Abstract
Bisphosphonates have demonstrated anti-tumour activity in preclinical studies of bone metastatic disease, thus it was natural to transition these agents into the adjuvant cancer therapy setting. Surprisingly, the results of adjuvant breast cancer trials have shown either modest to no benefit or even harm. We sought to explore whether the preclinical results supporting bisphosphonate use provided clues to help explain the current clinical data. Interestingly, the majority of preclinical data suggested that bisphosphonate treatment was more efficacious when administered after the establishment of osseous metastases. This is similar to the findings of one clinical study whereby patients with biopsy evidence of osseous micrometastases derive greater survival benefit from bisphosphonate treatment. Another clinical study found bisphosphonates were associated with increased incidence of visceral metastases, similar to what has been previously published in preclinical models using “preventative” dosing strategies. While the current clinical data suggest bisphosphonates may be more efficacious in post-menopausal or oestrogen depleted patients, or those with hormone receptor positive tumours, to date no appropriately designed preclinical studies have evaluated these effects. Furthermore, putative mechanisms that regulate response to bisphosphonates in other tumour types remain to be evaluated in breast cancer. Despite the initial optimism regarding adjuvant bisphosphonate therapy, the conflicting clinical results from large trials suggest that we should return to the bench to further investigate factors that may influence response to bisphosphonate treatment or identify appropriate characteristics that would indicate the sub-groups of patients most likely to benefit from bisphosphonate treatment.
Keywords: Bisphosphonate, Breast cancer, Bone metastasis, Adjuvant treatment, Xenograft, Preclinical models
1. Background
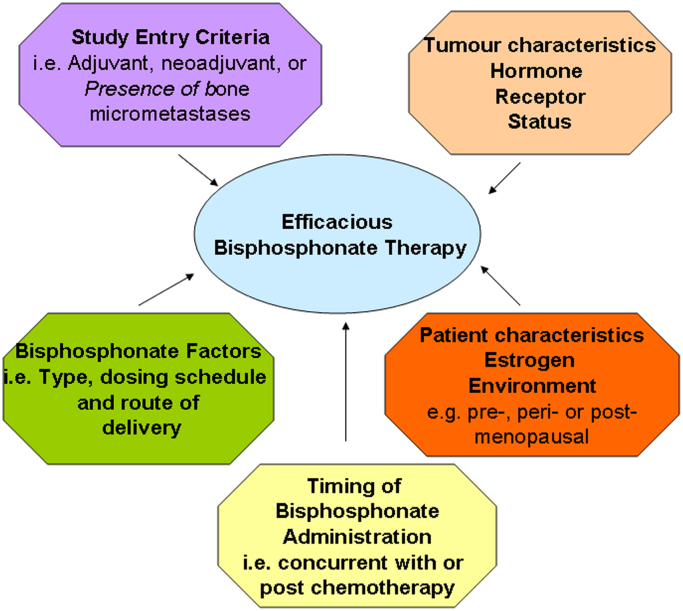
Following the publication of a number of preclinical studies suggesting that bisphosphonate treatment could significantly impair the growth of osseous breast tumours, and stabilise bone metastases, a number of clinical studies were initiated to evaluate the effects of adjuvant bisphosphonate treatment in newly diagnosed breast cancer patients. Now that many of these studies have reached clinical maturity, their published results have been either positive [1], [2], [3], [4] negative [5], [6], [7], [8], or even detrimental [9], (see Table 1). Although certain factors have been suggested to influence the clinical responses noted with bisphosphonate use (Fig. 1), formal demonstration of their association has yet to be determined. Given these conflicting clinical outcomes and the extensive preclinical data that was supposed to support the adjuvant development of these agents, it is time to revisit the published preclinical results in order to determine whether they predicted the current clinical outcomes.
Table 1.
