Abstract
Bone remodelling is related to coordinated phases of bone resorption and bone apposition allowing the maintenance of bone integrity, the phosphocalcic homoeostasis all along the life and consequently the bone adaptation to mechanical constraints or/and to endocrine fluctuations. Unfortunately, bone is a frequent site of tumour development originated from bone cell lineages (primary bone tumours: bone sarcomas) or from nonosseous origins (bone metastases: carcinomas). These tumour cells disrupt the balance between osteoblast and osteoclast activities resulting in a disturbed bone remodelling weakening the bone tissue, in a strongly altered bone microenvironment and consequently facilitating the tumour growth. At the early stage of tumour development, osteoclast differentiation and recruitment of mature osteoclasts are strongly activated resulting in a strong bone matrix degradation and release of numerous growth factors initially stored into this organic/calcified matrix. In turn these soluble factors stimulate the proliferation of tumour cells and exacerbate their migration and their ability to initiate metastases. Because Receptor Activator of NFκB Ligand (RANKL) is absolutely required for in vivo osteoclastogenesis, its role in the bone tumour growth has been immediately pointed out and has consequently allowed the development of new targeted therapies of these malignant diseases. The present review summarises the role of RANKL in the bone tumour microenvironment, the most recent pre-clinical and clinical evidences of its targeting in bone metastases and bone sarcomas. The following sections position RANKL targeted therapy among the other anti-resorptive therapies available and underline the future directions which are currently under investigations.
Keywords: Bone cancer, Bone metastases, Bone sarcomas, Osteoclasts, RANKL, Bone remodeling
1. Introduction
Bone is a very dynamic tissue resulting from coordinated phases of formation and resorption called bone remodelling. Additional to its role in phosphocalcic homoeostasis, bone remodelling process is necessary for bone growth, for renewal of cellular and extracellular matrix components to adapt bone organisation to the various biological and mechanical constraints [1], [2], [3]. Bone remodelling then leads to the renewal of around 10% of total bone mass each year in human. This metabolic process is based on a molecular crosstalk occurring between osteoblasts involved in bone apposition and osteoclasts specialized in bone resorption. Osteoclasts are multinucleated cells that originated from hematopoietic stem cells [4], [5], [6] whereas osteoblasts are derived from bone marrow mesenchymal stem cells [3], [7], [8]. Osteoblasts control osteoclast differentiation and activation through a very complex network of soluble factors which act in combination with various hormones produced by endocrine system even if contacts between both cell types also strongly contribute to full activation of osteoclasts [9], [10]. Reciprocity between osteoblasts and osteoclasts can be observed as shown by bidirectional signalling limiting osteoclast activities and stimulating osteoblast differentiation [11].
Bone remodelling can be dysregulated by oncologic events originated from bone cells (primary bone tumours: osteosarcoma, chondrosarcoma, Ewing's sarcoma, etc.) or from nonosseous origins (bone metastases). Large series revealed that around 0.2% of all neoplasms are bone sarcomas and two new primary bone tumours arise per 100,000 persons a year [12]. Bone tissue is then the most frequent site of their first relapse and consequently, the incidence of bone metastases is relatively high and is dependent on the cancer cell types (i.e. in 70–80% of patients with breast or prostate cancer, in 40% of patients with lung metastases or with kidney cancer). Bone metastases are frequently associated with numerous clinical complications named skeletal-related events (SREs) and have a strong deleterious impact on the quality of life. SREs include pathological fractures or spinal cord compression and exacerbated bone pains. All bone tumours disrupt the equilibrium between bone apposition and bone resorption leading to the first stop of the tumour development to an osteolytic process followed or not by bone forming lesions. Soluble mediators stored initially into the bone matrix contribute in turn to stimulate the tumour growth and to maintain the vicious cycle between bone and tumour cells [13]. The loss of equilibrium between bone formation and degradation combined with an osteomimetism behaviour of cancer cells (cancer cells acquire bone-like properties) explains the diversity of histological features (osteolytic or bone forming tumours) of bone metastases [14]. Additionally, the modulation of bone micro-environment (“niche” concept) by cancer cells is beneficial for their proliferation and also contributes to the drug resistance patterns [15].
In the late 1990s, two research groups in Japan and in USA have identified a truncated TNF receptor-like molecule (named OPG for osteoprotegerin, TNFRSF11B) inducing marked osteopetrosis phenotype when overexpressed in transgenic mice [16], [17]. One year later, RANKL (Receptor Activator of Nuclear Factor kB Ligand or TNFSF11) has been identified as a ligand for OPG [18], [19]. In a few years, OPG/RANKL couple became the principal system regulating osteoclastogenesis and bone resorption and has impressively stimulated the development of OPG/RANKL targeting agents for the treatment of osteolytic disorders in oncologic contexts or not competing with bisphosphonates, a well admitted drug class for the treatment of bone loss [13], [19], [20], [21], [22], [23].
In all bone cancers, a strong relationship between tumour cells and bone micro-environment has been then clearly established, facilitating the tumour development and/or the metastatic process. These specific communication pathways have strongly stimulated the research and development programs to design new drugs to treat oncologic bone diseases and have led specifically to the development of therapies targeting RANKL. The present review summarises the most recent progresses in the treatment of bone cancers based on RANKL targeting and underlines the future directions which are currently under pre-clinical investigations.
2. OPG, RANK and RANKL are key protagonists controlling osteoclast biology and bone remodelling
The critical function of OPG in osteoclastogenesis has been initially revealed by the osteopetrotic phenotype of mice overexpressing it [18], [19]. In contrast, OPG deficient mice exhibit osteoporotic phenotype which is totally reversed by administration of recombinant OPG [24]. RANKL has been identified as the main ligand of OPG known to bind RANK (TNFRSF11A), a transmembrane receptor of the TNFR superfamily [25]. RANKL transgenic mice and RANKL knockout mice are respectively osteoporotic and osteopetrotic (Fig. 1). In fact, membrane and soluble RANKL produced by osteoblasts interact with RANK expressed on monocyte lineage and osteoclast precursors, induces osteoclast differentiation and consequently activates bone resorption [23, Fig. 1]. Discovery of the RANK/RANKL signalling pathway through NFkB in the osteoclast has clearly provided new insights into the mechanisms of osteoclastogenesis and how hormonal networks impact bone remodelling [23], [24], [25], [26]. OPG is the third protagonists and acts as a decoy receptor, binds to RANKL, inhibits RANK–RANKL interactions and in fine is a strong anti-resorptive agent. The balance between bone resorption and bone apposition consequently depends on the ratio OPG/RANKL (Fig. 1). For instance, the relative equilibrium between OPG and RANKL levels results in a stable bone mass, and in contrast for instance to RANKL knockout where bone remodelling is in favour of excessive bone formation due a marked reduction of osteoclastogenesis (Fig. 1). Similarly, a clear relationship has been established between RANKL/OPG ratio and the severity of osteolysis in oncologic diseases as in benign diseases [27]. It is now admitted that RANKL is absolutely required for osteoclastogenesis in vivo even if RANKL can be substituted in vitro by other ligands such as TNFα [28]. As the other TNF members, OPG, RANK and RANKL exhibit very complex stoichiometric characteristics. Indeed, OPG is a dimeric molecule, but it can even act as a monomer and RANL and RANK are homotrimeric complexes [20], [28], [29], [30]. Additionally, OPG biology is more complex than those initially described and possesses numerous ligands such as other TNF Related Apoptosis Inducing Ligand (TRAIL) [31], proteoglycans [32] and glycosaminoglycans [33], [34], von Willebrand factor [35], complex VIII [36] which modulate its own activity.
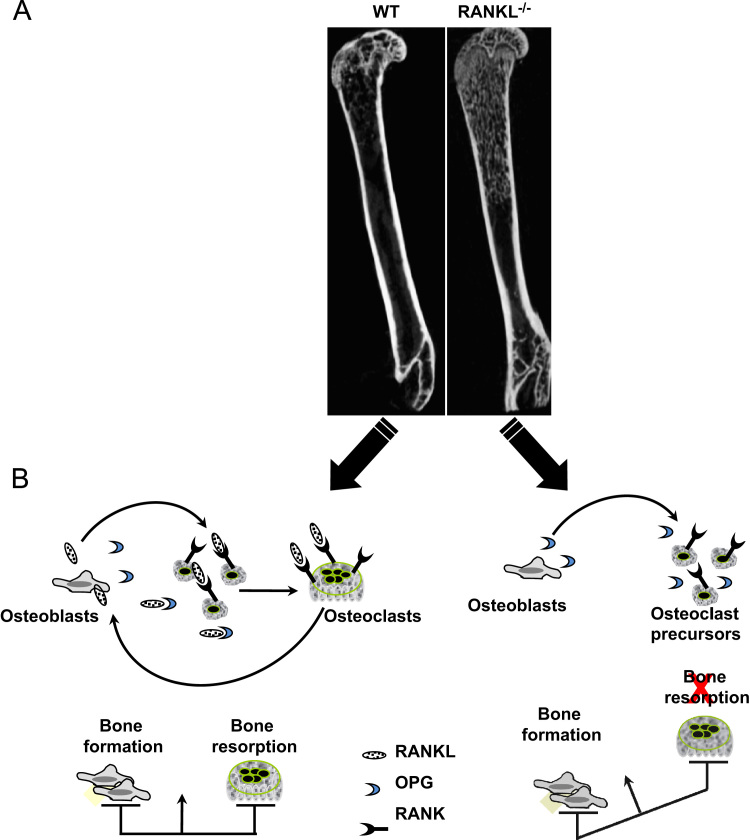
Fig. 1.
RANKL is absolutely required for osteoclast differentiation in vivo as revealed by the bone phenotype exhibited by RANKL knockout mice. (A) Osteopetrotic phenotype exhibited by RANKL knockout (RANKL−/−) compared to wild type (WT) C57BL6 mice analysed by μCT (skyscan 1076). (B) Osteoblasts produced RANKL (membrane and soluble forms) which binds to membrane RANK expressed by osteoclast precursors, OPG synthesised by osteoblasts acts as a decoy receptor, blocks the RANKL/RANK interactions and then inhibits bone resorption. The lack of RANKL results in a disturbed bone remodelling characterised by an excessive bone formation and a reduced bone resorption compared to the control mice.
3. OPG, RANK and RANKL contribute to the vicious cycle established between tumour cells and bone microenvironment: evidences for sarcomas and carcinomas
In bone microenvironment, OPG/RANK/RANKL molecular triad is not solely expressed by bone cells. Whether OPG is considered as a ubiquitary receptor [20], membrane RANK is expressed by various tumour cells that originate from primary bone tumours or bone metastases, from mesenchymal and epithelial origin (Fig. 2A and B). A recent study showed that more than 80% of bone metastases from solid tumours are RANK positive as revealed by immunohistochemistry [42], [43]. RANK is also expressed in more than 50% of human osteosarcoma specimens, with preferential expression in osteosarcomas that develop in pathological bone and are bad responders to chemotherapy [39]. These observations then identify tumour cells as potential RANKL targets. In addition to its expression by osteoblasts and bone marrow stromal cells, RANKL is produced similarly as RANK by numerous cancer cell types from various origins (Fig. 2C). RANKL expression is modulated by a lot of cytokines, hormones [20] and by hypoxia dysregulating bone remodelling, a common feature of malignant tumours [77]. Indeed, the invasion of bone tissue by a primary or metastatic tumour cell precociously affects the balance between bone resorption and bone formation. According to the tumour entities, tumour-derived factors (IGF, BMP, etc.) can stimulate osteoblast differentiation and activation and lead to tumour associated osteoblastic lesions (Fig. 3) or in contrast, RANKL released by tumour cells can activate osteoclastogenesis and the recruitment of mature osteoclasts resulting in osteolytic lesions. The co-existence of both phenomena leads to the formation of mixed osteoblastic/osteoclastic lesions. In turn, dysregulated bone cells-released extracellular matrix components and soluble mediators (TGFβ, etc.) are initially trapped in the bone matrix and stimulate proliferation of tumour cells and then the growth of tumour mass (Fig. 3). This mechanism is defined as the osteoclast-dependent role of RANK/RANKL axis in tumorigenesis. However, RANK/RANKL axis influences tumorigenesis through osteoclast-independent pathway (Fig. 3). RANKL produced by bone microenvironment constitutes a fertile soil for RANK-positive tumour cells. Initially proposed by Paget at the end of 1900 the concept of the seed and soil for primary and secondary bone tumours has been strengthened by the discovery of RANKL and RANKL partly explains why various tumours preferentially metastasise to bone. RANKL released by osteoblasts and bone marrow stromal cells creates a cytokine gradient between bone site and extraosseous sites and triggers the migration of RANK-positive tumours cells. Interestingly, numerous tumour cells expressed functional RANK as shown by the signal transduction (P-ERK1/2, P-P38, P-IkB, etc.) induced by RANKL [39], [47], [50]. The first evidence of this mechanism has been established by Jones et al. [45] and has been now described as prostate carcinoma [45], [47], [48], breast carcinoma [45], oral squamous carcinoma [50], lung cancer cells [52] and melanoma [45]. The implication of RANK/RANKL axis in tumour cell migration has been confirmed by exploration in human samples. Indeed, the levels of RANK expressed by primary tumour cells are directly related to the occurrence of bone metastases in solid tumours and more specifically in breast, prostate and melanoma [42], [43], [44]. Furthermore, RANK expression could be considered as an independent predictor of poor prognosis in breast cancer patients with bone metastasis in contrast with visceral metastasis for which no correlation has been shown [77]. Similarly, increased RANKL expression is related to the migration of renal carcinoma [53]. Whether the role of functional RANK expression has been clarified for carcinomas, its role in the pathogenesis of sarcomas is not fully understood. Indeed, it has been shown that osteosarcoma cells express the RANK protein [39]. RANK signalling, under the action of RANKL, results in the modulation of a panel of more than 70 specific genes demonstrating that osteosarcoma cells are therefore RANKL targets [40]. Wittrant et al. [38] showed that RANKL directly induces BMP-2 expression in RANK positive osteosarcoma cells and may contribute by this way to the osteoblastic lesions characteristic of osteosarcomas. More recently, Lee et al. observed that RANKL expression is correlated to clinical behaviour of patients suffering from high-grade osteosarcoma [57].
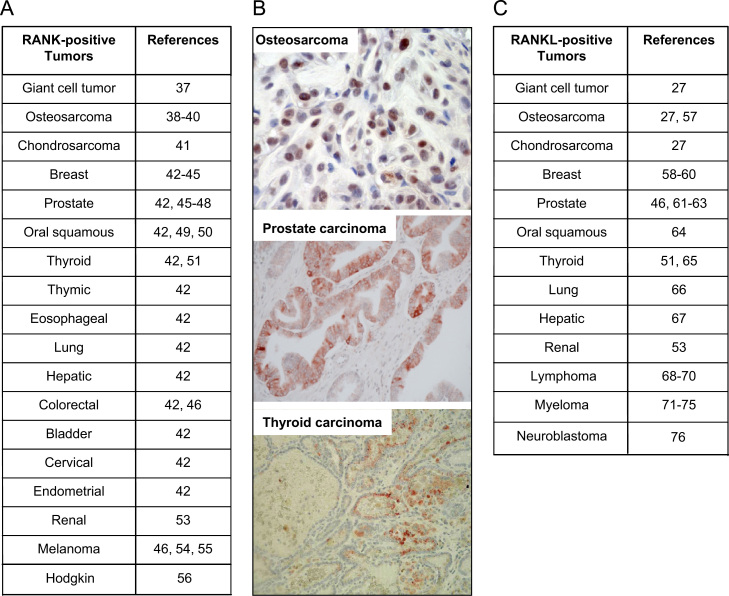
Fig. 2.
RANK is expressed by numerous tumour cell type. (A) Main tumour cell types expressing RANK [37], [41], [49], [55], [56]; (B) RANK immunostaining on osteosarcoma [39], prostate carcinoma and thyroid carcinoma [51]. (C) RANKL is also expressed by cancer cells [27], [46], [53], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79].
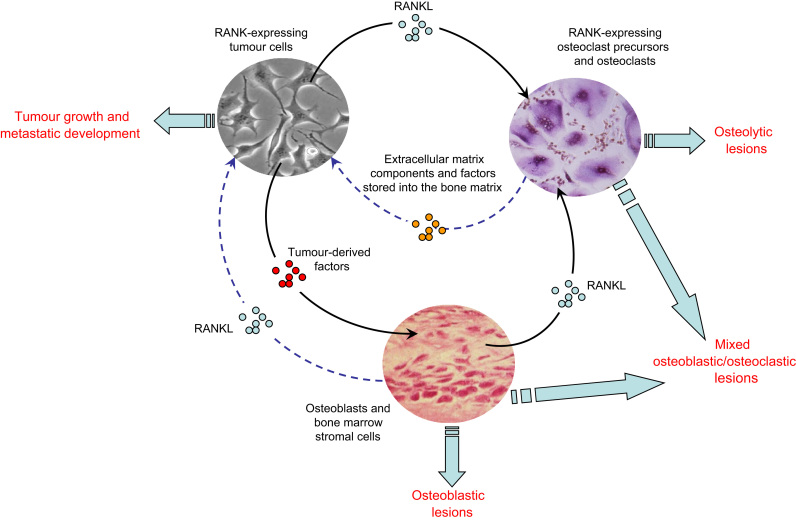
Fig. 3.
Direct and indirect role of RANKL in bone tumour development. RANKL contributes to the development of bone tumours via the activation of osteoclastogenesis and bone resorption defining an osteoclast-dependent pathway. RANKL also can bind directly RANK-expressing tumour cells, stimulating epithelial mesenchymal transition, cell migration and then identifying an independent osteoclast. Tumour cells dysregulate the balance between osteoblasts and osteoclasts, resulting in osteolytic, osteoblastic or osteoblastic–osteoclastic mixed lesions according the tumour cell type.
4. RANK/RANKL axis is involved in the tumorigenic process
RANK/RANKL axis is associated with the bone metastastic process, and as several arguments point out it is also involved in the tumorigenicity process itself. RANK/RANKL may participate in the initial oncogenic program as shown by high expression of RANK in melanoma-initiating cells compared to the other melanoma cells [54]. Epithelial mesenchymal transition (EMT) is the first step allowing the extravasation and migration of carcinoma cells and RANKL appears clearly involved in this process. Indeed, Yamada et al. show that RANKL promotes EMT and induces angiogenesis independent of VEGF in a human head and neck squamous carinoma [78]. RANKL has also a strong impact on normal epithelial cells as shown by its effect on mammary gland development evidenced by a lactation defect in RANKL knockout mice [79], [80]. In fact, RANKL promotes the proliferation and survival of mammary epithelial cells [79], [80], [81], [82] and RANK expression increases during the gestation more specifically at ductal branch points [82]. More interestingly, whether RANKL or RANK overexpression in the mammary epithelial cells results in aberrant proliferation and hyperplasia of mammary glands, it directly correlates with preneoplasias and the development of spontaneous mammary tumours [83], [84]. Several authors hypothesised that RANKL may act as a paracrine factor for mammary stem cells [85], [86]. Consequently, blockade of RANKL significantly reduces the occurrence of mammary tumours [84]. Overall, these data give clear evidences that the RANK/RANKL axis contributes to the initial steps of tumorigenesis at least for mammary glands, to the dissemination process of carcinoma cells and to the establishment of bone metastases.
5. Therapies targeting RANK/RANKL axis for patients suffering from bone tumours: pre-clinical and clinical arguments
Given this context, targeting of RANKL signalling with its decoy receptor OPG or with a soluble form of its membranous receptor RANK (RANK-Fc) inhibits tumour associated osteolysis in several experimental bone tumour models, including rat and mouse primary bone tumours and bone metastases. Indeed, OPG and RANK-Fc administered by nonviral gene transfer or as recombinant molecules are effective in preventing the formation of osteolytic lesions associated with osteosarcoma development and in reducing the tumour incidence leading to a significant increase of animal survival [87], [88]. Moreover, recent experiments demonstrated that RNA interference strategy targeting RANKL improved the tumour response to chemotherapy in a murine model of osteosarcoma [89]. Similarly, administration of recombinant OPG-Fc or RANK-Fc has been investigated in numerous murine models of bone metastases [20], [23], [79] and confirms that blockade of the RANK/RANKL axis is extremely efficient in preclinical assessment to prevent tumour-induced osteolysis, to reduce tumour growth and to improve the survival rate. According these pre-clinical proofs of concept, recombinant OPG (OPG-Fc) has been evaluated in postmenopausal [90] and in patients suffering from myeloma and osteolytic bone metastases (Table 1). Results demonstrated that OPG was well tolerated and demonstrated the efficacy of a single injection of OPG, which strongly reduced bone turnover for a sustained period and suppressed bone resorption as indicated by the decrease of bone resorption markers (urinary NTX/creatinine); these effects were comparable to those obtained with pamidronate. However, due to the risk of immune modulation of OPG through its binding to TRAIL [31] and other ligands [32], [33], [34], [35], [36], a fully human monoclonal antibody (IgG2) specifically targeting soluble and membrane RANKL has been developed [92], [93]. Clinical data in osteoporotic patients revealed that denosumab was well tolerated with no related serious adverse events occurred and that a single-dose (0.01–3.0 mg/kg) resulted in a dose-dependent sustained decrease from baseline in bone turnover [92], [93]. This antibody, named denosumab, only recognises the human protein and its nonhuman-primate homologue and its administration in chimeric mice expressing murine/human leads to a strong inhibition of bone resorption concomitantly to an increase of the bone mineral density [94].
Table 1.
Summary of the main clinical trials in oncology assessing anti-RANKL therapies.
| Clinical trials drug assessed | Cancer | Number of patients included | Doses | References |
|---|---|---|---|---|
| Phase I OPG recombinant | Bone metastases (Breast) | 26 | s.c. 0.1–3 mg/kg | [91] |
| Myeloma | 28 | |||
| Phase I, Denosumab | Bone metastases (Breast) | 29 | Denosumab s.c. 0.1–3 mg/Kg Pamidronate 90m g i.v. | [95] |
| Myeloma | 25 | |||
| Randomized, double blind | Bone metastases (excluding breast, prostate and myeloma) | 886 | Denosumab s.c. 120 mg monthly Zoledronate i.v. 4 mg monthly | [96] |
| Denosumab versus zoledronate | 890 | |||
| Randomized, double blind | Bone metastases (Breast) | 1026 | Denosumab s.c. 120 mg monthly Zoledronate i.v. 4 mg monthly | [97] |
| Denosumab versus zoledronate | 1020 | |||
| Phase II, Denosumab with and without bisphosphonate exposure | Bone metastases | 366 | Denosumab s.c. 60 or 180 mg every 12 weeks Denosumab s.c. 30, 120, or 180 mg every 4 weeks | [98], [99], [100] |
| Denosumab vs placebo and adjuvant aromatase inhibitors | Non-metastatic breast cancer | 127 (treated) 125 (placebo) | Denosumab s.c. 60 mg every 6 weeks | [101], [102] |
| Phase II, randomized trial Denosumab after i.v. bisphosphonates | Bone metastases (prostate, breast cancers and other neoplasms) | 111 | Denosumab s.c. 180 mg every 4 or 12 weeks | [103] |
| Phase II Denosumab | Myeloma | 96 | Denosumab s.c.120 mg on days 1, 8, and 15 (loading doses) of cycle 1 (28 day), and then on study day 29 (day 1 of cycle 2) and on day 1 of every cycle (28 day) thereafter | [104] |
| Phase II, Randomized Denosumad after i.v. bisphosphonates | Bone metastases (Prostate) | 111 | Denosumab s.c. 180 mg every 4 or 12 weeks | [105] |
| Double-blind study Denosumad and androgen-deprivation | Prostate cancer | 734 per group | Denosumab s.c. 60 mg every 6 months | [106] |
| Double-blind study Denosumab and androgen-deprivation | Prostate cancer | 734 per group | Denosumab s.c. 60 mg every 6 months | [107] |
| Phase III Denosumab in castration-resistant patients | Prostate cancer | 716 per group | Denosumab s.c. 120 mg every 4 weeks | [108] |
| Phase III, Denosumab versus zoledronate in castration-resistant patients | Prostate cancer | 950 per group | Denosumab s.c. 120 mg or zoledronate 4 mg i.v. every 4 weeks | [109] |
| Phase II Denosumab | Giant cell tumours of bone | 37 | Denosumab s.c. 120 mg monthly | [110] |
Numerous clinical trials (phase II and phase III) have been then designed to evaluate the efficacy of denosumab in oncology mainly in breast and prostate bone metastases (Table 1). These studies revealed that denosumab reduced significantly bone turnover markers similarly to osteoporotic patients. More specifically, it reduced levels of uNTX/Cr as well as serum TRAP5b thus showing a marked inhibition of osteoclastogenesis. According to the results obtained, the recommendations for the use of denosumab are 120 mg s.c. every 4 months in oncology. Using this dose, bone resorption markers are suppressed around 90% in most patients independent of the tumour types [103]. Various studies have been set up to compare denosumab versus bisphosphonate treatment mainly zoledronic acid [95], [96], [97], [103], [109]. Single dose of pamidronate for instance (90 mg i.v.) reduced in a similar intensity the levels of bone resorption markers but the effects of denosumab were more sustained [95]. Phase III study demonstrated that denosumab significantly delayed the time of first SRE (Skeletal Related Event) but also the risk of multiple SRE and whether zoledronic acid showed similar effects, statistical analyses are in favour of superiority for denosumab (Table 1). The time of disease progression and the overall survival rate were similar between anti-RANKL treatment and bisphosphonate. Zoledronic acid treatment requires a strict monitoring of kidney function due to its toxicity in contrast to denosumab even if a greater hypocalcemia requiring specific monitoring has been classically observed after denosumab treatment [111]. In addition to the phase acute phase reaction observed in patients after the first administration of zoledronic acid, osteonecrosis of the jaw occurred infrequently after long-term treatment by nitrogen-bisphosphonates, in around 2% of patients [97], [108], [109], [112], [113], [114], [115]. This incidence appears similar in bisphosphonate- and denosumab-treated patients. Consequently, the establishment of meticulous oral hygiene and surgical procedures prior to the administration of bisphosphonates and denosumab is the best method for preventing osteonecrosis of the jaw; prevention being better than treatment. In all studies, denosumab was well tolerated with the convenience of a subcutaneous administration and no requirement for renal monitoring. Overall, these clinical trials demonstrated that denosumab represents a potential treatment option economically viable for patients with bone metastases [116]. Very recently, a novel anti-RANKL antibodies derived from camelidae has been assessed in postmenopausal patients [117]. The results from this Phase I trial, including the one year follow-up information, indicate that ALX-0141 is well tolerated and can be administered safely over a wide range of doses. ALX-0141 exhibited a strong and sustained inhibitory effect on bone resorption markers.
6. The other anti-resorptive in therapies of bone cancer
Bisphosphonates have been used successfully for many years to treat the skeletal complications associated with the benign and malignant bone diseases [118], [119], [120], [121]. Bisphosphonates became progressively a standard treatment for cancer-associated with hypercalcemia and to control metastatic bone pain. Bisphosphonates are chemical compounds based on a phosphorus–carbon–phosphorus template and are characterised by their strong affinity for bone hydroxyapatite crystals and their anti-resorptive potency. Three families of bisphosphonate have been produced: the first possesses simple substituents attached to the central carbon and inhibits weakly the bone resorption; the second family possesses an aliphatic side chain containing a single nitrogen atom and exerts a more potent anti-resorptive activity; the third generation contains a heterocyclic substituent with one or two nitrogen atoms and are powerful bone resorption inhibitors and anti-tumour agents [118], [119]. The members of the first family which do not contain nitrogen atom are metabolised in cytotoxic analogues of ATP leading to cell death. Nitrogen-containing bisphosphonates inhibit the activity of two enzymes involved in the mevalonate pathway: farnesyl diphosphate synthase (FPP) and geranylgeranyl diphosphate synthase (GGPP). This inhibition results in osteoclast apoptosis by the strong reduction of the prenylation process, the loss of osteoclastic ruffled border and modifications of cell cytoplasmic actin ring [118], [119]. Additionally, nitrogen-containing bisphosphonates exert direct activities on tumour cells (breast, prostate, lung renal carcinoma, osteosarcoma, chondrosarcoma, etc.) through the inhibition of prenylation mechanim which induces tumour-cell apoptosis, inhibits cell proliferation, modulates tumour-cell adhesion and inhibits tumour-cell dissemination [118], [119], [120], [121], [122], [123], [124]. Thus, bisphosphonates inhibit the development of bone tumours through direct activity on tumour cells and indirect activity on osteoclasts. Pre-clinical studies revealed the therapeutic benefits of bisphosphonates for the treatments of primary bone tumours and bone metastases alone and in combination with chemotherapy or signalling pathway inhibitors [125], [126], [127], [128], [129], [130], [131], [132], [133]. Clinical trials have clearly confirmed their therapeutic interests (Table 1).
Since many decades, bone tumours have stimulated imagination of researchers and numerous therapeutic alternatives have been proposed [134], [135], Fig. 4]. The better knowledge of OPG/RANK/RANKL system leads to the development of peptides mimicking OPG and blocking RANK–RANKL interactions [136], [137], [138], [139]. Inhibitors of NF-KB signalling showed interesting anti-resorptive activities [140], [141], [142], [143]. The targeting of integrins more specifically αvβ3 strongly reduced the osteolytic process and the development of bone tumours [144], [145], [146], [147], [148], [149], [150]. Specific blockade of enzymatic activities has been envisaged with a great success. MMP9 involved in osteoclast migration and its targeting blocked by antisense oligodeoxyribonucleotide strongly affects osteoclast migration and resorption [151]. Cathepsin K, a key cysteine-proteinase related to osteolytic process [152], [153] stimulates a huge enthusiasm in the world of bone research. Several companies have then developed chemical inhibitors of cathepsin K to treat malignant and non-malignant bone loss with interesting results [154], [155], [156], [157]. Thus, forty-three women suffering from breast metastatic disease have been recently randomized in a double-blind study to evaluate the impact of oral cathepsin K inhibitors [odanacatib 5 mg daily for 4 weeks or 4 mg zoledronic acid i.v] on bone resorption markers [157]. Odanacatib appeared generally safe and well tolerated and has suppressed osteolytic markers similar to zoledronic acid after 4 weeks of treatment. These results strengthen the therapeutic interest of cathepsin K for oncologic bone loss.
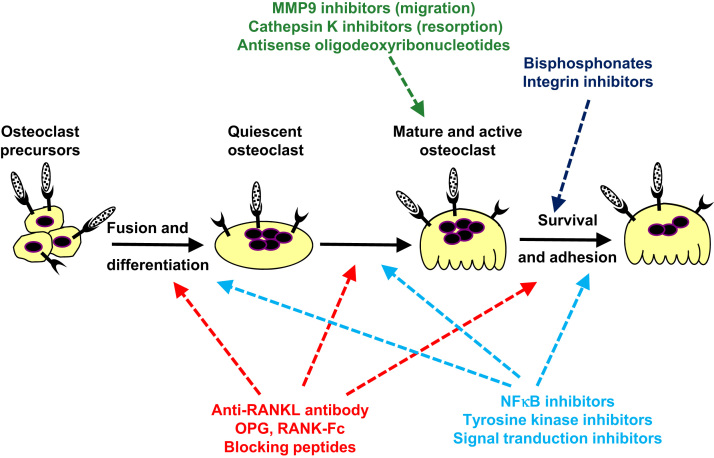
Fig. 4.
Therapeutic arsenals currently used or in development targeting osteoclast lineage to treat bone tumours. These therapeutic approaches target osteoclasts, their differentiation and/or their activation by blocking RANKL binding to RANK, by signal transduction, cell adhesion and migration or enzymatic activities.
7. Conclusions
Bone tissue massively attracts tumour cells where they find a favourable environment to maintain the stem cell dormancy and where they find a fertile ground for their development. This “fatal attraction” linked to the specific bone niche has boosted therapeutic innovations targeting the tumour cells and/or their microenvironment [158]. During the last past decade, RANK/RANKL axis emerged in bone biology as predominant protagonists of bone remodelling and as therapeutic targets of bone loss diseases. Better knowledge of RANK/RANKL biology will better define their relevance as biomarkers in bone oncology, and a complete cartography of RANK expression will be very useful to predict good responders to anti-RANKL therapies. Although anti-RANKL therapy progressively competes with approaches by bisphosphonates, a lot of prospect including signal transduction inhibitors, peptides or enzymatic inhibitors has been already identified and pre-clinical data as well as clinical trials allow personalised therapies in bone oncology.
Acknowledgments
Works presented in the present review were partly supported by Amgen Inc. Laboratories, by the Région des Pays de la Loire [Program entitled “Ciblage Moléculaire et Applications Thérapeutique” (CIMATH)] and by the Ligue Nationale Contre le Cancer.
References
- 1.Dempster D.W. Anatomy and functions of the adult skeleton. In: Favus M.J., editor. Primer of the metabolic bone diseases and disorders of mineral metabolism. 6th ed. American Society for Bone and Mineral Research Publication Office; Durham: 2006. pp. 7–11. [Google Scholar]
- 2.Favus M.J., Bushinsky D.A., Lemann J., Jr . Regulation of calcium, magnesium, and phosphate metabolism. In: Favus M.J., editor. Primer of the metabolic bone diseases and disorders of mineral metabolism. 6th ed. American Society for Bone and Mineral Research Publication Office; Durham: 2006. pp. 76–83. [Google Scholar]
- 3.Deschaseaux F., Sensebe L., Heymann D. Mechanisms of bone repair and regeneration. Trends in Molecular Medicine. 2009;15:417–429. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Ross F.P. Osteoclast biology and bone resorption. In: Favus Murray J., editor. Primer of the metabolic bone diseases and disorders of mineral metabolism. 6th ed. American Society for Bone and Mineral Research Publication Office; Durham: 2006. pp. 30–35. [Google Scholar]
- 5.Rousselle A.V., Heymann D. Osteoclastic acidification during bone resorption. Bone. 2002;30:533–540. doi: 10.1016/s8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- 6.Filgueira L. Osteoclast differentiation and function. In: Heymann D., editor. Bone Cancer. Academic Press; 2010. pp. 59–66. [Google Scholar]
- 7.Billiard J. Regulation of osteoblast differentiation and bone cancers by Wnt and PTH signaling pathways. In: Heymann D., editor. Bone Cancer. Academic Press; 2010. pp. 47–58. [Google Scholar]
- 8.Aubin J.E., Lian J.B., Stein G.S. Bone formation maturation and functional activities of osteoblast lineage cells. In: Favus Murray J., editor. Primer of the metabolic bone diseases and disorders of mineral metabolism. 6th ed. American Society for Bone and Mineral Research Publication Office; Durham: 2006. pp. 20–29. [Google Scholar]
- 9.Takahashi N. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 10.Jimi E. Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology. 1996;137:2187–2190. doi: 10.1210/endo.137.5.8612568. [DOI] [PubMed] [Google Scholar]
- 11.Mundy G.R., Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–443. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Hauben E.I., Hogendoorn P.C.W. Epidemiology of primary bone tumors and economical aspects of bone metastases. In: Heymann D., editor. Bone Cancer. Academic Press; 2010. pp. 3–8. [Google Scholar]
- 13.Wittrant Y. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochimica et Biophysica Acta. 2004;1704:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Koeneman K.S., Yeung F., Chung L.W. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. The Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.David E. The bone niche of chondrosarcoma: a sanctuary for drug resistance, tumour growth and also a source of new therapeutic targets. Sarcoma 2011; 932451 [ID 932451] [DOI] [PMC free article] [PubMed]
- 16.Simonet W.S. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda E. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 18.Lacey D.L. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda H. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of National Academy of Sciences USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theoleyre S., Wittrant Y.Kwan, Tat S., Fortun Y., Redini F., Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine & Growth Factor Reviews. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Heymann D., Fortun Y., Rédini F., Padrines M. Osteolytic bone diseases: physiological analogues of bone resorption effectors as alternative therapeutic tools to the standard bisphosphonates. Drug Discovery Today. 2005;10:242–247. doi: 10.1016/S1359-6446(04)03265-9. [DOI] [PubMed] [Google Scholar]
- 22.Mori K., Ando K., Heymann D., Redini F. Receptor activator of nuclear factor-kappa B ligand (RANKL) stimulates bone-associated tumours through functional RANK expressed on bone associated cancer cells? Histology and Histopathology. 2009;24:235–242. doi: 10.14670/HH-24.235. [DOI] [PubMed] [Google Scholar]
- 23.Baud'huin M., Lamoureux F., Duplomb L., Rédini F., RANKL Heymann D. RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cellular and Molecular Life Sciences. 2007;64:2334–2350. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min H. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. The Journal of Experimental Medicine. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson D.M. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 26.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Grimaud E. Receptor Activator of Nuclear Factor kB Ligand (RANKL)/Osteoprotegerin (OPG) ratio is increased in severe osteolysis. The American Journal of Pathology. 2003;163:2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwan Tat S., Padrines M., Theoleyre S., Heymann D., IL-6 Fortun Y. RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine & Growth Factor Reviews. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Liu C. Structural and functional insights of RANKL–RANK interaction and signaling. Journal of Immunology. 2010;184:6910–6919. doi: 10.4049/jimmunol.0904033. [DOI] [PubMed] [Google Scholar]
- 30.Ito S., Hata T. Crystal structure of RANK ligand involved in bone metabolism. Vitamins and Hormones. 2004;67:19–33. doi: 10.1016/S0083-6729(04)67002-6. [DOI] [PubMed] [Google Scholar]
- 31.Emery J.G. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. The Journal of Biological Chemistry. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 32.Standal T. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–3007. doi: 10.1182/blood-2002-04-1190. [DOI] [PubMed] [Google Scholar]
- 33.Theoleyre S. Cellular activity and signaling induced by osteoprotegerin in osteoclasts: involvement of receptor activator of nuclear factor kB Ligand and MAPK. Biochimica et Biophysica Acta: Molecular Cell Research. 2004;1644:1–7. doi: 10.1016/j.bbamcr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Lamoureux F. Glycosaminoglycans as potential regulators of osteoprotegerin therapeutic activity in osteosarcoma. Cancer Research. 2009;69:526–536. doi: 10.1158/0008-5472.CAN-08-2648. [DOI] [PubMed] [Google Scholar]
- 35.Zannettino A.C. Osteoprotegerin (OPG) is localized to the Weibel–Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. Journal of Cellular Physiology. 2005;204:714–723. doi: 10.1002/jcp.20354. [DOI] [PubMed] [Google Scholar]
- 36.Baud'huin M., Duplomb L., Télétchéa S., Charrier C., Maillasson M., Fouassier M., Heymann D. Factor VIII/von Willebrand factor complex controls RANKL-induced osteoclastogenesis and cell survival. The Journal of Biological Chemistry. 2009;264:31704–31713. doi: 10.1074/jbc.M109.030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkins G.J. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. Journal of Bone and Mineral Research. 2006;21:1339–1349. doi: 10.1359/jbmr.060604. [DOI] [PubMed] [Google Scholar]
- 38.Wittrant Y., Mori K., Riet A., Kamijo A., Heymann D., Rédini F. RANKL directly induces bone morphogenetic protein-2 expression in RANK-expression POS-1 osteosarcoma cells. International Journal of Oncology. 2006;28:261–269. [PubMed] [Google Scholar]
- 39.Mori K. Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. The Journal of Pathology. 2007;211:555–562. doi: 10.1002/path.2140. [DOI] [PubMed] [Google Scholar]
- 40.Mori K. Receptor activator of nuclear factor-kB Ligand (RANKL) directly modulates gene expression profile of RANK-positive Saos-2 human osteosarcoma cells. Oncology Reports. 2007;18:1365–1371. [PubMed] [Google Scholar]
- 41.Hsu C.J. Involvement of integrin up-regulation in RANKL/RANK pathway of chondrosarcomas migration. Journal of Cellular Biochemistry. 2010;111:138–147. doi: 10.1002/jcb.22677. [DOI] [PubMed] [Google Scholar]
- 42.Santini D. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One. 2011;6:e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santini D. Expression pattern of receptor activator of NFkB (RANK) in a series of primary solid tumors and related metastases. Journal of Cellular Physiology. 2011;226:780–784. doi: 10.1002/jcp.22402. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia P., Sanders M.M., Hansen M.F. Expression of receptor activator of nuclear factor-kappaB is inversely correlated with metastatic phenotype in breast carcinoma. Clinical Cancer Research. 2005;11:162–165. [PubMed] [Google Scholar]
- 45.Jones D.H. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 46.Chen G., Sircar K., Aprikian A., Potti A., Goltzman D., Rabbani S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 47.Mori K., Le Goff B., Charrier C., Battaglia S., Heymann D., Rédini F. DU145 human prostate cancer cells express functional receptor activator of NFkappaB: new insights in the prostate cancer bone metastasis process. Bone. 2007;40:981–990. doi: 10.1016/j.bone.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong A.P., Miller R.E., Jones J.C., Zhang J., Keller E.T., Dougall W.C. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. The Prostate. 2008;68:92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 49.Chuang F.H., Hsue S.S., Wu C.W., Chen Y.K. Immunohistochemical expression of RANKL, RANK, and OPG in human oral squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2009;38:753–758. doi: 10.1111/j.1600-0714.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 50.Shin M., Matsuo K., Tada T., Fukushima H., Furuta H., Ozeki S., Kadowaki T., Yamamoto K., Okamoto M., Jimi E. The inhibition of RANKL/RANK signaling by osteoprotegerin suppresses bone invasion by oral squamous cell carcinoma cells. Carcinogenesis. 2011;32:1634–1640. doi: 10.1093/carcin/bgr198. [DOI] [PubMed] [Google Scholar]
- 51.Heymann M.F., Riet A., Le Goff B., Battaglia S., Paineau J., Heymann D. OPG, RANK and RANK ligand expression in thyroid lesions. Regulatory Peptides. 2008;148:46–53. doi: 10.1016/j.regpep.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Chen L.M. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. Journal of Cellular Biochemistry. 2011;112:933–941. doi: 10.1002/jcb.23009. [DOI] [PubMed] [Google Scholar]
- 53.Mikami S. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. The Journal of Pathology. 2009;218:530–539. doi: 10.1002/path.2567. [DOI] [PubMed] [Google Scholar]
- 54.Kupas V. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. The Journal of Investigative Dermatology. 2011;131:944–955. doi: 10.1038/jid.2010.377. [DOI] [PubMed] [Google Scholar]
- 55.Mori K., Ando K., Lezot F., Heymann D. RANK/RANKL axis in melanoma. In: Tanaka Y., editor. Breakthroughs in Melanoma Research. InTech Publisher; 2011. [chapter 27] [Google Scholar]
- 56.Fiumara P. Functional expression of receptor activator of nuclear factor kappaB in Hodgkin disease cell lines. Blood. 2001;98:2784–2790. doi: 10.1182/blood.v98.9.2784. [DOI] [PubMed] [Google Scholar]
- 57.Lee J.A. RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatric Blood & Cancer. 2011;56:738–743. doi: 10.1002/pbc.22720. [DOI] [PubMed] [Google Scholar]
- 58.Rucci N. Receptor activator of NF-kappaB ligand enhances breast cancer-induced osteolytic lesions through upregulation of extracellular matrix metalloproteinase inducer/CD147. Cancer Research. 2010;70:6150–6160. doi: 10.1158/0008-5472.CAN-09-2758. [DOI] [PubMed] [Google Scholar]
- 59.Cross S.S. Expression of receptor activator of nuclear factor kappabeta ligand (RANKL) and tumour necrosis factor related, apoptosis inducing ligand (TRAIL) in breast cancer, and their relations with osteoprotegerin, oestrogen receptor, and clinicopathological variables. Journal of Clinical Pathology. 2006;59:716–720. doi: 10.1136/jcp.2005.030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Poznak C., Cross S.S., Saggese M., Hudis C., Panageas K.S., Norton L., Coleman R.E. Holen Expression of osteoprotegerin (OPG), TNF related apoptosis inducing ligand (TRAIL), and receptor activator of nuclear factor kappaB ligand (RANKL) in human breast tumours. Journal of Clinical Pathology. 2006;59:56–63. doi: 10.1136/jcp.2005.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabbota A.L., Kim H.R., Zhe X., Fridman R., Bonfil R.D., Cher M.L. Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Research. 2010;70:5558–5566. doi: 10.1158/0008-5472.CAN-09-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odero-Marah V.A. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Research. 2008;18:858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 63.Yuvaraj S., Griffin A.C., Sundaram K., Kirkwood K.L., Norris J.S., Reddy S.V. A novel function of CXCL13 to stimulate RANK ligand expression in oral squamous carcinoma cells. Molecular Cancer Research. 2009;7:1399–1407. doi: 10.1158/1541-7786.MCR-08-0589. [DOI] [PubMed] [Google Scholar]
- 64.Penno H., Nilsson O., Brändström H., Winqvist O., Ljunggren O. Expression of RANK-ligand in prostate cancer cell lines. Scandinavian Journal of Clinical & Laboratory Investigation. 2009;69:151–155. doi: 10.1080/00365510802460466. [DOI] [PubMed] [Google Scholar]
- 65.Sood S.K., Balasubramanian S., Higham S., Fernando M., Harrison B. Osteoprotegerin (OPG) and related proteins (RANK, RANKL and TRAIL) in thyroid disease. World Journal of Surgery. 2011;35:1984–1992. doi: 10.1007/s00268-011-1185-5. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura E.S. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clinical & Experimental Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki A. Receptor activator of nuclear factor-kappaB ligand (RANKL) expression in hepatocellular carcinoma with bone metastasis. Annals of Surgical Oncology. 2007;14:1191–1199. doi: 10.1245/s10434-006-9277-4. [DOI] [PubMed] [Google Scholar]
- 68.Barcala V. RANKL expression in a case of follicular lymphoma. European Journal of Haematology. 2003;70:417–419. doi: 10.1034/j.1600-0609.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 69.Shibata H. Malignant B-lymphoid cells with bone lesions express receptor activator of nuclear factor-kappaB ligand and vascular endothelial growth factor to enhance osteoclastogenesis. Clinical Cancer Research. 2005;11:6109–6115. doi: 10.1158/1078-0432.CCR-05-0181. [DOI] [PubMed] [Google Scholar]
- 70.Shu S.T., Martin C.K., Thudi N.K., Dirksen W.P., Rosol T.J. Osteolytic bone resorption in adult T-cell leukemia/lymphoma. Leukemia & Lymphoma. 2010;51:702–714. doi: 10.3109/10428191003646697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sezer O., Heider U., Jakob C., Eucker J., Possinger K. Human bone marrow myeloma cells express RANKL. Journal of Clinical Oncology. 2002;20:353–354. doi: 10.1200/JCO.2002.20.1.353. [DOI] [PubMed] [Google Scholar]
- 72.Roux S. RANK (receptor activator of nuclear factor-kappaB) and RANKL expression in multiple myeloma. British Journal of Haematology. 2002;117:86–92. doi: 10.1046/j.1365-2141.2002.03417.x. [DOI] [PubMed] [Google Scholar]
- 73.Farrugia A.N. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Research. 2003;63:5438–5445. [PubMed] [Google Scholar]
- 74.Heider U. Expression of receptor activator of NF-kappaB ligand (RANKL) mRNA in human multiple myeloma cells. Journal of Cancer Research and Clinical Oncology. 2004;130:469–474. doi: 10.1007/s00432-004-0578-3. [DOI] [PubMed] [Google Scholar]
- 75.Buckle C.H., Neville-Webbe H.L., Croucher P.I., Lawson M.A. Targeting RANK/RANKL in the treatment of solid tumours and myeloma. Current Pharmaceutical Design. 2010;16:1272–1283. doi: 10.2174/138161210791034021. [DOI] [PubMed] [Google Scholar]
- 76.Granchi D. In vitro blockade of receptor activator of nuclear factor-kappaB ligand prevents osteoclastogenesis induced by neuroblastoma cells. International Journal of Cancer. 2004;111:829–838. doi: 10.1002/ijc.20308. [DOI] [PubMed] [Google Scholar]
- 77.Tang Z.N., Zhang F., Tang P., Qi X.W., Jiang J. Hypoxia induces RANK and RANKL expression by activating HIF-1α in breast cancer cells. Biochemical and Biophysical Research Communications. 2011;48:411–416. doi: 10.1016/j.bbrc.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L. Receptor activator for nuclear factor kB expression predicts poor prognosis in breast cancer patients with bone metastasis but not in patients with visceral metastasis. Journal of Clinical Pathology. 2012;65:36–40. doi: 10.1136/jclinpath-2011-200312. [DOI] [PubMed] [Google Scholar]
- 79.Dougall W.C. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clinical Cancer Research. 2012;18:326–335. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 80.Fata J.E. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 81.Mukherjee A. Targeting RANKL to a specific subset of murine mammary eptithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. Federation of American Societies of Experimental Biology. 2010;24:4408–4419. doi: 10.1096/fj.10-157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez-Valdivia R. The RANKL signaling axis is sufficient to elicit ductal sidebranching and alveologenesis in the mammary gland of the virgin mouse. Developmental Biology. 2009;328:127–139. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Suarez E., Branstetter D., Armstrong A., Dinh H., Blumberg H., Dougall W.C. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Molecular and Cellular Biology. 2007;27:1442–1454. doi: 10.1128/MCB.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Suarez E. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–137. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 85.Joshi P.A. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 86.Asselin-Labat M.L. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 87.Lamoureux F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: prevention of bone resorption, inhibition of tumor progression, increase of animal survival. Cancer Research. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 88.Lamoureux F. Therapeutic efficacy of soluble receptor activator of NF-kB delivered by nonviral gene transfer in a mouse model of osteolytic osteosarcoma. Molecular Cancer Therapeutics. 2008;7:3389–3398. doi: 10.1158/1535-7163.MCT-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rousseau J. Formulated siRNAs targeting RANKL prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. Journal of Bone and Mineral Research. 2011;26:2452–2462. doi: 10.1002/jbmr.455. [DOI] [PubMed] [Google Scholar]
- 90.Bekker P.J. The effect of a single dose of osteoprotegerin in postmenopausal women. Journal of Bone and Mineral Research. 2001;16:348–360. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 91.Body J.J. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 92.Bekker P.J. A single-dose placebo-controlled study of AMG162, a fully human monoclonal antibody to RANKL, in postmenopausal women. Journal of Bone and Mineral Research. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 93.McClung M.R. Denosumab in postmenopausal women with low bone mineral density. The New England Journal of Medicine. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 94.Kostenuik P.J. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. Journal of Bone and Mineral Research. 2009;24:182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- 95.Body J.J. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clinical Cancer Research. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 96.Henry D.H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of Clinical Oncology. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 97.Stopeck A.T. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. Journal of Clinical Oncology. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 98.Body J.J. Effects of denosumab in patients with bone metastases with or without previous bisphosphonate exposure. Journal of Bone and Mineral Research. 2010;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 99.Lipton A. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clinical Cancer Research. 2008;14:6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 100.Lipton A. Randomized active-controlled phase II study of denosumad efficacy and safety in patients with breast cancer-related bone metatases. Journal of Clinical Oncology. 2007;45:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 101.Ellis G.K. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. Journal of Clinical Oncology. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 102.Ellis G.K. Effect of denosumab on bone and mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Research and Treatment. 2009;118:81–87. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 103.Fizazi K. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 104.Vij R. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. American Journal of Hematology. 2009;84:650–656. doi: 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- 105.Fizazi K. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. The Journal of Urology. 2009;182:509–515. doi: 10.1016/j.juro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 106.Smith M.R. Denosumab in mem receiving androgen-deprivation therapy for prostate cancer. The New England Journal of Medicine. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith M.R. Effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostated cancer. The Journal of Urology. 2009;182:2670–2675. doi: 10.1016/j.juro.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith M.R. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fizazi K. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration resistant prostate cancer: a randomized, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas D. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. The Lancet Oncology. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 111.Jamal S.A. Effects of denosumab on fracture and bone mineral density by level of kidney function. Journal of Bone and Mineral Research. 2011;26:1835. doi: 10.1002/jbmr.403. [DOI] [PubMed] [Google Scholar]
- 112.Coleman R. Zoledronic acid. Expert Opinion on Drug Safety. 2011;10:133–145. doi: 10.1517/14740338.2011.540387. [DOI] [PubMed] [Google Scholar]
- 113.Migliorati C.A., Epstein J.B., Abt E., Berenson J.R. Osteonecrosis of the jaw and bisphosphonates in cancer: a narrative review. Nature Reviews Endocrinology. 2011;7:34–42. doi: 10.1038/nrendo.2010.195. [DOI] [PubMed] [Google Scholar]
- 114.Filleul O, Crompot E, Saussez S Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases 2010;136:1117-24. [DOI] [PubMed]
- 115.Heymann D. Bisphosphonates and bone diseases: past, present and future. Current Pharmaceutical Design. 2010;16:2948–2949. doi: 10.2174/138161210793563572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie J. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. