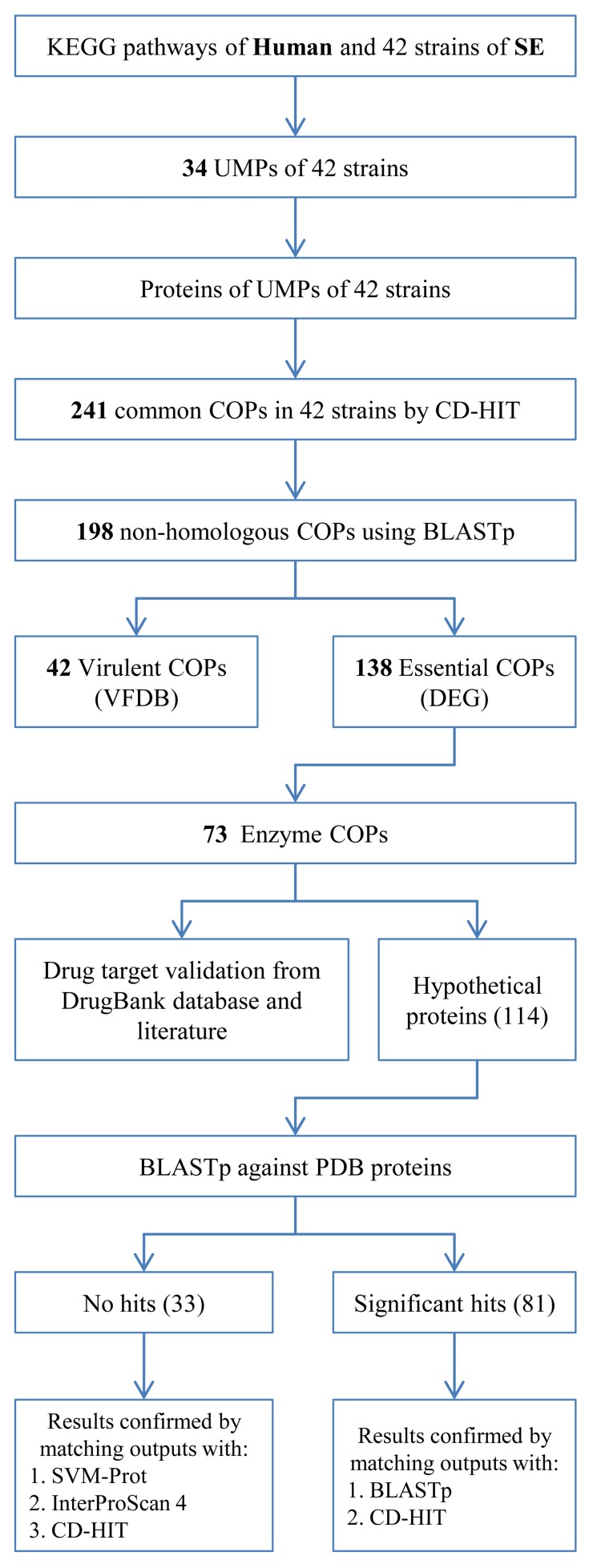
A schematic representation of the methodology is given in Fig 1. 88 biological datasets used in our analyses were downloaded from online sources, details of which are given in S1 Table.
Fig 1. Methodology.
1. Identification of UMPs of S. enterica
KEGG Brite Hierarchy files of H. sapiens and 42 strains of S. enterica containing information about the genes of respective metabolic pathways were downloaded from the KEGG database [21]. The metabolic pathways unique to the serovars (i.e. missing in human host) were identified using KEGG Orthology (KO) IDs, and the corresponding genes were sorted out. The UMPs absent in some strains were listed out using in-house AWK scripts.
2. Clustering common proteins of UMPs of 42 strains
The KEGG IDs of all the genes from UMPs were converted to corresponding NCBI GIs using KEGG-API service [21]. Amino acid sequences were retrieved from the respective strains available on NCBI FTP server [22] using Fastblast [23]. The genes encoding tRNA and rRNA were excluded since the aim was to propose enzymes as the drug targets. Further plasmid-encoded genes were not considered to be essential for the survival of cell, as per information available in the Database of Essential Genes (DEG) [24]. We noticed that some NCBI GIs were discontinued and therefore, updated to the new GIs. We linked the new GIs with the old one and retrieved the sequence. CD-HIT [25] is a standalone command-based application which groups a set of sequences of a database on the basis of sequence identity. Orthologs within the 42 strains were identified by using CD-HIT (updated on August 27, 2012) to group protein sequences with at least 80% sequence identity in to Clusters of Proteins (COPs) so that each COP will be analyzed at once for further steps of subtractive proteomics. The results were verified by comparison to the online server of ElimDupes [26].
3. Searching of non-homologous essential enzymes
To process all COPs for subtractive proteomic analyses at once, a novel strategy was applied which comprised of two approaches. In first approach, proteins of all COPs were subjected to BLASTp [27] against Homo sapiens downloaded from NCBI FTP server [28] and the output was analyzed for non-homologous proteins. In second approach, 3 strains out of 42 were selected at random and proteins of those strains were subjected to BLASTp against human proteome. Both approaches are illustrated in S1 Fig. The parameter details for BLASTp are mentioned in Table 1 (a). The results of both approaches were observed by BioPerl module SearchIO [29] and the better approach was adapted to the next steps considering the criteria of time processing. The non-homologous COPs from the previous step were subjected to BLASTp of DEG V. 10 [24] to identify essential genes of the pathogen. The parameter details are mentioned in Table 1 (b). The KEGG Brite hierarchy is one of the important features of KEGG server containing the information of enzymes of metabolic pathways. The enzymes were sorted out from non-homologous essential COPs of S. enterica using the hierarchy files of 42 strains [21].
Table 1. Parameters for BLASTp.
| a | b | c | d | |
|---|---|---|---|---|
| Program | BLAST+ 2.2.28 | BLASTp of DEG 10 | BLAST+ 2.2.28 | BLAST+ 2.2.28 |
| Query name | COPs | Non-homologous COPs | Non-homologous COPs | Hypothetical proteins |
| No. of queries | 241 | 198 | 198 | 114 |
| Subject name | Human proteome | DEG | VFDB | PDB proteins |
| No. of subjects | 68,939 | 12,379 | 2,447 | 252,484 |
| E-value | 1.00E-03 | 1.00E-05 | 1.00E-04 | 1.00E-05 |
4. Searching the virulent genes
VFDB (Virulence Factors Database) [30]containing protein sequences of all virulent genes was downloaded and non-homologous COPs from 3 randomly selected strains were subjected to standalone BLASTp against VFDB sequences to find out virulent genes with sequence identity of 70% or more. Table 1 (c) contained the parameter details.
5. Characterization of the hypothetical proteins
The hypothetical proteins were identified among the enzymes to characterize their structure and/or function. All the hypothetical protein sequences were subjected to standalone BLASTp against protein sequences available in PDB (Protein Data Bank) [31] obtained from PDB FTP server [32]. The parameter details are mentioned in Table 1 (d). The queries with significant hits against PDB database were verified from CD-HIT output and those with ‘no hits’ were subjected to SVM-Prot [33] and InterProScan version 4.0 [34] for protein family prediction. The results were manually cross-checked with CD-HIT output.
6. Validation from the literature:
The non-homologous catalytic proteins considered as putative drug targets were validated from DrugBank database [35] and published results of Becker et. al. [20]. In order to do so, the gene symbols of essential enzymes [20] were converted to full form using DAVID Bioinformatics tool [36], and then searched in both sources manually.
Results and Discussion
1. Identification of UMPs of S. enterica
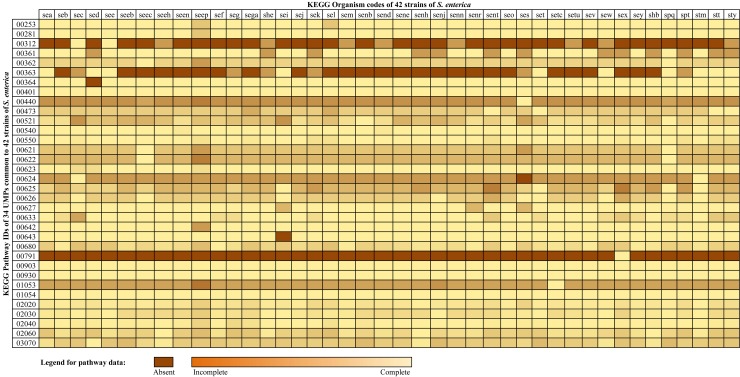
Each of the metabolic pathways of 42 strains of the S. enterica was compared with the complete human metabolic pathway. On average, each strain has 117 metabolic pathways and at least 34 UMPs (Table 2) with all UMPs present in almost all strains. A heatmap containing the percentage presence of proteins in each pathway and totally absent pathways in individual strains is illustrated in Fig 2, while its corresponding quantitative data is provided as S2 Table. In the studied strains of S. enterica, we found that only the strain (Typhi P-stx-12) was predicted to metabolize the Atrazine, thus may be resistant to it. However the dataset lacked the pathway information of β-Lactam resistance and Bisphenol degradation which were also the next most frequent absent pathways among all studied strains. The strains Heidelberg CFSAN002069 and Typhi CT18 needed to update in KEGG since the data was not updated and hence 22 and 11 NCBI GIs were appended, respectively in both strains and mentioned in S3 Table.