| Characteristic | Diel et al.[2], Annals of Oncology, 19:2007 | Powles et al.[1], Breast Cancer Research, 8:R13 | Gnant et al.[3], [4], The Lancet, 12:631 | Coleman et al.[5], New Eng J of Med, 365:1396 | Kristensen et al.[6], Acta Oncologica, 47:740 | Saarto et al.[9], Acta Oncologica, 43:650 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bisphosphonate used | Clodronate | Placebo | Clodronate | Placebo | Zoledronic acid | Placebo | Zoledronic acid | Placebo | Pamidronate | Placebo | Clodronate | Placebo |
| Dosing schedule and route | 1600 mg orally daily for 2 years | No | 1600 mg orally daily for 2 years | Yes | 4 mg IV every 6 month for 3 years | No | 4 mg IV every 3–4 weeks for 6 cycles then every 3–6 months for 5 years | No | 150 mg orally twice daily for 4 yrs | No | 1,600 mg orally daily for 3 yrs | No |
| Use of anti-estrogens | 47% received tamoxifen | 45% received tamoxifen | 80% of cohort received tamoxifen | 100% of cohort received either Tamoxifen or anastrozole | 78.3% on endocrine or endocrine+chemotherapy | 78.6% on endocrine or endocrine+chemotherapy | Endocrine therapy excluded | Endocrine therapy excluded | 100% of cohort received tamoxifen or toremifene | |||
| Cohort size | 157 | 145 | 530 | 539 | 900 | 903 | 1681 | 1678 | 460 | 493 | 139 | 143 |
| Mean age | NR | NR | 52.8 | 52.7 | 44.5 | 44.5 | NR | NR | b | b | ||
| 52 | ||||||||||||
| T-stage | ||||||||||||
| T1 | 38% | 37% | 26% | 26% | 75.7% | 76.7% | 32.2% | 31.2% | 41% | 44% | 51% | 46% |
| T2 | 45% | 46% | 57% | 57% | 21.2% | 21.7% | 50.6% | 51.7% | 50% | 50% | 42% | 46% |
| T3 or greater | 17% | 16% | 9% | 10% | 17.0% | 17.1% | 7% | 5% | 7% | 6% | ||
| Unknown | 8% | 7% | 2.1% | 2.6% | 0.2% | 0.1% | 2% | 1% | 0 | 3 | ||
| Lymph node positive | 51% | 54% | 37% | 38% | 30.5% | 30.5% | 97.8% | 97.7% | 75% | 75% | 99% | 99% |
| Menopausal status | ||||||||||||
| Pre-menopausal | 36% | 39% | 50% | 49% | NR | NR | 44.7% | 44.8% | 67% | 66% | 48% | 57% |
| Post-menopausal | 64% | 61% | 50% | 51% | NR | NR | 33% | 34% | 52% | 43% | ||
| Post-menopausal <5yrs | 14.7% | 14.5% | ||||||||||
| Post-menopausal >5yrs | 30.9% | 31.1% | ||||||||||
| Unknown | 9.8% | 9.5% | 0% | 0.2% | ||||||||
| ER status | ||||||||||||
| Positive | 75% | 71% | 46% | 45% | 94.6% | 93.3% | 78.5% | 78.4% | 13.5% | 17.2% | 61% | 68% |
| Negative | 25%a | 29%a | 26% | 25% | 3.3% | 3.9% | 20.8% | 21.1% | 60.4% | 52.9% | 35% | 23% |
| Unknown | 28% | 30% | 2.1% | 2.6% | 0.8% | 0.4% | 26.1% | 29.8% | 4% | 9% | ||
| PR status | ||||||||||||
| Positive | 62% | 63% | 21% | 22% | 89.9% | 89.5% | NR | NR | 11% | 11% | 50% | 60% |
| Negative | 38%a | 67%a | 15% | 14% | 7.6% | 8.3% | NR | NR | 29% | 28% | 45% | 31% |
| Unknown | 64% | 65% | 2.5% | 2.2% | NR | NR | 60% | 61% | 5% | 9% | ||
| Pretreatment evidence of bone metastasis | Yes | Yes | No | No | No | No | No | No | No | No | No | No |
| Positive | Positive | Positive | Negative | Negative | Negative | |||||||
|
|
|
|
|
|
|||||||
NR—Not Reported.
Not originally reported therefore may contain negative and unknown categories.
Mean age for treatment groups was not reported however was stratified across 4 groups originally; for pamidronate and control cohorts there were 61.3% and 63.1% of patients younger than age 50 respectively.
Fig. 1.
Factors influencing efficacy of bisphosphonate therapy in clinical investigations.
2. Preclinical studies: Of mice, rats and women?
2.1. Preclinical animal models and dosing regimens
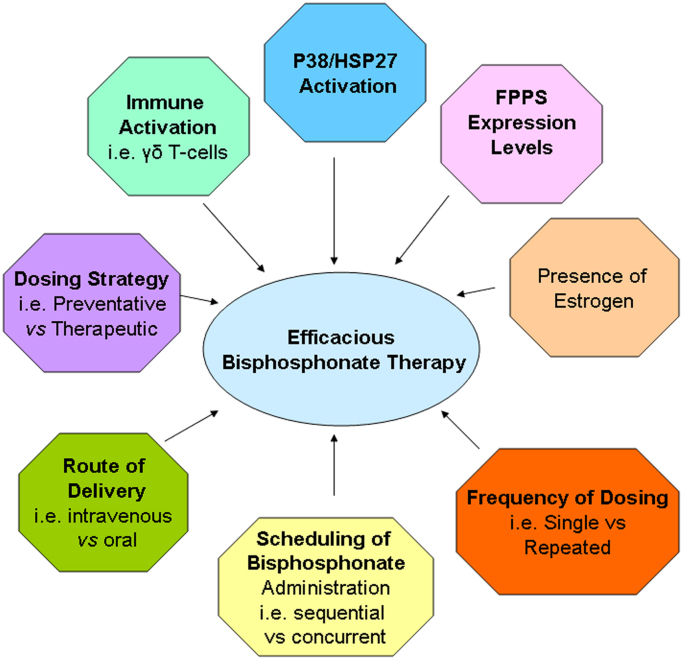
To date, a number of factors that may influence tumour response to bisphosphonates have been suggested by preclinical studies (Fig. 2). The studies referenced by the published clinical trials cite data restricted to 4 different preclinical models of osteolytic bone metastases, one human, one mouse and two rat-derived tumour cell lines. While in all cases inhibitory effects on skeletal metastases were observed (summarised in [10]), the models differed in their results with respect to their effects on extraskeletal metastases. The studies using preclinical models of human breast cancer, involved intracardiac injection of tumour cells into immunocompromised mouse models where tumours would subsequently form in the bone. It is important to remember that as these studies are performed in immunocompromised animals, the effects of tumour-elicited immune responses on the efficacy of bisphosphonate treatment are not evaluable. A number of the studies using human xenografts have employed two strategies for bisphosphonate delivery. Bisphosphonates were given after bone metastases had been established following intracardiac injection of breast cancer cells, termed “therapeutic dosing”. Alternatively, bisphosphonate administration was prior to injection of tumour cells, and hence prior to establishment of bone metastases, termed “preventative dosing”. Although osseous metastases appeared to be inhibited by both types of intervention with bisphosphonates, there were unexplained increases in soft tissue metastases in a number of studies when the preventative dosing strategy was followed [11], [12], [13]. This is similar to the effects seen in the clinical study by Saarto et al. [9] in which patients received clodronate from the start of their systemic therapy. Of all the clinical studies reported to date, the study by Saarto et al. [9] contained a relatively larger proportion of hormone receptor negative patients (Table 1) compared to most of the other clinical studies who have not reported adverse effects on the incidence of extraskeletal metastases. Thus, it would be of great interest to determine whether the enhanced incidence of extraskeletal metastases is a predominant feature of hormone receptor negative as compared to hormone receptor positive disease.
Fig. 2.
Factors shown to influence bisphosphonate therapy in preclinical studies.
A few studies have been performed using oestrogen receptor (ER) positive murine 4T1 breast cancer cells. In these orthotopic models, cells implanted into the mammary fat pad will metastasise to the bones as well as to other extraskeletal sites. Using this model system, the inhibitory effects of bisphosphonate treatment on osseous metastases were observed when drug was given in a therapeutic dosing regimen, i.e. after radiologic detection of bone metastases [11]. Importantly, no effects of a single administration of bisphosphonate were observed on lung metastases, highlighting the fact that in this immunocompetent orthotopic model bisphosphonates appeared to have a primary effect in the bone microenvironment as previously suggested [12], [14], [15]. Interestingly, in the same model system, repeated dosing of zoledronic acid in a therapeutic dosing regimen did result in significant decreases in both lung and liver metastatic burden [16]. However, in contrast to zoledronic acid, repeated dosing of the bisphosphonates clodronate or pamidronate did not inhibit lung metastases growth in the same model [16]. Unfortunately, the effects of bisphosphonates on the growth and metastasis of breast tumours using a preventative dosing regimen were not evaluated in this orthotopic model in any of the above-mentioned studies, hence their effects in an “adjuvant therapy” setting were not evaluated in this context. It is noteworthy that the clinical trial that has demonstrated the greatest impact on survival following use of bisphosphonates in the “adjuvant” setting, that by Diel et al. [2] would have also used a therapeutic dosing strategy, as study entry required biopsy evidence of tumour cells in their bone prior to treatment. Taken together with numerous preclinical studies demonstrating significant inhibition of bone metastases with a therapeutic dosing regimen, these clinical results suggest that future trials should possibly be designed with this prescreening for micrometastases in mind.
2.2. Patient Hormonal status
When one examines all the clinical trial results published to date, there appears to be a possible enhanced benefit derived from adjuvant bisphosphonate treatment in a post-menopausal or oestrogen depleted cohort of patients [1], [2], [3]. To date, there have been no preclinical animal model studies evaluating the efficacy of bisphosphonates in the menopausal setting. Evaluation of the efficacy of bisphosphonates in either ovarectomized or 4-vinylcyclohexene diepoxide (VCD)-treated mice, which induces menopause in mice that most closely mimics that in women [17], would thus be of great interest. However, population studies of bisphosphonate use in post-menopausal women being treated for osteoporosis, have shown significant reductions in the incidence of invasive breast cancer [18], [19]. This supports the argument that perhaps the use of bisphosphonates as a strategy for prevention of development of bone metastases should only be pursued in post-menopausal cohorts. A similar finding however, has also been reported in younger cohorts (mean age of ∼54 years with a range from 20–70) [20], thus raising more potential questions than answers.
2.3. Tumour receptor status
The clinical trials also suggest that patients with hormone positive tumours may derive more benefit from adjuvant bisphosphonate treatment [1], [2], [3]. Interestingly, the majority of preclinical studies evaluating the efficacy of bisphosphonates using human breast cancer cells have generally been performed using a single cell line, MDA-MB-231 breast tumour cells. Although these cells are known to readily form osteolytic lesions when injected intracardially, they are an ER and Her2 negative cell line [12], [13], [15]. The fact that these tumour cells are oestrogen non-responsive may suggest that preclinical responses noted using these cells are not reflective of responses in the clinical setting, where breast cancer patients who develop bone metastases predominantly have ER positive tumours [21], [22], [23]. There is a lack of preclinical data directly comparing the efficacy of bisphosphonates in ER positive vs negative breast cancer cells. We have been able to find only one published article describing the effects of bisphosphonates on tumour growth using the ER positive, Her2 negative human breast tumour cell line, MCF7 which primarily forms osteoblastic as opposed to osteolytic lesions, after intracardiac injection [15]. This article also suggested that treatment with bisphosphonates prior to injection of MCF7 tumour cells, i.e. in a preventative dosing regimen, inhibited development of osteosclerotic metastases. In contrast, treatment with bisphosphonates following development of bone metastases, i.e. in a therapeutic dosing regimen, had no effect on osseous metastases. Unfortunately, within this article, the experimental data are not presented.
As mentioned above, a few studies have also been performed using ER positive murine 4T1 breast cancer cells. However, as bisphosphonate treatment was initiated after radiologic detection of bone metastases in these studies [11], their efficacy in a preventative adjuvant setting was not evaluated. As such, the efficacy of bisphosphonates in the inhibition of bone metastases growth derived from ER positive vs negative tumours in an adjuvant therapy setting has not been examined. It would be of interest to perform studies in genetically modified cells of the same parental background, e.g. ER negative MDA-MB-231 cells modified to re-express the ER, and evaluate their response to bisphosphonate therapy head to head in preclinical animal models.
There is also a lack of preclinical literature evaluating the effects of sex steroids (e.g. oestrogen) on the efficacy of bisphosphonates. We were able to find only one study which has specifically evaluated the effects of steroids on bisphosphonate inhibition of tumour cell growth. Using in vitro steroid free culture medium conditions, the authors observed that instead of inhibiting tumour growth, administration of clodronate actually enhanced proliferation of the ER positive MCF7 breast tumour cell line [24]. The authors further demonstrate that administration of tamoxifen in combination with clodronate, reversed the tumour promoting capabilities of clodronate previously observed in the steroid free culture conditions, hence showing a dependence of bisphosphonate inhibition on ER activity. In contrast, treatment of the ER negative cell line MDA-MB-231 with clodronate inhibited tumour growth regardless of whether cells were cultured in steroid containing versus steroid free culture media [24]. These data highlight that not only may the ER status of the tumour be important for patient overall response to bisphosphonate therapy, but that simultaneous regulation of oestrogen activity in these treated patients may be of equal importance. This is clearly a line of investigation that warrants further preclinical and clinical evaluation.
3. Factors that may modulate response to bisphosphonate therapy
3.1. Immunomodulation
Preclinical studies also suggest that a number of alternative factors may modulate the efficacy of bisphosphonates in patients. It has been shown that treatment of tumour cells with bisphosphonates can result in the cellular production of isopentenyl pyrophosphate/triphosphoric acid I-adenosin-5′-yl ester 3-3-methylbut-3-enyl ester (IPP/ApppI) metabolites which are subsequently recognised by a subpopulation of γδ T-cells (specifically Vγ9Vδ2) as phospho-antigens (recently reviewed in [25]). As γδ T-cells can recognise non-peptide antigens independently of association with MHC molecules, they can readily recognise and lyse tumour cells expressing these non-peptide antigens despite the downregulated MHC expression that is often observed in tumour cells. In fact, a correlation between the levels of bisphosphonate-induced expression of IPP/ApppI in breast tumour cells and the resulting effective γδ T-cell mediated killing of these cells has been demonstrated [26]. Treatment of MCF7 breast cancer cells with either of the nitrogen-containing bisphosphonates pamidronate or zoledronic acid was shown to induce Vγ9Vδ2 activation and subsequent lysis of treated MCF7 cells [27]. It has also been shown that luminal breast cancer cell lines (ER and/or PR positive, MCF or T47D cells) were induced to express high levels of these metabolites and were readily killed by γδ T-cells [26]. In contrast, basal breast cancer cell lines (ER, PR and HER2 negative MDA-MB-231/B02 cells) failed to accumulate IPP/ApppI metabolites following bisphosphonate treatment and were not killed by γδ T-cells [26]. Moreover, γδ T-cells were found to readily infiltrate subcutaneous ER+breast cancer xenograft tumours in mice upon zoledronic acid treatment, and this correlated with tumour shrinkage, while similar infiltration of γδ T-cells did not occur in zoledronic treated ER-xenograft tumours [26]. This leads to the intriguing hypothesis that the enhanced benefit derived from bisphosphonate treatment noted in many of the breast cancer patients with ER positive tumours in the clinical trials, could be due in part to an enhanced anti-tumour immunity state induced following activation of these γδ T-cells in this patient cohort.
