Abstract
Purpose
The ARIBON trial is a double blind, randomised, placebo controlled study designed to evaluate the impact of ibandronate on bone mineral density (BMD) in women taking anastrozole for adjuvant treatment of breast cancer.
Methods
131 postmenopausal women with early breast cancer were recruited to the study. Of these, 13 had osteoporosis, 50 osteopenia and 68 normal BMD. Patients with osteoporosis at baseline were treated with monthly oral ibandronate 150 mg for 5 years; osteopenic patients were randomised to receive either ibandronate or placebo for two years and offered open label ibandronate depending upon the results of their 2-year BMD result.
Results
Of the 20 patients with osteopenia who were randomised to ibandronate and evaluable at the 2 year visit, 17/20 were not offered a bisphosphonate and the improvements in BMD accrued during the first 2 years were lost both at the LS (−3.21%) and TH (−5.0%). Of the 16 patients randomised to placebo 8/16 with high rates of bone loss during years 0–2 received ibandronate over the next 3 years with improvements in BMD of +5.01 and +1.19 at the LS and TH respectively. The 8 patients who were not offered a bisphosphonate experienced relatively little change in BMD throughout the 5 years of the study (LS +0.15%, TH −2.72%). BMD increased steadily in the 9/13 patients initially identified as having osteoporosis (LS +9.65%, TH +2.72%).
Conclusions
Monthly oral ibandronate provides an option to clinicians considering use of a bisphosphonate to prevent bone loss during aromatase inhibitor therapy.
Keywords: Osteoporosis, Breast, Aromatase inhibitor, Bone, Cancer
1. Introduction
Following the results of several large randomised trials of endocrine treatment strategies, adjuvant treatment with an aromatase inhibitor (AI) has become part of standard care for most post-menopausal patients with oestrogen receptor positive (ER+) breast cancer [1], [2], [3], [4], [5]. Whilst the side effect profile of AIs is generally favourable in comparison to tamoxifen, with fewer thrombo-embolic and gynaecological adverse events, musculoskeletal side effects including accelerated bone loss and arthralgias are more frequent with the AIs.
In the ATAC trial bone sub-protocol, patients treated with anastrozole lost on average 2.3%, 4.0% and 6.1% bone mineral density (BMD) at the lumbar spine (LS) and 1.5%, 3.9% and 7.2% at the hip (TH) after 1, 2 and 5 years respectively (p<0.001) [6]. This degree of bone loss is much higher than the normal rate of decline in postmenopausal women of 0.6–1.0% per year [7]. Similar effects have been seen with letrozole [8] and exemestane [9]. Additionally, the effects of AIs on bone turnover and the associated increased bone loss results in a 40–50% increase in on treatment fracture rate compared to the use of tamoxifen [10]. In order to prevent clinically important adverse bone events, a number of guidelines have been developed providing advice on how to monitor patients, assess the risk of fragility fractures and treat at risk patients with bone targeted drugs to prevent bone loss [11], [12], [13], [14].
Bisphosphonates are the cornerstone of management in postmenopausal osteoporosis with randomised trial evidence of reductions in vertebral fractures for ibandronate [15] and both vertebral and non-vertebral fracture rates for alendronate [16], risedronate [17], and zoledronic acid [18]. Ibandronate is a highly potent nitrogen containing bisphosphonate that can be administered both intravenously and orally. Using the oral route of administration, a single dose every month in post menopausal women with osteoporosis led to similar changes in BMD and bone turnover markers as daily treatment [19]. This simple treatment is potentially highly attractive for cancer patients and offers an alternative to 6 monthly intravenous treatment with zoledronic acid [20], [21] or weekly oral therapy with risedronate [22].
Given the concerns about the long-term effects of anastrozole on bone health, the ARIBON study was designed to evaluate the effect of bisphosphonate treatment on patients considered at risk for the development of osteoporosis during 5 years of anastrazole therapy. The 2-year results have been published previously [23] and showed that oral ibandronate was able to prevent bone loss and reduce markers of bone turnover in patients with osteopenia and osteoporosis. Here we present the final 5 year study results.