Table 2. Details of Metabolic Pathways and Genes of human and 42 strains of S. enterica.
| S.No. | Organism name | Organism KEGG Code | No. of Pathways | Unique Pathways | KEGG ID | NCBI RefSeq ID | NCBI Gis | Sequences |
|---|---|---|---|---|---|---|---|---|
| Homo sapiens | has | 286 | - | - | H_sapiens | - | - | |
| 1 | Agona SL483 | sea | 118 | 32 | 428 | NC_011149 | 426 | 417 |
| 2 | Arizonae 62 z4 z23 | ses | 117 | 31 | 407 | NC_010067 | 406 | 406 |
| 3 | Bareilly CFSAN000189 | see | 119 | 33 | 429 | NC_021844 | 428 | 426 |
| 4 | Bovismorbificans 3114 | senb | 117 | 31 | 414 | NC_022241 | 414 | 414 |
| 5 | Choleraesuis SC B67 | sec | 119 | 33 | 410 | NC_006905 | 410 | 408 |
| 6 | Cubana CFSAN002050 | seeb | 117 | 31 | 416 | NC_021818 | 416 | 415 |
| 7 | Dublin CT 02021853 | sed | 116 | 31 | 425 | NC_011205 | 425 | 416 |
| 8 | Enteritidis P125109 | setc | 117 | 31 | 435 | NC_011294 | 435 | 430 |
| 9 | Gallinarum 287 91 | sega | 116 | 31 | 399 | NC_011274 | 399 | 399 |
| 10 | Gallinarum Pullorum CDC1983 67 | seg | 117 | 32 | 409 | NC_022221 | 409 | 409 |
| 11 | Gallinarum pullorum RKS5078 | sel | 116 | 31 | 395 | NC_016831 | 395 | 395 |
| 12 | Heidelberg 41578 | seec | 117 | 31 | 430 | NC_021810 | 430 | 426 |
| 13 | Heidelberg B182 | shb | 116 | 31 | 442 | NC_017623 | 440 | 430 |
| 14 | Heidelberg CFSAN002069 | senh | 116 | 31 | 451 | NC_021812 | 451 | 440 |
| 15 | Heidelberg SL476 | seh | 119 | 33 | 433 | NC_011083 | 431 | 431 |
| 16 | Javiana CFSAN001992 | senj | 115 | 31 | 419 | NC_020307 | 419 | 419 |
| 17 | Newport SL254 | seeh | 118 | 32 | 444 | NC_011080 | 444 | 433 |
| 18 | Newport USMARC S3124 1 | senn | 118 | 32 | 425 | NC_021902 | 425 | 425 |
| 19 | Paratyphi A AKU 12601 | sek | 117 | 32 | 405 | NC_011147 | 404 | 404 |
| 20 | Paratyphi A ATCC 9150 | spt | 117 | 32 | 408 | NC_006511 | 407 | 407 |
| 21 | Paratyphi B SPB7 | spq | 118 | 32 | 428 | NC_010102 | 427 | 427 |
| 22 | Paratyphi C RKS4594 | sei | 116 | 31 | 418 | NC_012125 | 418 | 418 |
| 23 | Pullorum S06004 | seep | 116 | 31 | 375 | NC_021984 | 375 | 375 |
| 24 | Schwarzengrund CVM19633 | sew | 119 | 33 | 436 | NC_011094 | 435 | 424 |
| 25 | Thompson RM6836 | sene | 117 | 31 | 421 | NC_022525 | 421 | 421 |
| 26 | Typhi CT18 | sty | 119 | 33 | 409 | NC_003198 | 409 | 408 |
| 27 | Typhi P stx 12 | sex | 117 | 32 | 407 | NC_016832 | 407 | 406 |
| 28 | Typhi Ty2 | stt | 117 | 32 | 409 | NC_004631 | 409 | 409 |
| 29 | Typhi Ty21a | sent | 116 | 31 | 406 | NC_021176 | 406 | 406 |
| 30 | Typhimurium 08–1736 | seen | 117 | 31 | 420 | NC_021820 | 420 | 420 |
| 31 | Typhimurium 14028S | seo | 117 | 31 | 433 | NC_016856 | 433 | 433 |
| 32 | Typhimurium 798 | sef | 117 | 31 | 430 | NC_017046 | 430 | 430 |
| 33 | Typhimurium D23580 | sev | 117 | 31 | 434 | NC_016854 | 434 | 434 |
| 34 | Typhimurium DT104 | send | 119 | 32 | 426 | NC_022569 | 426 | 426 |
| 35 | Typhimurium DT2 | senr | 117 | 31 | 428 | NC_022544 | 428 | 428 |
| 36 | Typhimurium LT2 | stm | 118 | 32 | 447 | NC_003197 | 447 | 447 |
| 37 | Typhimurium SL1344 | sey | 117 | 31 | 435 | NC_016810 | 435 | 435 |
| 38 | Typhimurium ST4 74 | seb | 117 | 31 | 437 | NC_016857 | 437 | 437 |
| 39 | Typhimurium T000240 | sem | 119 | 32 | 438 | NC_016860 | 438 | 438 |
| 40 | Typhimurium U288 | setu | 119 | 32 | 434 | NC_021151 | 434 | 433 |
| 41 | Typhimurium UK 1 | sej | 117 | 31 | 430 | NC_016863 | 430 | 430 |
| 42 | Typhimurium var 5 CFSAN001921 | set | 117 | 32 | 422 | NC_021814 | 422 | 422 |
Fig 2. Heatmap of genes in UMPs of S. enterica strains.
The heatmap contains percentage presence and absence of genes of in each metabolic pathway of 42 strains of S. enterica.
2. Clustering common proteins of UMPs of 42 strains and searching of non-homologous essential enzymes
The CD-HIT resulted in 537 COPs and each cluster was comprised of more than 1 protein. Out of total, 241 COPs contained at least 42 proteins belonging to the 42 strains of S. enterica. S4 Table contained the NCBI-GIs of orthologous proteins (genes) clustered in groups.
The complete human proteome was obtained from NCBI FTP server (details in S1 Table). The non-homologous proteins could be potential drug targets with reduced possible side effects or cross reactivity of the drug with the host proteins. It is essential to find the similarity of the shortlisted sequences with the human host. In order to do so, we compared each COP with the individual human proteins. We performed this comparison by two separate approaches (details in methods section). As stated earlier that the COPs were consisted of up to 80% similar proteins; therefore, if we compare either (i) each single entry of the COPs with the host proteins or (ii) comparing few randomly selected entries of the COPs with human host proteins, the outcome would remain same. We used both of the approaches to see if the statement maintains. Both approaches of searching non-homologous sequences in the pathogen revealed exactly same results i.e. 198 out of 241 COPs were identified as non-homologous to humans (Table 3). The second approach was selected for the further steps of subtractive proteomics as the approach was accurate and relatively fast. The COP names mentioned in Table 3 were allocated by the authors following the criteria of maximum or common occurrences of that name in a respective cluster. One important aspect was observed during the tabulation of data (Table 3) that despite having exactly the same or closely similar names within the COPs, the member proteins of the respective COPS showed low similarity among them. These COPs include Cytochrome BD-II Ubiquinol Oxidase (COP # 139 and 221), D-alanyl-D-alanine Carboxypeptidase (COP # 127 and 190), Lipopolysaccharide core biosynthesis protein (COP # 250, 339 and 384), Peptidoglycan Synthetase FtsI (COP # 65 and 67), PTS system Ascorbate-specific transporter IIC (COP # 129 and 164), Transcriptional regulator (COP # 17 and 167), Tricarboxylate transport membrane protein (COP # 109 and 476), Two component response regulator (COP # 378, 410 and 411) and Type III Secretion apparatus protein SpaR (COP # 341 and 344). From the similar named COPs, we randomly selected the few proteins and subjected to online BLASTp which resulted in low similarity in each case. There might be two possibilities for the outcome; either these sets of COPs were isozymes or might be human error during the GenBank submission. For instance BLASTp of NCBI GI 194443076 and 194443845 have only 29% identity though they both have same name and belong to the same strain. The beta subunit of the subtype 1 and 2 of the enzyme Nitrate reductase shared more than 80% sequence similarity and hence clustered in a single COP. The enzyme Succinate Dehydrogenase Cytochrome b556 large membrane was somehow not characterized as an enzyme during KEGG analysis hence its UniProt ID was mentioned in Table 3.