While still not extensively studied, there is some supporting clinical evidence that activated γδ T-cells may play a role in patient response to bisphosphonate therapy. In a small phase I trial with terminal metastatic breast cancer patients, treatment with zoledronic acid and low dose IL-2 resulted in mobilisation of peripheral Vγ9Vδ2T-cells [28]. Although the patient cohort was small, a significant correlation with sustained levels of circulating Vγ9Vδ2T-cells was noted for patients with improved outcomes, while patients who did not have sustained levels continued to clinically decline. Although not reported in any of the published literature regarding the large adjuvant bisphosphonate breast cancer trials, it is well recognised that some patients get flu like symptoms following their first dose of bisphosphonates, suggesting that a priming of the immune system may occur in some cases. It would be very interesting to determine whether those patients that develop these flu-like symptoms also derive the greatest benefit from bisphosphonate therapy. Alternatively, clinical correlative studies could be performed to determine whether a correlation between the level of activation of γδ T-cells in bisphosphonate treated patients and efficacy of treatment in the adjuvant setting exists.
3.2. Sequencing of additional therapies
The effects of concurrent or sequential chemotherapy on the efficacy of bisphosphonates should also be considered. Induction of apoptosis of the ER positive MCF7 or ER negative MDA-MB-231 cells was substantially increased when the bisphosphonate zoledronic acid was used in combination with paclitaxel compared to administration of either drug alone [29]. It has also been reported that treatment with zoledronic acid prior to doxorubicin treatment in preclinical animal models of breast cancer resulted in no significant inhibition of subcutaneous human MDA-MB-436 xenograft tumour growth [30]. In contrast, if the doxorubicin and zoledronic acid were given concurrently, or if zoledronic acid was given following doxorubicin treatment, subcutaneous tumour growth was significantly impaired [30]. Another study evaluated the effect of similar dosing strategies using the highly osseotrophic MDA-MB-231/BO2 derivative cell line that forms bone metastases following intravenous injection. In this case, the authors saw slight but statistically significant inhibition of bone metastases growth when doxorubicin and zoledronic acid were given concurrently, however more substantial inhibition of osseous tumour growth was observed when zoledronic acid was given 24 h following doxorubicin treatment [14]. In contrast, when the authors quantified the extraosseous lesions, they observed no significant effect on tumour growth with any of the dosing regimens [14]. Considering that these lesions were formed by the same tumour cells injected via the same route, these results further support the notion that it is the ability of the bisphosphonates to modify the bone microenvironment that contributes to their predominant anti-tumour activity in vivo.
For the most part, the clinical trials we have discussed within this manuscript have used concurrent administration of bisphosphonates with adjuvant treatments. Given that most of these studies have not demonstrated significant clinical benefit, and preclinical data suggesting that bisphosphonates may be most efficacious when given following chemotherapy, future studies dissecting out the most efficacious dosing regimens should be performed both preclinically and clinically.
3.3. Mechanisms of resistance to bisphosphonates
Other factors that may contribute to a lack of patient response which warrant further examination involve the induction of potential mechanisms of resistance to bisphosphonate treatment. Although some preclinical studies have suggested that bisphosphonates have direct anti-tumour activities and can induce tumour cell apoptosis, it is not likely that this is playing a significant role in the patient setting, given that the doses required for these effects are extremely high (at either 10 μM [31], or in excess of 50–100 μM [32], [33], [34], [35] in the majority of reports) and likely not achievable within the local patient tumour microenvironment. Moreover, factors produced by breast cancer cells can promote osteoclast survival and overcome the direct apoptotic-inducing effects of bisphosphonates on osteoclasts [36], which may also in part contribute to a lack of response to bisphosphonates in a patient setting. In osteosarcoma cells, response to zoledronic acid is dependent on the level of expression of farnesyl diphosphate synthetase (FPPS), one of the major targets of bisphosphonates in tumour cells [37]. Bisphosphonate resistance in myeloma cells following long-term exposure, has been shown to correlate with up regulation of FPPS [38]. Thus it remains possible that patient response could be predicted following evaluation of the levels of expression of factors such as this, although to date no clear evidence demonstrating a link between sensitivity to bisphosphonates and levels of FPPS expression has been shown in breast cancer.