2. Methods
131 postmenopausal women with a histologically confirmed diagnosis of oestrogen receptor positive breast cancer from two cancer centres (Sheffield and Leeds) were recruited. Details of the study eligibility and methods have been published previously [23]. Briefly, patients were excluded if menopause had been induced by either prior chemotherapy or drug therapy (e.g. goserelin). Other exclusion criteria included concurrent administration of medications with effects on bone, abnormal renal function, disorders of bone metabolism and previous bilateral hip fractures or bilateral hip prostheses that would have hindered reliable BMD assessments.
Following informed consent, patients underwent a baseline assessment of BMD at the LS and TH before commencing anastrozole and study entry. All patients received anastrozole 1 mg once a day and calcium (500 mg) and vitamin D (400 IU) supplements daily. Patients with normal BMD (T score >−1 at both the LS and TH) were allocated to an observation group with a follow-up BMD assessment at 2 years. No further protocol-directed follow-up of these patients after year 2 was required, and they were treated according to current clinical guidelines at the two participating institutions. Patients with osteoporosis (T score <−2.5 at either the LS or TH) received open label ibandronate 150 mg every 28 day by mouth for the 5 years of the study. Patients classified as osteopenic (T-scores of >−2.5 and <−1.0 at either the LS and TH) were randomly assigned in a double blind manner on a 1:1 basis to receive either ibandronate tablets 150 mg every 28 day or placebo tablets of identical appearance also every 28 day for two years. Ibandronate/placebo capsules were taken in an upright position first thing in the morning on an empty stomach and washed down with 100 mls water to minimise the risk of oesophageal irritation; no food or drink (other than water) was consumed for at least 30 min after taking the study medication.
LS and TH BMD scans were repeated after 1 year (12±1 month), 2 years (24±2 months) and 5 years (60±2 months). Throughout the study the same densitometer was used at each of the two centres to optimise the accuracy of measurements. A phantom spine was scanned on a minimum of three days per week in each centre to ensure precision over time. T-score results were calculated using the Lunar (DPX Series Operator's Manual 1998) manufacturer's reference ranges for the lumbar spine (L2-L4) and the NHANES III reference range for the total hip region.
Patients (n=5) who developed osteoporosis whilst taking ibandronate/placebo medication were un-blinded and offered open label ibandronate treatment. After 2 years the randomised treatment was stopped and the treating clinician, depending upon the results of the BMD result at 2 years, offered treatment with open label ibandronate.
Local ethics committee approval was obtained at both centres prior to commencing recruitment. The study was sponsored by the University of Sheffield, and funded through unrestricted academic grants from both Roche and Astra Zeneca who also provided ibandronate/placebo and anastrazole study medications respectively. All data were held within the Cancer Clinical Trials Centre at the University of Sheffield. All analyses were performed by the investigators. Neither pharmaceutical company had any input into the analysis or reporting of results. The ARIBON study is registered on the UK department of health national research register. Publication ID N0276137347 (http://www.nrr.nhs.uk).
3. Statistical power and analysis
A description of the statistical analysis of the study has been published previously [23]. For the analysis of the results at the 5-year time point, comparisons were expressed graphically using 95% confidence intervals. Statistical analysis was performed using SPSS version 19.0.0, 2010.