Table 3. Functional characterization of non-homologous COPs.
| COP Name | Subtype | COP # | Virulent | Essential | Enzyme | Becker 2006 |
|---|---|---|---|---|---|---|
| [Citrate (pro-3S)-lyase] ligase | 247 | |||||
| 2-(5''-triphosphoribosyl)-3'-dephospho-CoA synthase | 432 | |||||
| 2-dehydro-3-deoxyphosphooctonate aldolase | 328 | Yes | Yes | Yes | ||
| 3-deoxy-D-manno-octulosonic-acid transferase | 192 | Yes | Yes | Yes | ||
| 3-deoxy-manno-octulosonate cytidylyltransferase | 361 | Yes | Yes | Yes | ||
| Acetate kinase | 205 | Yes | Yes | |||
| ADP-heptose—LPS heptosyltransferase | I | 291 | Yes | Yes | Yes | |
| II | 261 | Yes | Yes | Yes | ||
| Aerotaxis receptor | 104 | Yes | ||||
| Alanine racemase | 245 | Yes | Yes | |||
| Alkylphosphonate utilization operon protein PhnA | 498 | Yes | Yes | |||
| Anti-sigma-28 factor | FlgM | 507 | ||||
| Aspartate racemase | 366 | |||||
| Bifunctional chorismate mutase/prephenate dehydrogenase | 227 | |||||
| Carbon storage regulator | 527 | Yes | ||||
| Chemotaxis methyltransferase | CheR | 321 | Yes | Yes | ||
| Chemotaxis protein | CheA | 49 | Yes | Yes | Dispensable | |
| CheW | 276 | Yes | ||||
| CheZ | 409 | |||||
| CheY | 486 | Yes | Yes | |||
| Chemotaxis-specific methylesterase | 259 | Yes | Yes | Yes | ||
| Chromosomal replication initiation protein | 136 | Yes | ||||
| Citrate lyase | Gamma | 505 | Yes | Yes | ||
| Colanic acid capsular biosynthesis activation protein | A | 416 | ||||
| Cytochrome BD-II ubiquinol oxidase | 1 | 221 | Yes | Yes | ||
| 1 | 139 | Yes | Yes | |||
| 2 | 273 | Yes | Yes | |||
| D-alanyl-D-alanine carboxypeptidase | 127 | Yes | Yes | |||
| 190 | Yes | Yes | ||||
| DNA-binding transcriptional activator | DcuR | 376 | Yes | |||
| KdpE | 395 | Yes | ||||
| SdiA | 355 | |||||
| UhpA | 404 | Yes | ||||
| DNA-binding transcriptional regulator | BaeR | 374 | Yes | |||
| BasR | 390 | Yes | Yes | |||
| CpxR | 385 | Yes | ||||
| PhoP | 398 | Yes | Yes | |||
| QseB | 336 | Yes | ||||
| RstA | 368 | Yes | ||||
| D-ribose transporter | RbsB | 310 | Yes | |||
| Flagella synthesis protein | FlgN | 478 | ||||
| Flagellar assembly protein | FliH | 370 | ||||
| Flagellar basal body L-ring protein | 382 | Yes | ||||
| Flagellar basal body P-ring biosynthesis protein | FlgA | 401 | ||||
| Flagellar basal body rod modification protein | 383 | |||||
| Flagellar basal body rod protein | FlgB | 479 | Yes | |||
| FlgC | 484 | Yes | ||||
| FlgF | 354 | |||||
| FlgG | 343 | Yes | ||||
| Flagellar biosynthesis protein | FliJ | 471 | Yes | |||
| FliO | 487 | Yes | ||||
| FliP | 364 | Yes | ||||
| FliQ | 517 | Yes | ||||
| FliR | 340 | |||||
| FliT | 490 | |||||
| Flagellar hook protein | FlgE | 201 | ||||
| Flagellar hook-associated protein | FlgL | 290 | Yes | |||
| Flagellar hook-basal body protein | FliE | 503 | ||||
| Flagellar hook-length control protein | 199 | |||||
| Flagellar motor protein | MotA | 312 | Yes | |||
| Flagellar motor switch protein | FliM | 275 | Yes | |||
| Flagellar motor switch protein | G | 279 | Yes | |||
| Flagellar MS-ring protein | 68 | |||||
| Flagellar protein | FliS | 481 | Yes | |||
| Formate dehydrogenase-O | Gamma | 412 | ||||
| Fructose 1,6-bisphosphate aldolase | 244 | Yes | Yes | |||
| Fumarate reductase | C | 485 | ||||
| D | 492 | Yes | ||||
| Glutamate/aspartate ABC transporter permease | GltK | 397 | Yes | |||
| Hydrogenase 2 | Large | 72 | ||||
| Small | 230 | Yes | Yes | |||
| Integral membrane protein | MviN | 90 | Yes | |||
| Invasion protein | InvA | 48 | Yes | |||
| Isochorismatase | 326 | Yes | Yes | Yes | ||
| Isochorismate synthase | 174 | Yes | Yes | |||
| Lipid A biosynthesis lauroyl acyltransferase | 280 | Yes | Yes | Yes | ||
| Lipid-A-disaccharide synthase | 218 | Yes | Yes | Yes | ||
| Lipopolysaccharide 1,2-glucosyltransferase | 272 | Yes | Yes | |||
| Lipopolysaccharide 1,3-galactosyltransferase | 271 | Yes | Yes | |||
| Lipopolysaccharide core biosynthesis protein | 250 | Yes | Yes | |||
| 339 | Yes | Yes | Yes | |||
| 384 | Yes | Yes | ||||
| RfaG | 226 | Yes | Yes | |||
| Maltose ABC transporter substrate-binding protein | 132 | Yes | ||||
| Monofunctional biosynthetic peptidoglycan transglycosylase | 369 | Yes | Yes | |||
| Multidrug efflux system | MdtC | 12 | Yes | |||
| Nitrate reductase 1 | Alpha | 4 | Yes | Yes | ||
| Nitrate reductase molybdenum cofactor assembly chaperone 1 | 381 | |||||
| Nitrate reductase (81 duplicates of 1 and 2) | Beta | 98 | Yes | Yes | ||
| Nitrogen regulation protein | NR(I) | 135 | Yes | |||
| NR(II) | 260 | Yes | Yes | |||
| P-II 1 | 497 | Yes | ||||
| O-antigen ligase | 166 | Yes | Yes | Yes | ||
| Osmolarity response regulator | OmpR | 367 | Yes | |||
| Osmolarity sensor protein | EnvZ | 160 | Yes | Yes | ||
| Outer membrane channel protein | TolC | 120 | Yes | |||
| Outer membrane lipoprotein | 482 | |||||
| Outer membrane porin protein | C | 223 | Yes | |||
| Outer membrane protease | 293 | Yes | ||||
| Outer membrane protein | F | 238 | Yes | |||
| Penicillin-binding protein | 1b | 33 | Yes | Yes | Yes | |
| 2 | 54 | Yes | ||||
| Peptide transport periplasmic protein | SapA | 76 | Yes | |||
| Peptidoglycan synthetase | 1a | 31 | Yes | Yes | Yes | |
| FtsI | 65 | Yes | Yes | Yes | ||
| FtsI | 67 | Yes | Yes | |||
| Phosphate ABC transporter substrate-binding protein | 251 | Yes | ||||
| Phosphate acetyltransferase | 43 | Yes | Yes | |||
| Phosphate regulon sensor protein | PhoR | 186 | Yes | Yes | ||
| Phosphoenolpyruvate carboxylase | 29 | Yes | Yes | |||
| Phosphoenolpyruvate-protein phosphotransferase | 40 | Yes | Yes | |||
| Phosphoglyceromutase | 93 | Yes | Yes | |||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase | 242 | Yes | Yes | Yes | ||
| PII uridylyl-transferase | 23 | Yes | Yes | |||
| Preprotein translocase | SecA | 22 | Yes | |||
| SecB | 459 | Yes | ||||
| SecD | 60 | Yes | ||||