Bisphosphonate treatment of breast tumour cells has also been shown to induce activation of p38 which impairs bisphosphonate-induced apoptosis via its ability to promote progression through the cell cycle G2/M checkpoint [39], [40]. Use of p38 inhibitors was able to overcome resistance to bisphosphonates in osteosarcoma [41] and breast cancer [39] cells. One of the downstream targets of p38, heat shock protein 27 (HSP27), has also been shown to be significantly up-regulated in bisphosphonate resistant osteosarcoma cell lines [42]. The authors further demonstrated that upon silencing of HSP27, sensitivity to bisphosphonates could be restored in these resistant cells. Elevated levels of HSP27 have been associated with decreased overall survival and decreased survival after first recurrence in breast cancer patients [43]. A role for HSP27 in mediating resistance of breast cancer cells to drugs, including doxorubicin [44], [45], and herceptin [46] has also been previously demonstrated. Thus these data suggest that patients with high p38 activity or elevated HSP27 expression may be more refractory to bisphosphonate treatment. Clearly, given the lack of disease free and overall survival benefit seen in the largest adjuvant bisphosphonate trial to date [5], investigation into these alternative mechanism regulating response to bisphosphonates, strategies to enhance their efficacy in vivo, and putative biomarkers that can predict patient response to these agents should be pursued.
4. Discussion
Despite considerable initial excitement, the negative outcomes of many large randomized clinical trials evaluating efficacy of bisphosphonates in the adjuvant breast cancer setting have been disappointing. However, due to the osteotrophic nature of the bisphosphonates, they remain an exciting area of treatment strategy for breast cancer patients at risk of developing bone metastases. In order to make future progress, correctly interpreting the currently reported clinical results and identifying the treatment population and strategy most likely to achieve benefit are critically important. Clearly analysis of the benefit derived from bisphosphonates in certain subpopulations of breast cancer patients needs to be fully elucidated. It appears that few of the clinical studies were designed around the strategies that were found to be the most efficacious in preclinical evaluations. We also noted that the majority of preclinical studies utilise model systems that are not reflective of the phenotype of the majority of human breast tumours that are osteotrophic. Unless we begin to design our preclinical evaluations based on what is most reflective of the disease in a patient setting and not necessarily around what is the most reliable and convenient preclinical model system, we may continue to observe unexpected results in patients. Similarly, we must design rational clinical studies that use similar strategies to what has been shown to be the most efficacious in the most relevant preclinical models, otherwise contradictory results are sure to follow. Furthermore, we should not miss the opportunity to understand how bone-targeted agents such as bisphosphonates can change the risk of relapse outside bone in some subpopulations: is this a host modification phenomena or a new pathway to eliminate micrometastatic disease? While we digest the current literature regarding bisphosphonate use in adjuvant breast cancer treatment, it will also be important to await the outcome of other bone-targeted agents like denosumab, in order to determine whether this approach will be more efficacious or will show similar results to the bisphosphonates in the adjuvant setting.
References
- 1.Powles T., Paterson A., McCloskey E., Schein P., Scheffler B., Tidy A. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Research. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diel I.J., Jaschke A., Solomayer E.F., Gollan C., Bastert G., Sohn C. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Annals of Oncology. 2008;19:2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Heck D., Menzel C. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. The Lancet Oncology. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 4.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Stoeger H, Dubsky P, Jakesz R, et al. Long-term follow-up in ABCSG-12: Significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor-positive early breast cancer. In San Antonio Breast Cancer Symposium. December 6–10 2011, San Antonio, Texas; 2011.
- 5.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M. Breast-cancer adjuvant therapy with zoledronic acid. New England Journal of Medicine. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen B., Ejlertsen B., Mouridsen H.T., Jensen M.B., Andersen J., Bjerregaard B. Bisphosphonate treatment in primary breast cancer: results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncologica. 2008;47:740–746. doi: 10.1080/02841860801964988. [DOI] [PubMed] [Google Scholar]
- 7.Paterson AHG, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, et al. NSABP protocol B-34: A clinical trial comparing adjuvant clodronate vs. placebo in early stage breast cancer patients receiving systemic chemotherapy and/or tamoxifen or no therapy-final analysis. In San Antonio Breast Cancer Symposium. December 6-10 2011, San Antonio, Texas. Edited by CTRC-AACR; 2011.