4. Results
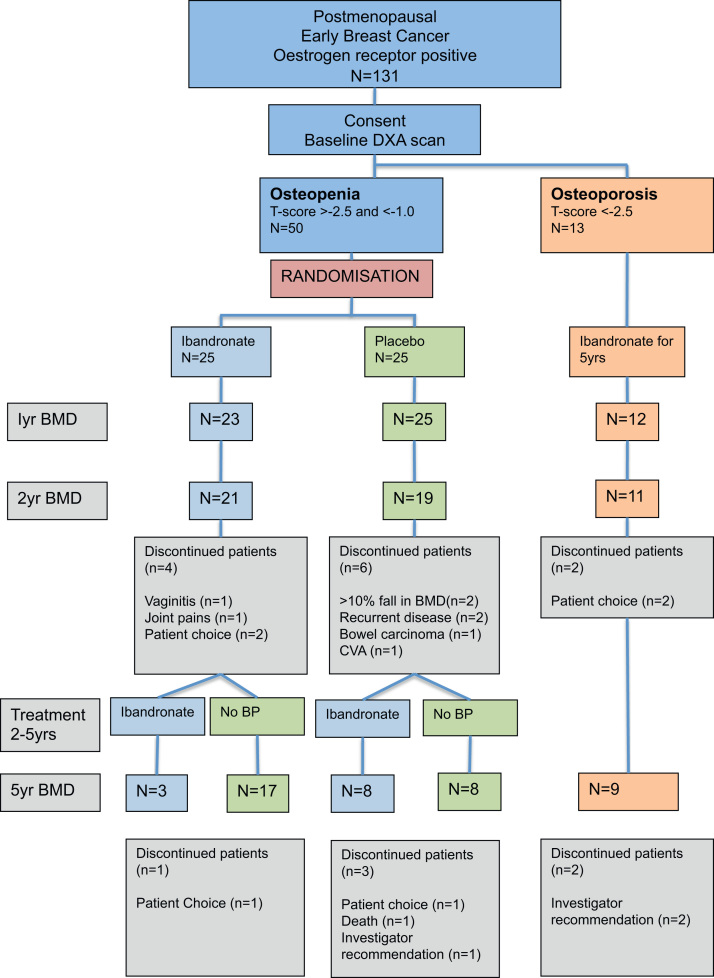
Between December 2003 and October 2005, 131 postmenopausal, surgically treated breast cancer patients were recruited from oncology clinics in Leeds and Sheffield, UK. A total of 50 recruited patients were identified as having osteopenia at the time of the baseline scan. 68 had normal BMD and 13 had osteoporosis prior to starting anastrozole. The CONSORT diagram in Fig. 1 shows the disposition of patients at baseline and their subsequent course on study. The baseline characteristics of the patients recruited to the trial have been previously published [23]. The median age of the study population was 64.8 years and the stratification factors (centre, time from menopause, previous HRT and previous chemotherapy) for the randomised patients were well balanced between the two treatment groups.
Fig. 1.
Consort diagram showing the number of patients recruited and those included in the analysis.
5. BMD results
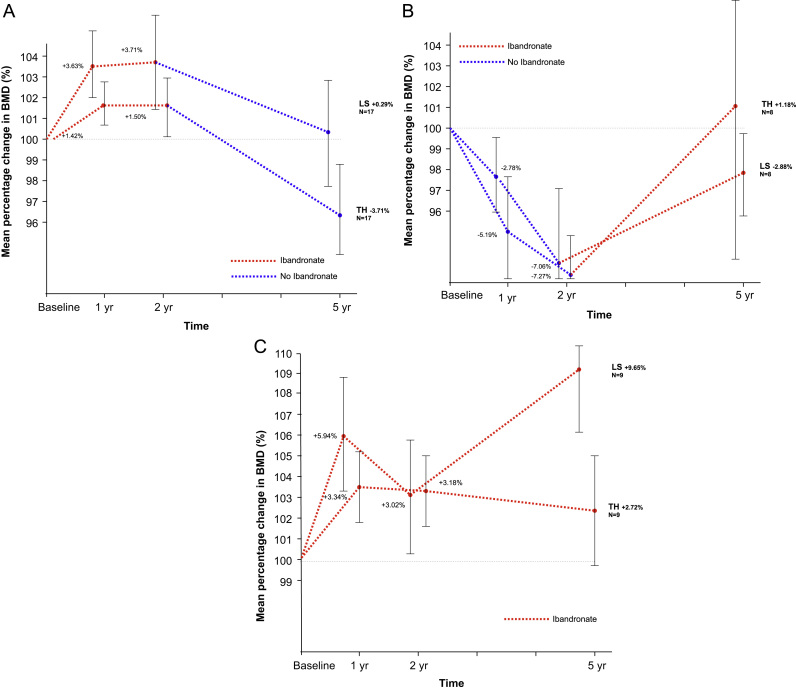
The BMD changes from baseline for the osteopenic and osteoporotic patients are shown in Fig. 2a–c and Table 1.
Fig. 2.