| SecE | 477 | Yes | ||||
| SecF | 270 | Yes | ||||
| SecG | 439 | Yes | ||||
| SecY | 169 | Yes | ||||
| YajC | 500 | Yes | ||||
| PTS system ascorbate-specific transporter | IIC | 129 | Yes | |||
| IIC | 164 | Yes | ||||
| PTS system fructose-specific transporter | IIBC | 74 | Yes | Yes | ||
| PTS system glucitol/sorbitol-specific transporter | IIA | 491 | ||||
| IiB | 284 | |||||
| PTS system glucose-specific transporter | IIA | 447 | Yes | Yes | ||
| IIBC | 119 | Yes | Yes | |||
| PTS system lactose/cellobiose-specific transporter | IIB | 515 | ||||
| PTS system L-ascorbate-specific transporter | IIA | 460 | Yes | Yes | ||
| PTS system mannitol-specific transporter | IIA | 465 | Yes | Yes | ||
| IIABC | 50 | Yes | Yes | |||
| PTS system mannose-specific transporter | IiAB | 285 | Yes | Yes | ||
| IIC | 338 | |||||
| IID | 324 | |||||
| PTS system N,N'-diacetylchitobiose-specific transporter | IIA | 496 | Yes | Yes | ||
| IIB | 499 | Yes | Yes | |||
| IIC | 158 | |||||
| PTS system phosphohistidinoprotein-hexose phosphotransferase | Hpr | 514 | Yes | |||
| Npr | 516 | Yes | ||||
| PTS system transporter subunit IIA-like nitrogen-regulatory protein | PtsN | 452 | Yes | Yes | ||
| Purine-binding chemotaxis protein | 449 | Yes | Yes | |||
| Respiratory nitrate reductase 1 | Gamma | 396 | ||||
| RNA polymerase sigma factor for flagellar biosynthesis | 377 | Yes | Yes | |||
| RNA polymerase sigma-54 factor | 128 | Yes | ||||
| Sec-independent translocase | 434 | |||||
| Secretion system apparatus protein | SsaU | 255 | Yes | |||
| SsaV | 45 | Yes | ||||
| Sensor protein | PhoQ | 123 | Yes | Yes | Yes | |
| BasS/ PmrB | 246 | Yes | Yes | Yes | ||
| RstB | 179 | Yes | Yes | |||
| Signal transduction histidine-protein kinase | BaeS | 140 | Yes | Yes | ||
| Succinate dehydrogenase cytochrome b556 large membrane | 483 | Yes | K8TKP2 | |||
| Surface presentation of antigens protein | SpaO | 305 | Yes | |||
| SpaP | 400 | Yes | ||||
| SpaQ | 521 | Yes | Yes | |||
| SpaS | 249 | Yes | ||||
| Tetraacyldisaccharide 4'-kinase | 282 | Yes | Yes | Yes | ||
| Tetrathionate reductase complex | A | 13 | Yes | |||
| Transcriptional activator | FlhC | 423 | Yes | |||
| FlhD | 494 | Yes | ||||
| Transcriptional regulator | PhoB | 387 | Yes | |||
| 167 | Yes | |||||
| 17 | Yes | Yes | ||||
| RcsB | 386 | Yes | ||||
| Tricarboxylate transport membrane protein | 109 | |||||
| 476 | ||||||
| Twin arginine translocase | A | 522 | Yes | |||
| E | 525 | Yes | ||||
| Twin-arginine protein translocation system | TatC | 345 | Yes | |||
| Two component response regulator | 410 | Yes | Yes | |||
| 411 | Yes | Yes | ||||
| 378 | Yes | |||||
| Two-component sensor kinase protein | 152 | Yes | Yes | |||
| Type III secretion apparatus lipoprotein YscJ/HrcJ family | 352 | Yes | Yes | |||
| Type III secretion apparatus needle protein | PrgI | 523 | Yes | |||
| SsaG | 519 | Yes | ||||
| Type III secretion apparatus protein | SpaR | 341 | Yes | Yes | ||
| SpaR | 344 | Yes | Yes | |||
| Type III secretion outer membrane pore | 111 | Yes | ||||
| Type III secretion outer membrane protein YscC/HrcC family | 73 | Yes | ||||
| Type III secretion system protein | 286 | Yes | Yes | |||
| FliP | 407 | Yes | ||||
| InvE | 229 | Yes | ||||
| UDP pyrophosphate phosphatase | 333 | Yes | Yes | |||
| UDP-2,3-diacylglucosamine hydrolase | 372 | Yes | Yes | Yes | ||
| UDP-3-O-[3-hydroxymyristoyl] glucosamine N-acyltransferase | 265 | Yes | Yes | Yes | ||
| UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | 301 | Yes | Yes | Yes | ||
| UDP-N-acetylenolpyruvoylglucosamine reductase | 263 | Yes | Yes | Yes | ||
| UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 194 | Yes | Yes | Yes | ||
| UDP-N-acetylglucosamine acyltransferase | 342 | Yes | Yes | Yes | ||
| UDP-N-acetylmuramate—L-alanine ligase | 121 | Yes | Yes | Yes | ||
| UDP-N-acetylmuramoylalanyl-D-glutamate—2,6-diaminopimelate ligase | 113 | Yes | Yes | Yes | ||
| UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | 177 | Yes | Yes | Yes | ||
| UDP-N-acetylmuramoyl-tripeptide—D-alanyl-D-alanine ligase | 157 | Yes | Yes | Yes | ||
| Virulence membrane protein | PagC | 430 | ||||
| Zinc resistance protein | 467 | Yes |
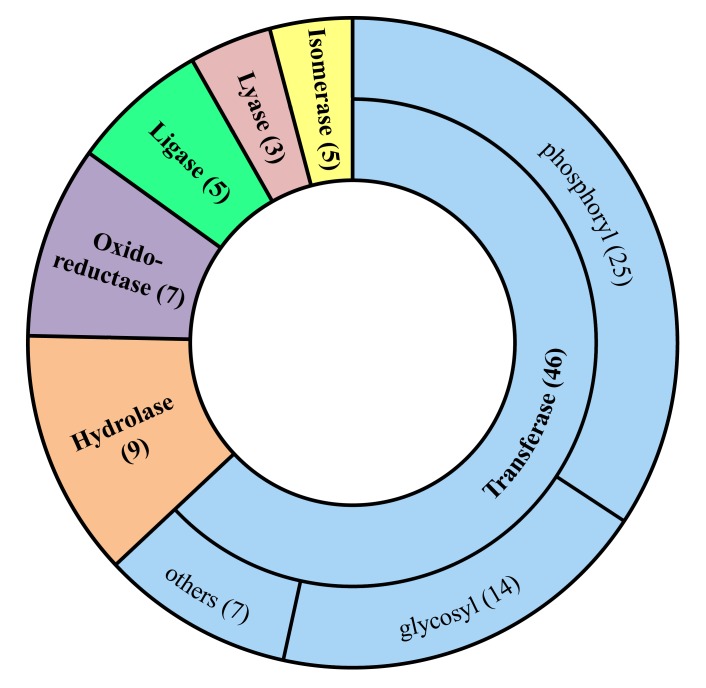
Additionally, we searched for the essential and virulent genes from the 198 COPs by applying the same subtractive proteomics approach. The database of essential genes (DEG) is a well curated open-access database consisting of essential genes from various organisms ranging from single-cell prokaryotes to multicellular eukaryotes. The bacteria harbor various virulent genes which lead to pathogenecity. Therefore, identifying virulent factors in the genome could lead us to elucidate the molecular mechanism of bacterial pathogenecity. The VFDB [30] is an online server containing information about virulent genes present in various microorganisms. Similar results were obtained from 3 randomly selected strains and it was found out that 138 out of 198 COPs were essential for the bacteria as per the prediction of DEG (Table 3), and 42 out of 198 COPs were identified as virulent genes (Table 3). There were 73 enzymes in the 138 non-humongous essential COPs (Table 3). The NCBI GIs of each respective COP was presented in S5 Table. The S1 Text contained important information regarding the accessibility of NCBI GIs mentioned in S5 Table. The data illustrated through pie chart in Fig 3 and tabulated in Table 4 revealed that most of the targets (34%) belonged to the subclass ‘phosphoryl transferases’ or ‘kinases’ which are the most favorable targets in drug discovery research [37].
Fig 3. Enzyme classification of 73 potential drug targets.
The pie chart reveals that 63% of the enzyme targets belong to Transferase class which is subdivided into phosphoryl (34%), glycosyl (19%) and other (10%) transferases.
Table 4. Enzyme Classification of 73 drug targets.
| Enzyme name | E.C. Number | Enzyme Class | Enzyme Sub-class |
|---|---|---|---|
| Cytochrome BD-II ubiquinol oxidase 1 | 1.10.3.10 | Oxidoreductase | diphenols as donors |
| Cytochrome BD-II ubiquinol oxidase 2 | 1.10.3.10 | Oxidoreductase | diphenols as donors |
| Cytochrome BD-II ubiquinol oxidase 3 | 1.10.3.10 | Oxidoreductase | diphenols as donors |
| Hydrogenase 3 | 1.12.-.- | Oxidoreductase | hydrogen as donor |
| UDP-N-acetylenolpyruvoylglucosamine reductase | 1.3.1.98 | Oxidoreductase | CH-CH group of donors |
| Nitrate reductase 1 | 1.7.99.4 | Oxidoreductase | nitrogenous compounds as donors |
| Nitrate reductase 2 | 1.7.99.4 | Oxidoreductase | nitrogenous compounds as donors |
| Chemotaxis methyltransferase | 2.1.1.80 | Transferase | One-Carbon group |
| Lipid A biosynthesis lauroyl acyltransferase | 2.3.1.- | Transferase | acyl |
| UDP-N-acetylglucosamine acyltransferase | 2.3.1.129 | Transferase | acyl |
| UDP-3-O-[3-hydroxymyristoyl] glucosamine N-acyltransferase | 2.3.1.191 | Transferase | acyl |
| Phosphate acetyltransferase | 2.3.1.8 | Transferase | acyl |
| ADP-heptose—LPS heptosyltransferase 1 | 2.4.-.- | Transferase | glycosyl |
| ADP-heptose—LPS heptosyltransferase 2 | 2.4.-.- | Transferase | glycosyl |
| Lipopolysaccharide core biosynthesis protein 1 | 2.4.-.- | Transferase | glycosyl |
| Lipopolysaccharide core biosynthesis protein 2 | 2.4.-.- | Transferase | glycosyl |
| Lipopolysaccharide core biosynthesis protein 3 | 2.4.-.- | Transferase | glycosyl |
| Lipopolysaccharide core biosynthesis protein 4 | 2.4.-.- | Transferase | glycosyl |
| Peptidoglycan synthetase 1 | 2.4.1.129 | Transferase | glycosyl |
| Peptidoglycan synthetase 2 | 2.4.1.129 | Transferase | glycosyl |
| Peptidoglycan synthetase 3 | 2.4.1.129 | Transferase | glycosyl |
| Lipid-A-disaccharide synthase | 2.4.1.182 | Transferase | glycosyl |
| Lipopolysaccharide 1,3-galactosyltransferase | 2.4.1.44 | Transferase | glycosyl |
| Lipopolysaccharide 1,2-glucosyltransferase | 2.4.1.58 | Transferase | glycosyl |
| Monofunctional biosynthetic peptidoglycan transglycosylase | 2.4.2.- | Transferase | glycosyl |
| 3-deoxy-D-manno-octulosonic-acid transferase | 2.4.99.12 | Transferase | glycosyl |
| 2-dehydro-3-deoxyphosphooctonate aldolase | 2.5.1.55 | Transferase | alkyl |
| UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 2.5.1.7 | Transferase | alkyl |
| Tetraacyldisaccharide 4'-kinase | 2.7.1.130 | Transferase | phosphorus |
| PTS system fructose-specific transporter | 2.7.1.69 | Transferase | phosphorus |
| PTS system glucose-specific transporter 1 | 2.7.1.69 | Transferase | phosphorus |
| PTS system glucose-specific transporter 2 | 2.7.1.69 | Transferase | phosphorus |
| PTS system L-ascorbate-specific transporter | 2.7.1.69 | Transferase | phosphorus |
| PTS system mannitol-specific transporter 1 | 2.7.1.69 | Transferase | phosphorus |
| PTS system mannitol-specific transporter 2 | 2.7.1.69 | Transferase | phosphorus |
| PTS system mannose-specific transporter | 2.7.1.69 | Transferase | phosphorus |
| PTS system N,N'-diacetylchitobiose-specific transporter 1 | 2.7.1.69 | Transferase | phosphorus |
| PTS system N,N'-diacetylchitobiose-specific transporter 2 | 2.7.1.69 | Transferase | phosphorus |
| PTS system transporter subunit IIA-like nitrogen-regulatory protein | 2.7.1.69 | Transferase | phosphorus |
| Chemotaxis protein | 2.7.13.3 | Transferase | phosphorus |
| Osmolarity sensor protein | 2.7.13.3 | Transferase | phosphorus |
| Phosphate regulon sensor protein | 2.7.13.3 | Transferase | phosphorus |
| Sensor protein 1 | 2.7.13.3 | Transferase | phosphorus |
| Sensor protein 2 | 2.7.13.3 | Transferase | phosphorus |
| Sensor protein 3 | 2.7.13.3 | Transferase | phosphorus |
| Signal transduction histidine-protein kinase | 2.7.13.3 | Transferase | phosphorus |
| Acetate kinase | 2.7.2.1 | Transferase | phosphorus |
| Nitrogen regulation protein | 2.7.3.- | Transferase | phosphorus |
| Two-component sensor kinase protein | 2.7.3.- | Transferase | phosphorus |
| Phosphoenolpyruvate-protein phosphotransferase | 2.7.3.9 | Transferase | phosphorus |
| 3-deoxy-manno-octulosonate cytidylyltransferase | 2.7.7.38 | Transferase | phosphorus |
| PII uridylyl-transferase | 2.7.7.59 | Transferase | phosphorus |
| Phospho-N-acetylmuramoyl-pentapeptide-transferase | 2.7.8.13 | Transferase | phosphorus |
| Chemotaxis-specific methylesterase | 3.1.1.61 | Hydrolase | Ester bond |
| Alkylphosphonate utilization operon protein PhnA | 3.11.1.2 | Hydrolase | phosphonoacetate |
| Isochorismatase | 3.3.2.1 | Hydrolase | Ether bond |
| D-alanyl-D-alanine carboxypeptidase 1 | 3.4.16.4 | Hydrolase | peptidase |
| D-alanyl-D-alanine carboxypeptidase 2 | 3.4.16.4 | Hydrolase | peptidase |
| Penicillin-binding protein | 3.4.16.4 | Hydrolase | peptidase |
| UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | 3.5.1.- | Hydrolase | linear amides |
| UDP pyrophosphate phosphatase | 3.6.1.27 | Hydrolase | acid anhydrides |
| UDP-2,3-diacylglucosamine hydrolase | 3.6.1.54 | Hydrolase | acid anhydrides |
| Phosphoenolpyruvate carboxylase | 4.1.1.31 | Lyase | Carbon-Carbon |
| Fructose 1,6-bisphosphate aldolase | 4.1.2.13 | Lyase | Carbon-Carbon |
| Citrate lyase | 4.1.3.6 | Lyase | Carbon-Carbon |
| Alanine racemase | 5.1.1.1 | Isomerase | Epimerases |
| Phosphoglyceromutase | 5.4.2.- | Isomerase | Intramolecular transfer |
| Isochorismate synthase | 5.4.99.6 | Isomerase | Intramolecular transfer |
| O-antigen ligase | 6.-.-.- | Ligase | Ligase |
| UDP-N-acetylmuramoyl-tripeptide—D-alanyl-D-alanine ligase | 6.3.2.10 | Ligase | Peptide Synthases |
| UDP-N-acetylmuramoylalanyl-D-glutamate—2,6-diaminopimelate ligase | 6.3.2.13 | Ligase | Peptide Synthases |
| UDP-N-acetylmuramate—L-alanine ligase | 6.3.2.8 | Ligase | Peptide Synthases |
| UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | 6.3.2.9 | Ligase | Peptide Synthases |
3. Characterization of the hypothetical proteins
Hypothetical proteins are those for which the sequences are available but their family and functional classification has not been established. As such they may represent unidentified drug targets [38, 39]. The computational methods (for e.g. Blast2GO, HMMscan, KEGG Automatic Annotation Server (KAAS), ProtParam server, PSORTb, SVMProt, etc) are effective in annotating the functional and family classes of the big number of hypothetical sequences present in bacterial genomes [40–42]. The functional classification may lead us to predict the mechanism of the possible metabolic pathway in which the protein is involved. In order to characterize the hypothetical proteins among the shortlisted COPs, we first looked how many proteins were hypothetical. We found out that there were 3,105 proteins in 73 COPs, out of which 114 proteins were hypothetical (Table 5). The identifier details of these 3,105 enzymes are provided in S6 Table.
Table 5. BLASTp of Hypothetical Proteins in non-homologous COPs in PDB.