- 8.Thomssen Mobus V, Harbeck C, Untch N, Jackisch M, Diel C, Elling IJ, et al., GAIN (German Adjuvant Intergroup Node Positive) study: A phase III-multicenter trial to compare dose dense, dose intense etc (iddETC) vs. EC-TX and ibandronate vs. observation in patients with node-positive primary breast cancer—1st interim efficacy analysis. In San Antonio Breast Cancer Symposium. December 6–10 2011, San Antonio, Texas. Edited by CTRC-AACR; 2011.
- 9.Saarto T., Vehmanen L., Virkkunen P., Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncologica. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 10.Padalecki S.S., Guise T.A. Actions of bisphosphonates in animal models of breast cancer. Breast Cancer Research. 2002;4:35–41. doi: 10.1186/bcr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michigami T., Hiraga T., Williams P.J., Niewolna M., Nishimura R., Mundy G.R. The effect of the bisphosphonate ibandronate on breast cancer metastasis to visceral organs. Breast Cancer Research and Treatment. 2002;75:249–258. doi: 10.1023/a:1019905111666. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki A., Boyce B.F., Story B., Wright K.R., Chapman M., Boyce R. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Research. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 13.Sasaki A., Kitamura K., Alcalde R.E., Tanaka T., Suzuki A., Etoh Y. Effect of a newly developed bisphosphonate, YH529, on osteolytic bone metastases in nude mice. International Journal of Cancer. 1998;77:279–285. doi: 10.1002/(sici)1097-0215(19980717)77:2<279::aid-ijc18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Ottewell P.D., Deux B., Monkkonen H., Cross S., Coleman R.E., Clezardin P. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clinical Cancer Research. 2008;14:4658–4666. doi: 10.1158/1078-0432.CCR-07-1545. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda T., Michigami T., Yi B., Williams P.J., Niewolna M., Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88:2979–2988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Hiraga T., Williams P.J., Ueda A., Tamura D., Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clinical Cancer Research. 2004;10:4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 17.Mayer L.P., Devine P.J., Dyer C.A., Hoyer P.B. The follicle-deplete mouse ovary produces androgen. Biology of Reproduction. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski R.T., Chen Z., Cauley J.A., Anderson G., Rodabough R.J., McTiernan A. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. Journal of Clincal Oncology. 2010;28:3582–3590. doi: 10.1200/JCO.2010.28.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennert G., Pinchev M., Rennert H.S. Use of bisphosphonates and risk of postmenopausal breast cancer. Journal of Clinical Oncology. 2010;28:3577–3581. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb P.A., Trentham-Dietz A., Hampton J.M. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. British Journal of Cancer. 2010;102:799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wedin R., Skoog L., Bauer H.C. Proliferation rate, hormone receptor status and p53 expression in skeletal metastasis of breast carcinoma. Acta Oncologica. 2004;43:460–466. doi: 10.1080/02841860410033721. [DOI] [PubMed] [Google Scholar]
- 22.Koenders P.G., Beex L.V., Langens R., Kloppenborg P.W., Smals A.G., Benraad T.J. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The breast cancer study group. Breast Cancer Research and Treatment. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- 23.Wei S., Li Y., Siegal G.P., Hameed O. Breast carcinomas with isolated bone metastases have different hormone receptor expression profiles than those with metastases to other sites or multiple organs. Annals of Diagnostic Pathology. 2011;15:79–83. doi: 10.1016/j.anndiagpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Journe F., Chaboteaux C., Dumon J.C., Leclercq G., Laurent G., Body J.J. Steroid-free medium discloses oestrogenic effects of the bisphosphonate clodronate on breast cancer cells. British Journal of Cancer. 2004;91:1703–1710. doi: 10.1038/sj.bjc.6602181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton E., Clay T.M., Blackwell K.L. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Cancer Investigation. 2011;29:533–541. doi: 10.3109/07357907.2011.605413. [DOI] [PubMed] [Google Scholar]
- 26.Benzaid I., Monkkonen H., Stresing V., Bonnelye E., Green J., Monkkonen J. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Research. 2011;71:4562–4572. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- 27.Dhar S., Chiplunkar S.V. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vgamma9Vdelta2 T cells. Cancer Immunity. 2010;10:10. [PMC free article] [PubMed] [Google Scholar]