Mean percentage change in LS and TH BMD from baseline (95% Confidence Intervals). (A) Ibandronate-Placebo. (B) Placebo-Ibandronate. (C) Osteoporosis.
Table 1.
BMD Changes for the 5 years of the study.
| Treatment 0–2yrs | Treatment 2–5yrs | N |
Mean % change from baseline (Range) |
||||||
| 0–1 yr | 0–2 yrs | 2–5 yrs | 0–5 yrs | ||||||
| Osteopenic | Randomisation | Ibandronate | Ibandronate | 3 | LS | +0.23 (−3.8,+2.3) | +0.10 (−8.9,+10.3) | +2.33 (−3.7,+7.3) | +2.60 (−7.8,+18.4) |
| TH | −0.10 (−2.1,+3.4) | −2.95 (−9.0,+3.1) | −3.08 (−6.6,+0.1) | −6.07 (−8.9,−3.7) | |||||
| No BP | 17 | LS | +3.63 (−2.3,+15.1) | +3.71 (−6.1,+19.9) | −3.21 (−10.9,+5.6) | +0.29 (−10.4,+17.6) | |||
| TH | +1.42 (−4.1,+5.6) | +1.50 (−5.8,+6.9) | −5.00 (−19.9,+0.0) | −3.71 (−16.5,+3.5) | |||||
| Placebo | Ibandronate | 8 | LS | −2.77 (−6.3,+2.0) | −7.06 (−16.0,+0.7) | +5.01 (−4.3,+17.0) | −2.88 (−15.4,+17.7) | ||
| TH | −5.19 (−9.9,-0.4) | −7.27 (−12.3,-4.0) | +1.19 (−5.5,+5.5) | −6.22 (−10.3,−0.1) | |||||
| No BP | 8 | LS | −1.88 (−4.7,+0.5) | −1.00 (−4.4, +3.3) | +1.22 (−4.3,+9.8) | +0.15 (−3.5,+5.0) | |||
| TH | −0.41 (−5.2,+5.2) | −0.52 (−5.9,+7.2) | −1.82 (−5.4,+1.5) | −2.72 (−5.6,+2.1) | |||||
| Osteoporosis | Ibandronate for 5 yrs | 9 | LS | +5.94 (−+0.7,+15.2) | +3.03 (−2.3,+12.1) | +6.43 (−+2.1,+13.5) | +9.65 (−+4.9,+18.9) | ||
| TH | +3.34 (−0.4,+8.5) | +3.18 (−3.7,+8.1) | −0.46 (−2.5,+5.9) | +2.72 (−4.0,+9.6) | |||||
BP=Bisphosphonate.
Osteopenic patients (ibandronate group): Of the 20 patients randomised to ibandronate and evaluable at the 2 year visit, BMD increased in the majority (13/20) with a mean overall increase in LS BMD of +3.19%. In 17/20 patients initially randomised to ibandronate with a mean T-score of −1.11 (range 0.0 to −2.2) at 2 years, study medication was stopped and the patients followed up on anastrozole alone till year 5 because BMD T scores were >−2. The improvements in BMD accrued during the first 2 years were lost at both the LS (−3.21%) and TH (−5.0%). As a consequence, at 5 years, 14 patients had T-scores in the osteopenic range and one had an osteoporotic T-score. Of the three offered an open label bisphosphonate for the remaining 3 years of the study; at the 2 year visit, two of these patients had a T-score of between −2 and −2.5 and in one the T-score was <−2.5. In these three patients, BMD increased in 2 and 1 and fell in 1 and 2 at the LS and TH respectively (Data not shown). Two patients still had a T-score of <−2.5 at LS and/or TH after 5 years of ibandronate.
Osteopenic patients (placebo group): The mean change in LS BMD at the 2 year time-point in the 16 patients who were randomised to placebo and who remained on study until the 5 year visit was −4.52%. Eight patients with high rates of bone loss during years 0–2 (LS −7.1%, TH −7.3%) and a BMD result at 2 years showing either severe osteopenia (T-score <−2.0 to −2.5; n=5) or osteoporosis (t-score <−2.5; n=3) went on to receive open label ibandronate in years 2–5 alongside continued anastrazole, while 8 with less marked bone loss (LS −1.0%, TH −0.5%) were observed on anastrazole alone.