| NCBI-GI | Protein name | COP # | KEGG Organism code | NCBI RefSeq ID | PDB Best Hit | ||
|---|---|---|---|---|---|---|---|
| PDB ID | Bit Score | Percent identity | |||||
| 161503125 | SARI_01190 | 4 | ses | NC_010067 | 1q16_A | 1247 | 95.1 |
| 161613744 | SPAB_01469 | 4 | spq | NC_010102 | 1q16_A | 1247 | 95.3 |
| 538362953 | BN855_24210 | 43 | senb | NC_022241 | 1xco_F | 313 | 46.6 |
| 378959111 | STBHUCCB_10250 | 49 | sex | NC_016832 | 2lp4_A | 227 | 84.1 |
| 538362544 | BN855_20090 | 49 | senb | NC_022241 | 2lp4_A | 227 | 84.1 |
| 161505779 | SARI_03955 | 50 | ses | NC_010067 | 1j6t_A | 146 | 97.9 |
| 161616759 | SPAB_04578 | 50 | spq | NC_010102 | 1j6t_A | 146 | 97.9 |
| 161504756 | SARI_02879 | 65 | ses | NC_010067 | 4kqr_B | 549 | 45.7 |
| 161612466 | SPAB_00156 | 65 | spq | NC_010102 | 4kqr_B | 549 | 45.7 |
| 161503040 | SARI_01104 | 67 | ses | NC_010067 | 4kqr_B | 539 | 43.0 |
| 161613654 | SPAB_01378 | 67 | spq | NC_010102 | 4kqr_B | 539 | 43.4 |
| 538362430 | BN855_18930 | 67 | senb | NC_022241 | 4kqr_B | 539 | 43.4 |
| 161503126 | SARI_01191 | 98 | ses | NC_010067 | 3ir7_B | 511 | 92.8 |
| 161613745 | SPAB_01470 | 98 | spq | NC_010102 | 3ir7_B | 511 | 93.2 |
| 161613976 | SPAB_01714 | 98 | spq | NC_010102 | 3ir7_B | 506 | 80.2 |
| 378984138 | STMDT12_C15970 | 98 | sem | NC_016860 | 3ir7_B | 506 | 80.0 |
| 538360694 | BN855_1290 | 113 | senb | NC_022241 | 1e8c_B | 471 | 92.4 |
| 538361709 | BN855_11640 | 119 | senb | NC_022241 | 1o2f_B | 90 | 93.3 |
| 161504450 | SARI_02563 | 139 | ses | NC_010067 | No hit | - | - |
| 161615466 | SPAB_03237 | 139 | spq | NC_010102 | No hit | - | - |
| 538362753 | BN855_22200 | 140 | senb | NC_022241 | 4i5s_B | 226 | 30.5 |
| 378961722 | STBHUCCB_37440 | 152 | sex | NC_016832 | 4i5s_B | 224 | 31.3 |
| 538364097 | BN855_35800 | 160 | senb | NC_022241 | 1bxd_A | 161 | 90.7 |
| 29144086 | t3806 | 166 | stt | NC_004631 | No hit | - | - |
| 62182206 | SC3636 | 166 | sec | NC_006905 | No hit | - | - |
| 161505752 | SARI_03928 | 166 | ses | NC_010067 | No hit | - | - |
| 161616791 | SPAB_04610 | 166 | spq | NC_010102 | No hit | - | - |
| 488656245 | TY21A_19335 | 166 | sent | NC_021176 | No hit | - | - |
| 378959497 | STBHUCCB_14250 | 179 | sex | NC_016832 | 4i5s_B | 222 | 27.9 |
| 538360809 | BN855_2450 | 218 | senb | NC_022241 | No hit | - | - |
| 161504097 | SARI_02195 | 221 | ses | NC_010067 | No hit | - | - |
| 161615026 | SPAB_02786 | 221 | spq | NC_010102 | No hit | - | - |
| 161505743 | SARI_03919 | 226 | ses | NC_010067 | 2iw1_A | 374 | 85.8 |
| 161616800 | SPAB_04619 | 226 | spq | NC_010102 | 2iw1_A | 374 | 86.4 |
| 538364332 | BN855_38170 | 226 | senb | NC_022241 | 2iw1_A | 374 | 86.1 |
| 378962383 | STBHUCCB_44400 | 246 | sex | NC_016832 | 4i5s_B | 225 | 29.8 |
| 538364321 | BN855_38060 | 261 | senb | NC_022241 | 1psw_A | 346 | 92.5 |
| 161505747 | SARI_03923 | 271 | ses | NC_010067 | 1ss9_A | 273 | 26.0 |
| 161616796 | SPAB_04615 | 271 | spq | NC_010102 | 1ga8_A | 273 | 26.0 |
| 161505748 | SARI_03924 | 272 | ses | NC_010067 | 3tzt_B | 252 | 27.0 |
| 161616795 | SPAB_04614 | 272 | spq | NC_010102 | 3tzt_B | 252 | 25.8 |
| 161504449 | SARI_02562 | 273 | ses | NC_010067 | No hit | - | - |
| 161615465 | SPAB_03236 | 273 | spq | NC_010102 | No hit | - | - |
| 378960680 | STBHUCCB_26520 | 273 | sex | NC_016832 | No hit | - | - |
| 379699575 | STM474_0375 | 273 | seb | NC_016857 | No hit | - | - |
| 538361476 | BN855_9270 | 282 | senb | NC_022241 | 4itn_A | 316 | 27.5 |
| 161503046 | SARI_01110 | 285 | ses | NC_010067 | 2jzh_A | 170 | 94.7 |
| 161613660 | SPAB_01384 | 285 | spq | NC_010102 | 2jzh_A | 170 | 95.3 |
| 378959115 | STBHUCCB_10290 | 321 | sex | NC_016832 | 1af7_A | 274 | 99.3 |
| 161504232 | SARI_02339 | 326 | ses | NC_010067 | 2fq1_B | 285 | 87.4 |
| 161615198 | SPAB_02966 | 326 | spq | NC_010102 | 2fq1_B | 285 | 88.1 |
| 538361140 | BN855_5890 | 326 | senb | NC_022241 | 2fq1_B | 285 | 88.4 |
| 538363806 | BN855_32850 | 333 | senb | NC_022241 | No hit | - | - |
| 161505744 | SARI_03920 | 339 | ses | NC_010067 | No hit | - | - |
| 161616799 | SPAB_04618 | 339 | spq | NC_010102 | No hit | - | - |
| 538364331 | BN855_38160 | 339 | senb | NC_022241 | No hit | - | - |
| 528818715 | SN31241_20010 | 361 | senn | NC_021902 | 1vh1_D | 480 | 94 |
| 378960112 | STBHUCCB_20620 | 361 | sex | NC_016832 | 1vh1_D | 479 | 94 |
| 16759510 | Conserved | 372 | sty | NC_003198 | No hit | - | - |
| 56414314 | SPA2188 | 372 | spt | NC_006511 | No hit | - | - |
| 378698495 | SL1344_0528 | 372 | sey | NC_016810 | No hit | - | - |
| 378956078 | SPUL_2424 | 372 | sel | NC_016831 | No hit | - | - |
| 378444035 | None | 372 | sev | NC_016854 | No hit | - | - |
| 383495341 | UMN798_0581 | 372 | sef | NC_017046 | No hit | - | - |
| 537437644 | SPUCDC_2410 | 372 | seg | NC_022221 | No hit | - | - |
| 549723245 | Conserved | 372 | senr | NC_022544 | No hit | - | - |
| 550899973 | Conserved | 372 | send | NC_022569 | No hit | - | - |
| 525841289 | CFSAN001921_21865 | 384 | set | NC_021814 | No hit | - | - |
| 525860398 | CFSAN002050_25550 | 384 | seeb | NC_021818 | No hit | - | - |
| 526221794 | SE451236_02340 | 384 | seen | NC_021820 | No hit | - | - |
| 525949065 | SEEB0189_01285 | 384 | see | NC_021844 | No hit | - | - |
| 529222678 | I137_18460 | 384 | seep | NC_021984 | No hit | - | - |
| 549482315 | IA1_18065 | 384 | sene | NC_022525 | No hit | - | - |
| 161502511 | SARI_00555 | 465 | ses | NC_010067 | 3oxp_B | 147 | 44.2 |
| 161612923 | SPAB_00629 | 465 | spq | NC_010102 | 3oxp_B | 147 | 44.2 |
| 378984906 | STMDT12_C23650 | 465 | sem | NC_016860 | 3oxp_B | 147 | 44.2 |
| 16767539 | STM4289 | 498 | stm | NC_003197 | 2akl_A | 110 | 68.2 |
| 16762971 | Conserved | 498 | sty | NC_003198 | 2akl_A | 110 | 68.2 |
| 29144458 | t4196 | 498 | stt | NC_004631 | 2akl_A | 110 | 68.2 |
| 56416088 | SPA4107 | 498 | spt | NC_006511 | 2akl_A | 110 | 68.2 |
| 62182738 | SC4168 | 498 | sec | NC_006905 | 2akl_A | 110 | 68.2 |
| 161505231 | SARI_03369 | 498 | ses | NC_010067 | 2akl_A | 92 | 66.3 |
| 161617431 | SPAB_05288 | 498 | spq | NC_010102 | 2akl_A | 110 | 68.2 |
| 194444767 | SNSL254_A4635 | 498 | seeh | NC_011080 | 2akl_A | 110 | 68.2 |
| 194448085 | SeHA_C4635 | 498 | seh | NC_011083 | 2akl_A | 110 | 68.2 |
| 194735822 | SeSA_A4544 | 498 | sew | NC_011094 | 2akl_A | 110 | 68.2 |
| 197365014 | SSPA3814 | 498 | sek | NC_011147 | 2akl_A | 110 | 68.2 |
| 197249113 | SeAg_B4551 | 498 | sea | NC_011149 | 2akl_A | 110 | 68.2 |
| 198243014 | SeD_A4684 | 498 | sed | NC_011205 | 2akl_A | 110 | 68.2 |
| 205355060 | SG4134 | 498 | sega | NC_011274 | 2akl_A | 110 | 67.3 |
| 207859443 | SEN4060 | 498 | setc | NC_011294 | 2akl_A | 110 | 67.3 |