- 28.Meraviglia S., Eberl M., Vermijlen D., Todaro M., Buccheri S., Cicero G. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clinical and Experimental Immunology. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagdev S.P., Coleman R.E., Shipman C.M., Rostami H.A., Croucher P.I. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. British Journal of Cancer. 2001;84:1126–1134. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottewell P.D., Monkkonen H., Jones M., Lefley D.V., Coleman R.E., Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. Journal of the National Cancer Institute. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 31.Senaratne S.G., Pirianov G., Mansi J.L., Arnett T.R., Colston K.W. Bisphosphonates induce apoptosis in human breast cancer cell lines. British Journal of Cancer. 2000;82:1459–1468. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraga T., Williams P.J., Mundy G.R., Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Research. 2001;61:4418–4424. [PubMed] [Google Scholar]
- 33.Hiraga T., Ueda A., Tamura D., Hata K., Ikeda F., Williams P.J. Effects of oral UFT combined with or without zoledronic acid on bone metastasis in the 4T1/luc mouse breast cancer. International Journal of Cancer. 2003;106:973–979. doi: 10.1002/ijc.11330. [DOI] [PubMed] [Google Scholar]
- 34.Oades G.M., Senaratne S.G., Clarke I.A., Kirby R.S., Colston K.W. Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. Journal of Urology. 2003;170:246–252. doi: 10.1097/01.ju.0000070685.34760.5f. [DOI] [PubMed] [Google Scholar]
- 35.Coxon J.P., Oades G.M., Kirby R.S., Colston K.W. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. British Journal of Urology International. 2004;94:164–170. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- 36.Hussein O., Tiedemann K., Komarova S.V. Breast cancer cells inhibit spontaneous and bisphosphonate-induced osteoclast apoptosis. Bone. 2011;48:202–211. doi: 10.1016/j.bone.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Ory B., Moriceau G., Trichet V., Blanchard F., Berreur M., Redini F. Farnesyl diphosphate synthase is involved in the resistance to zoledronic acid of osteosarcoma cells. Journal of Cellular and Molecular Medicine. 2008;12:928–941. doi: 10.1111/j.1582-4934.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomo M., Jurlander J., Nielsen L.B., Gimsing P. How myeloma cells escape bisphosphonate-mediated killing: development of specific resistance with preserved sensitivity to conventional chemotherapeutics. British Journal of Haematology. 2003;122:202–210. doi: 10.1046/j.1365-2141.2003.04437.x. [DOI] [PubMed] [Google Scholar]
- 39.Merrell M., Suarez-Cuervo C., Harris K.W., Vaananen H.K., Selander K.S. Bisphosphonate induced growth inhibition of breast cancer cells is augmented by p38 inhibition. Breast Cancer Research and Treatment. 2003;81:231–241. doi: 10.1023/a:1026126430905. [DOI] [PubMed] [Google Scholar]
- 40.Merrell M.A., Wakchoure S., Lehenkari P.P., Harris K.W., Selander K.S. Inhibition of the mevalonate pathway and activation of p38 MAP kinase are independently regulated by nitrogen-containing bisphosphonates in breast cancer cells. European Journal of Pharmacology. 2007;570:27–37. doi: 10.1016/j.ejphar.2007.05.075. [DOI] [PubMed] [Google Scholar]
- 41.Kubo T., Shimose S., Matsuo T., Sakai A., Ochi M. Efficacy of a nitrogen-containing bisphosphonate, minodronate, in conjunction with a p38 mitogen activated protein kinase inhibitor or doxorubicin against malignant bone tumor cells. Cancer Chemotherapy and Pharmacology. 2008;62:111–116. doi: 10.1007/s00280-007-0580-y. [DOI] [PubMed] [Google Scholar]
- 42.Morii T., Ohtsuka K., Ohnishi H., Mochizuki K., Satomi K. Inhibition of heat-shock protein 27 expression eliminates drug resistance of osteosarcoma to zoledronic acid. Anticancer Research. 2010;30:3565–3571. [PubMed] [Google Scholar]
- 43.Thanner F., Sutterlin M.W., Kapp M., Rieger L., Morr A.K., Kristen P. Heat shock protein 27 is associated with decreased survival in node-negative breast cancer patients. Anticancer Research. 2005;25:1649–1653. [PubMed] [Google Scholar]
- 44.Hansen R.K., Parra I., Lemieux P., Oesterreich S., Hilsenbeck S.G., Fuqua S.A. Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Research and Treatment. 1999;56:187–196. doi: 10.1023/a:1006207009260. [DOI] [PubMed] [Google Scholar]
- 45.Oesterreich S., Weng C.N., Qiu M., Hilsenbeck S.G., Osborne C.K., Fuqua S.A. The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Research. 1993;53:4443–4448. [PubMed] [Google Scholar]
- 46.Kang S.H., Kang K.W., Kim K.H., Kwon B., Kim S.K., Lee H.Y. Upregulated HSP27 in human breast cancer cells reduces herceptin susceptibility by increasing Her2 protein stability. BMC Cancer. 2008;8:286. doi: 10.1186/1471-2407-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]