For those treated with ibandronate, BMD improved on average during years 2–5 by +5.01% and +1.19% at the LS and TH respectively. Over the 5 years of the study however BMD remained below baseline measurements at both the LS (−2.88%) and TH (−6.22%). At the end of 5 years, 4 patients had T-scores ≥−2 but in the other 4 the BMD at either the LS or TH was in the osteoporotic range.
The 8 patients who were not offered a bisphosphonate experienced relatively little change in BMD throughout the study. At the end of 5 years, the overall change in BMD from baseline was +0.15% and −2.72% at the LS and TH respectively. All patients in this group had an end of treatment BMD T score of >−2. Although the number of patients is small, these results suggest that some patients are less susceptible to BMD loss from the AI and relatively small changes in BMD after 2 years appear to predict relatively stable BMD for the remaining 3 years of AI treatment.
Osteoporotic patients: Nine of the 13 patients initially identified as having osteoporosis were assessed at the 5 year visit. These patients showed steady increases in BMD through the 5 years of the study. Mean changes in BMD at the LS were +3.03% and +9.65% after 2 and 5 years respectively. At the TH BMD improved by +3.18% after 2 years and stabilised at +2.72% after 5 years. After 5 years only 2 out of the 9 patients remaining on study still had a T score of <−2.5.
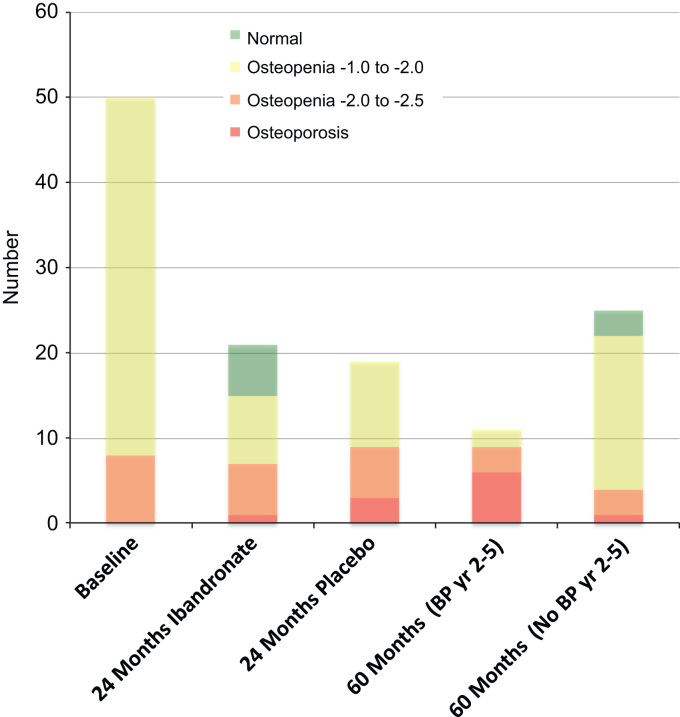
Fig. 3 shows the changes in BMD grouping for the 50 patients with osteopenia through the 5 years of the study. The number of patients with osteoporosis at either the LS or TH increased from 0 to 4 to 7 at the baseline, 2 year and 5 year endpoints respectively. Six out of the 11 patients (54%) who were offered ibandronate from 2–5 years developed or still had osteoporosis at 5 years.
Fig. 3.
Changes in BMD groupings for all osteopenic patients.
6. Study withdrawals
From 2 to 5 years on study, one patient out of the 21 patients who were initially randomised to ibandronate withdrew from the study (patient choice). After 2 years, 19 patients who were randomised to placebo remained on study. Of these 3 patients came off study prior to the 5 year visit (patient choice n=1, death n=1, investigator recommendation n=1). 2 osteoporotic patients came off study following the 2 year visit (investigator recommendation n=2). Three patients withdrew from the study following their 2 year visit due following a recommendation from the treating clinician. In each of these 3 cases the treatment was discontinued because of problems related to the anastrozole rather than any changes in BMD.
7. Adverse events
Treatment reported toxicities were similar to those previously reported [23].