| 224586054 | SPC_4352 | 498 | sei | NC_012125 | 2akl_A | 110 | 68.2 |
| 378702132 | SL1344_4226 | 498 | sey | NC_016810 | 2akl_A | 110 | 68.2 |
| 378957845 | SPUL_4281 | 498 | sel | NC_016831 | 2akl_A | 110 | 67.3 |
| 378962381 | STBHUCCB_44380 | 498 | sex | NC_016832 | 2akl_A | 110 | 68.2 |
| 378447608 | None | 498 | sev | NC_016854 | 2akl_A | 110 | 68.2 |
| 378453234 | STM14_5159 | 498 | seo | NC_016856 | 2akl_A | 110 | 68.2 |
| 378986964 | STMDT12_C44240 | 498 | sem | NC_016860 | 2akl_A | 110 | 68.2 |
| 378991557 | STMUK_4274 | 498 | sej | NC_016863 | 2akl_A | 110 | 68.2 |
| 383498867 | UMN798_4648 | 498 | sef | NC_017046 | 2akl_A | 110 | 68.2 |
| 452121975 | CFSAN001992_12425 | 498 | senj | NC_020307 | 2akl_A | 110 | 68.2 |
| 482906826 | STU288_21535 | 498 | setu | NC_021151 | 2akl_A | 110 | 68.2 |
| 488656631 | TY21A_21335 | 498 | sent | NC_021176 | 2akl_A | 110 | 68.2 |
| 525815577 | SEEH1578_07620 | 498 | seec | NC_021810 | 2akl_A | 110 | 68.2 |
| 525828145 | CFSAN002069_10645 | 498 | senh | NC_021812 | 2akl_A | 110 | 68.2 |
| 525839753 | CFSAN001921_18970 | 498 | set | NC_021814 | 2akl_A | 110 | 68.2 |
| 525856209 | CFSAN002050_04690 | 498 | seeb | NC_021818 | 2akl_A | 110 | 68.2 |
| 526218734 | SE451236_04480 | 498 | seen | NC_021820 | 2akl_A | 110 | 68.2 |
| 525948743 | SEEB0189_20995 | 498 | see | NC_021844 | 2akl_A | 110 | 68.2 |
| 529221780 | I137_20500 | 498 | seep | NC_021984 | 2akl_A | 110 | 67.3 |
| 537439413 | SPUCDC_4267 | 498 | seg | NC_022221 | 2akl_A | 110 | 67.3 |
| 549481441 | IA1_20890 | 498 | sene | NC_022525 | 2akl_A | 110 | 68.2 |
| 549726803 | Conserved | 498 | senr | NC_022544 | 2akl_A | 110 | 68.2 |
| 550903633 | Conserved | 498 | send | NC_022569 | 2akl_A | 110 | 68.2 |
Later on, we performed a BLASTp search using 114 hypothetical sequences as ‘query’ and sequences of PDB as ‘database’. It was performed so that if there is any homology in already well characterized PDB database then it may lead us to classify the hypothetical proteins. The BLASTp showed hits against 81 queries with the PDB database while rest (i.e. 33) queries showed no hits (Table 5). The names of obtained hits for 81 queries were manually matched with the corresponding 24 COPs. The leftover 33 queries for which no similarity was found in PDB database were subjected to the bioinformatics tools i.e SVM–Prot and InterProScan. The obtained results for the 33 ‘no hits’ were confirmed by matching their names with the respective COPs. All results verified the output of CD-HIT clustering.
4. Validation from the literature
A similar study was performed by Becker et. al. using experimental techniques, so we have compared our results obtained from in silico approach. We also looked in the DrugBank of the possible entry of any drug target(s) against Salmonella. The DrugBank [35] reported 19 drug targets of S. enterica. 11 out of 19 belonged to the human, while remaining 8 belonged to the bacteria. The oxygen-insensitive NADPH Nitro reductase was common in 35 strains only. Other five did not belong to UMP. Only one (i.e. Penicillin-binding protein) out of 8 genes was present in the output of current strategy. Results are summarized in Table 6. Becker and his coworkers [20] have reported 155 essential enzymes for S. enterica serovar Typhimurium strain LT2, and compared those with various strains of S. enterica by performing extensive experimental study. We compared our identified 73 enzymes with the results of Becker and observed that 24 enzymes were shared by the reports of Becker et. al. (Table 3). Furthermore, the enzyme CheA (Chemotaxis Protein, COP # 49) was found as essential in current study while Backer et. al. suggested it as non-essential. This discrepancy may arise due to the recent updates in the DEG.
Table 6. S. enterica eight genes as drug targets–data from DrugBank.
| Genes | Molecule | Output | Reason |
|---|---|---|---|
| 16S rRNA | Nucleic Acid | excluded | Not the aim |
| 30S ribosomal protein S10 | Protein | X | Not in UMPs of SE |
| 30S ribosomal protein S12 | Protein | X | Not in UMPs of SE |
| DNA gyrase subunit A | Enzyme | X | Not in UMPs of SE |
| DNA topoisomerase 4 subunit A | Enzyme | X | Not in UMPs of SE |
| Oxygen-insensitive NADPH nitroreductase | Enzyme | X | In 35/42 strains |
| Penicillin-binding protein 2 | Enzyme | present | Included |
| Probable pyruvate-flavodoxin oxidoreductase | Enzyme | X | Not in UMPs of SE |
Conclusion
We have performed extensive computational analysis of S. enterica at the level of core proteome to identify new potential drug targets. Subtractive proteomics through a novel approach was applied, i.e. by considering only proteins of the unique metabolic pathways of the pathogens and mining the proteomic data of all completely sequenced strains of the pathogen, thus improving the quality and application of the results. We identified 73 enzymes that are common to 42 strains of S. enterica, belong to unique metabolic pathways, are essential for pathogen survival and which have no human homologs. These four characteristics suggest that the enzymes are potential drug targets and should be tested experimentally. We compared them to experimental data [Becker et. al] showing that 24 out of the 73 (~33%) enzymes are current drug targets. The remaining 49 enzymes are new potential drug targets. We have annotated the function of 114 hypothetical proteins unique to S. enterica, providing additional new potential drug targets. Finally, our organization of the available core proteomic data (available in S2, S4, S5 and S6 Tables) in different categories e.g. clusters, organism codes, NCBI RefSeq IDs etc, provide a basis for further studies.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
The authors would like to gratefully acknowledge the Higher Education Commission of Pakistan to provide fellowship during the study.
Abbreviations
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- CD-HIT
Cluster Database at High Identity with Tolerance
- DEG
Database of Essential Genes
- UMP
Unique Metabolic Pathways
- SVM
Support Vector Machine
- KO
KEGG Orthology
- FTP
File Transfer Protocol
- NCBI-GI
National Center for Biotechnology Information—GenInfo Identifier
- COP
Cluster of Proteins
- API
Program Interface
- BLAST
Basic Local Alignment Search Tool
- BLASTp
Protein-Protein BLAST
- VFDB
Virulence Factors Database
- PDB
Protein Databank
- SE
Salmonella enterica
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by International Foundation for Science (IFS) grant# F/5378-1. The authors would also like to gratefully acknowledge the Higher Education Commission of Pakistan for providing fellowship during the study.
References
- 1.Popoff MY, Bockemuhl J, Gheesling LL. Supplement 2001 (no. 45) to the Kauffmann-White scheme. Research in microbiology. 2003;154(3):173–4. Epub 2003/04/23. 10.1016/s0923-2508(03)00025-1 . [DOI] [PubMed] [Google Scholar]
- 2.Betancor L, Yim L, Martinez A, Fookes M, Sasias S, Schelotto F, et al. Genomic Comparison of the Closely Related Salmonella enterica Serovars Enteritidis and Dublin. The open microbiology journal. 2012;6:5–13. Epub 2012/03/01. 10.2174/1874285801206010005 ; PubMed Central PMCID: PMCPmc3282883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunology and cell biology. 2007;85(2):112–8. Epub 2006/12/06. 10.1038/sj.icb.7100007 . [DOI] [PubMed] [Google Scholar]
- 4.Sun JS, Hahn TW. Comparative proteomic analysis of Salmonella enterica serovars Enteritidis, Typhimurium and Gallinarum. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2012;74(3):285–91. Epub 2011/10/15. . [DOI] [PubMed] [Google Scholar]
- 5.Fierer J, Guiney DG. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. The Journal of clinical investigation. 2001;107(7):775–80. Epub 2001/04/04. 10.1172/jci12561 ; PubMed Central PMCID: PMCPmc199580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella—all in the name of entry. Current opinion in microbiology. 2005;8(1):10–5. Epub 2005/02/08. 10.1016/j.mib.2004.09.001 . [DOI] [PubMed] [Google Scholar]
- 7.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nature reviews Microbiology. 2008;6(1):53–66. Epub 2007/11/21. 10.1038/nrmicro1788 . [DOI] [PubMed] [Google Scholar]
- 8.Wangdi T, Winter SE, Baumler AJ. Typhoid fever: "you can't hit what you can't see". Gut microbes. 2012;3(2):88–92. Epub 2011/12/14. 10.4161/gmic.18602 ; PubMed Central PMCID: PMCPmc3370952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter PB, Collins FM. The route of enteric infection in normal mice. The Journal of experimental medicine. 1974;139(5):1189–203. Epub 1974/05/01. ; PubMed Central PMCID: PMCPmc2139651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastroeni P, Grant A. Dynamics of spread of Salmonella enterica in the systemic compartment. Microbes and infection / Institut Pasteur. 2013;15(13):849–57. Epub 2013/11/05. 10.1016/j.micinf.2013.10.003 . [DOI] [PubMed] [Google Scholar]
- 11.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial "pan-genome". Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13950–5. Epub 2005/09/21. 10.1073/pnas.0506758102 ; PubMed Central PMCID: PMCPmc1216834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng X, Phillippy AM, Li Z, Salzberg SL, Zhang W. Probing the pan-genome of Listeria monocytogenes: new insights into intraspecific niche expansion and genomic diversification. BMC genomics. 2010;11:500 Epub 2010/09/18. 10.1186/1471-2164-11-500 ; PubMed Central PMCID: PMCPmc2996996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donati C, Hiller NL, Tettelin H, Muzzi A, Croucher NJ, Angiuoli SV, et al. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome biology. 2010;11(10):R107 Epub 2010/11/03. 10.1186/gb-2010-11-10-r107 ; PubMed Central PMCID: PMCPmc3218663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao P, Zheng H, Yu Y, Ding G, Gu W, Chen S, et al. Complete sequencing and pan-genomic analysis of Lactobacillus delbrueckii subsp. bulgaricus reveal its genetic basis for industrial yogurt production. PloS one. 2011;6(1):e15964 Epub 2011/01/26. 10.1371/journal.pone.0015964 ; PubMed Central PMCID: PMCPmc3022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, et al. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. Journal of bacteriology. 2008;190(20):6881–93. Epub 2008/08/05. 10.1128/jb.00619-08 ; PubMed Central PMCID: PMCPmc2566221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilburn TG, Cai H, Gu J, editors. The Core and Pan-Genome of the Vibrionaceae. Bioinformatics, Systems Biology and Intelligent Computing, 2009 IJCBS'09 International Joint Conference on; 2009: IEEE.
- 17.Yang L, Tan J, O'Brien EJ, Monk JM, Kim D, Li HJ, et al. Systems biology definition of the core proteome of metabolism and expression is consistent with high-throughput data. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(34):10810–5. 10.1073/pnas.1501384112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Xiao D, Pang B, Zhang Q, Zhou H, Zhang L, et al. The core proteome and pan proteome of Salmonella Paratyphi A epidemic strains. PloS one. 2014;9(2):e89197 Epub 2014/03/04. 10.1371/journal.pone.0089197 ; PubMed Central PMCID: PMCPmc3933413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen A, Hendriksen RS, Aaresturp FM, Ussery DW, Friis C. The Salmonella enterica pan-genome. Microbial ecology. 2011;62(3):487–504. Epub 2011/06/07. 10.1007/s00248-011-9880-1 ; PubMed Central PMCID: PMCPmc3175032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker D, Selbach M, Rollenhagen C, Ballmaier M, Meyer TF, Mann M, et al. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature. 2006;440(7082):303–7. Epub 2006/03/17. 10.1038/nature04616 . [DOI] [PubMed] [Google Scholar]
- 21.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic acids research. 2014;42(Database issue):D199–205. Epub 2013/11/12. 10.1093/nar/gkt1076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCBI. NCBI FTP server 2013 [cited 2013 December 21]. Available: ftp://ftp.ncbi.nlm.nih.gov/genomes/Bacteria/.
- 23.Hallam S. Fast Blast 2013 [cited December, 2014]. Available: http://www.cmde.science.ubc.ca/hallam/fastblast.php.
- 24.Luo H, Lin Y, Gao F, Zhang CT, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic acids research. 2014;42(Database issue):D574–80. Epub 2013/11/19. 10.1093/nar/gkt1131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics (Oxford, England). 2012;28(23):3150–2. Epub 2012/10/13. 10.1093/bioinformatics/bts565 ; PubMed Central PMCID: PMCPmc3516142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HCV-Sequence-Database. ElimDupes [December, 2014]. Available: http://hcv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html.
- 27.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC bioinformatics. 2009;10:421 Epub 2009/12/17. 10.1186/1471-2105-10-421 ; PubMed Central PMCID: PMCPmc2803857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NCBI. NCBI FTP server 2014 [updated January 6; cited 2014 January 11]. Available: ftp://ftp.ncbi.nlm.nih.gov/refseq/H_sapiens/mRNA_Prot/.
- 29.Stajich JE, Block D, Boulez K, Brenner SE, Chervitz SA, Dagdigian C, et al. The Bioperl toolkit: Perl modules for the life sciences. Genome research. 2002;12(10):1611–8. Epub 2002/10/09. 10.1101/gr.361602 ; PubMed Central PMCID: PMCPmc187536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Xiong Z, Sun L, Yang J, Jin Q. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic acids research. 2012;40(Database issue):D641–5. Epub 2011/11/10. 10.1093/nar/gkr989 ; PubMed Central PMCID: PMCPmc3245122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose PW, Bi C, Bluhm WF, Christie CH, Dimitropoulos D, Dutta S, et al. The RCSB Protein Data Bank: new resources for research and education. Nucleic acids research. 2013;41(Database issue):D475–82. Epub 2012/11/30. 10.1093/nar/gks1200 ; PubMed Central PMCID: PMCPmc3531086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PDB. RCSB PDB FTP server 2014 [updated October 1; cited 2014 January 18]. Available: ftp://ftp.wwpdb.org/pub/pdb/derived_data/.
- 33.Cai CZ, Han LY, Ji ZL, Chen X, Chen YZ. SVM-Prot: Web-based support vector machine software for functional classification of a protein from its primary sequence. Nucleic acids research. 2003;31(13):3692–7. Epub 2003/06/26. ; PubMed Central PMCID: PMCPmc169006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis Tool Web Services from the EMBL-EBI. Nucleic acids research. 2013;41(Web Server issue):W597–600. Epub 2013/05/15. 10.1093/nar/gkt376 ; PubMed Central PMCID: PMCPmc3692137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, et al. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic acids research. 2011;39(Database issue):D1035–41. Epub 2010/11/10. 10.1093/nar/gkq1126 ; PubMed Central PMCID: PMCPmc3013709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang da W, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic acids research. 2007;35(Web Server issue):W169–75. Epub 2007/06/20. 10.1093/nar/gkm415 ; PubMed Central PMCID: PMCPmc1933169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen P, Alessi DR. Kinase drug discovery—what's next in the field? ACS chemical biology. 2013;8(1):96–104. Epub 2013/01/02. 10.1021/cb300610s ; PubMed Central PMCID: PMCPmc4208300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teh BA, Choi SB, Musa N, Ling FL, Cun ST, Salleh AB, et al. Structure to function prediction of hypothetical protein KPN_00953 (Ycbk) from Klebsiella pneumoniae MGH 78578 highlights possible role in cell wall metabolism. BMC structural biology. 2014;14:7 Epub 2014/02/07. 10.1186/1472-6807-14-7 ; PubMed Central PMCID: PMCPmc3927764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naqvi AA, Shahbaaz M, Ahmad F, Hassan MI. Identification of Functional Candidates amongst Hypothetical Proteins of Treponema pallidum ssp. pallidum. PloS one. 2015;10(4):e0124177 Epub 2015/04/22. 10.1371/journal.pone.0124177 ; PubMed Central PMCID: PMCPmc4403809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravooru N, Ganji S, Sathyanarayanan N, Nagendra HG. Insilico analysis of hypothetical proteins unveils putative metabolic pathways and essential genes in Leishmania donovani. Frontiers in genetics. 2014;5:291 Epub 2014/09/11. 10.3389/fgene.2014.00291 ; PubMed Central PMCID: PMCPmc4144268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahbaaz M, Hassan MI, Ahmad F. Functional annotation of conserved hypothetical proteins from Haemophilus influenzae Rd KW20. PloS one. 2013;8(12):e84263 Epub 2014/01/07. 10.1371/journal.pone.0084263 ; PubMed Central PMCID: PMCPmc3877243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui T, Zhang L, Wang X, He ZG. Uncovering new signaling proteins and potential drug targets through the interactome analysis of Mycobacterium tuberculosis. BMC genomics. 2009;10:118 Epub 2009/03/21. 10.1186/1471-2164-10-118 ; PubMed Central PMCID: PMCPmc2671525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.