A total of 10 patients developed a fracture following low energy trauma during the course of the 5 years of the study. Of these, four patients were initially randomised to ibandronate (wrist=1, hip=3) and three were randomised to placebo (wrist=1, shoulder=1, rib=1). A further three fractures occurred in the osteoporotic group (wrist=1, hip=1, humerus=1).
Cancer recurrence rates were very low because most patients had an excellent prognosis and no patients recruited to the study were considered for adjuvant chemotherapy. Only 2 patients developed recurrent disease during the study, both patients recurred within the first 2 years and were osteopenic receiving placebo. One patient had a local recurrence and the other developed widespread metastatic disease including bone metastasis.
Tablet counts suggested that patient adherence to monthly ibandronate was high with greater than 90% of study patients taking all of their monthly doses.
8. Discussion
Bone mineral density monitoring is now an important part of the management of breast cancer patients treated with an aromatase inhibitor. Several groups have now published guidance on how best to monitor and treat patients at risk. In this small study, a strategy of monitoring and ultimately treating patients who have developed or who are at risk of developing osteoporosis has proven effective in at least maintaining bone density over the 5 year course of aromatase inhibitor therapy. An oral bisphosphonate is therefore a reasonable option to treat bone loss from an aromatase inhibitor.
Several other studies have addressed the same issue using different bisphosphonates. The SABRE study randomised breast cancer patients taking anastrozole with a T-score of between −1 and −2 to risedronate (35 mg weekly) or placebo. After 2 years, BMD increased by 2.2% at the LS and by 1.8% at the TH [22].
3 large, very similar studies (Z-FAST, ZO-FAST and E-ZO-FAST), compared the efficacy of 6 monthly intravenous zoledronic acid given either from the start of AI therapy with letrozole or if there was either significant bone loss or the development of a non-traumatic fracture in patients taking letrozole for their breast cancer. In all three studies [20], [24], [25] immediate zoledronic acid prevented bone loss. The Z-FAST study has recently published the final results after 5 years of treatment [20], and showed that treatment with zoledronic acid resulted in a difference in BMD after 5 years on study at the LS and TH of 8.9% and 6.7% respectively (p<0.0001). These differences appear somewhat greater than we observed with oral ibandronate, but no direct comparison of ibandronate and zoledronic acid is planned to determine whether there are any meaningful differences in the two treatment approaches.
The ARIBON study has not evaluated BMD changes after the 5 years of aromatase inhibitor therapy but a degree of recovery of BMD would be expected as was seen in the extended results of the ATAC [26] and IES [27] trials. Although the small number of patients and the fact that some patients on placebo required subsequent bisphosphonate limits our study, some important conclusions can nevertheless be drawn. Firstly a strategy of introducing a bisphosphonate in patients with severe osteopenia can prevent osteoporosis but patients still need to be monitored for changes in bone density. Secondly patients who have relatively stable BMD for the first 2 years of an aromatase inhibitor without a bisphosphonate may require little further treatment or monitoring because the risk of subsequent accelerated bone loss appears to be low. Further research is important to investigate why their bone density is so stable and also how these patients can be prospectively identified. Thirdly patients with osteoporosis at the diagnosis of breast cancer can be safely treated with an aromatase inhibitor provided they are also prescribed a bisphosphonate.
References
- 1.Howell A., Cuzick J., Baum M., Buzdar A., Dowsett M., Forbes J.F. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J., Sestak I., Baum M., Buzdar A., Howell A., Dowsett M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncology. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 3.Coombes R.C., Kilburn L.S., Snowdon C.F., Paridaens R., Coleman R.E., Jones S.E. Survival and safety of exemestane versus tamoxifen after 2-3 years tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 4.Thurlimann B., Keshaviah A., Coates A.S., Mouridsen H., Mauriac L., Forbes J.F. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. New England Journal of Medicine. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 5.Coates A.S., Keshaviah A., Thurlimann B., Mouridsen H., Mauriac L., Forbes J.F. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. Journal of Clinical Oncology. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R., Adams J.E., Coleman R.E., Howell A., Hannon R.A., Cuzick J. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. Journal of Clinical Oncology. 2008;26(7):1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 7.Mazess R.B., Barden H. Bone density of the spine and femur in adult white females. Calcified Tissue International. 1999;65:91–99. doi: 10.1007/s002239900663. [DOI] [PubMed] [Google Scholar]
- 8.Perez E.A., Josse R.G., Pritchard K.I., Ingle J.N., Martino S., Findlay B.P. Effect of anastrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. Journal of Clinical Oncology. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 9.Coleman R.E., Banks L.M., Girgis S.I., Kilburn L.S., Vrdoljak E., Fox J. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmanopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncology. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 10.Forbes J.F., Cuzick J., Buzdar A., Howell A., Tobias J.S., Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncology. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 11.Hillner B.E., Ingle J.N., Chlebowski R.T., Gralow J., Yee G.C., Janjan N.A. American society of clinical oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. Journal of Clinical Oncology. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Reid D.M., Doughty J., Eastell R., Heys S.D., Howell A., McCloskey E.V. Guidance on the managemnet of breast cancer induced bone loss: a consensus position statement from a UK expert group. Cancer Treatment Reviews. 2008;34(1):S3–S18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Hadji P., Aapro M.S., Body J.J., Brufsky A., Coleman R.E., Guise T. Practical guidance for the management of aromatase inhibitor-associated bone loss. Annals of Oncology. 2008;19:1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 14.Hadji P., Aapro M.S., Body J.J., Bundred N.J., Brufsky A., Coleman R.E. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance on prevention and treatment. Annals of Oncology. 2011;22(12):2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 15.Chesnut C., Skag A., Christiansen C., Recker R., Stakkestad J.A., Hoiseth A. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. Journal of Bone and Mineral Research. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 16.Liberman U., Weiss S., Broll J., Minne H.W., Quan H., Bell N.H. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. New England Journal of Medicine. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 17.Harris S., Watts N., Gennant H., McKeever C.D., Hangartner T., Keller M. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. Journal of the American Medical Association. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 18.Black D., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. New England Journal of Medicine. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 19.Reginster J., Adami S., Lakatos P., Greenwald M., Stepan J.J., Silverman S.L. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the mobile study. Annals of the Rheumatic Diseases. 2006;65:654–661. doi: 10.1136/ard.2005.044958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brufsky A.M., Harker W.G., Beck J.T., Bosserman L., Vogel C., Seidler C. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 21.Gnant M., Mlineritsch B., Luschin-Ebengreuth G., Grampp S., Kaessmann H., Schmid M. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian breast and colorectal cancer study group. Journal of Clinical Oncology. 2007;25:820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 22.Van Poznak C., Hannon R.A., Mackey J.R., Campone M., Apffelstaedt J.P., Clack G. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. Journal of Clinical Oncology. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 23.Lester J.E., Dodwell D., Purohit O., Gutcher S.A., Ellis S.P., Thorpe R. Prevention of anastrozole-induced bone loss with oral monthly ibandronate during aromatase inhibitor therapy for breast cancer. Clinical Cancer Research. 2008;14:6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 24.Eidtmann H., de Boer R., Bundred N., Llombart-Cussac A., Davidson N., Neven P. Efficacy of zoledronic acid in postmanopausal women with early breast cancer receiving adjuvant letrozole 36-month results of the ZO-FAST study. Annals of Oncology. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 25.Llombart A., Frassoldati A., Paija O., Sleeboom H.P., Jerusalem G., Mebis J. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmanopausal women with early breast cancer: 12 month analysis of the EZO-FAST trial. Clinical Breast Cancer. 2012;12:40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R., Adams J., Clack G., Howell a., Cuzick J., Mackey J. Long term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Annals of Oncology. 2011;22:857–862. doi: 10.1093/annonc/mdq541. [DOI] [PubMed] [Google Scholar]
- 27.Coleman R.E., Banks L.M., Girgis S.I., Vrdoljak E., Fox J., Cawthorn S.J. Reversal of skeletal effects of endocrine treatments in the intergroup exemestane study. Breast Cancer Research and Treatment. 2011;124:153–161. doi: 10.1007/s10549-010-1121-7. [DOI] [PubMed] [Google Scholar]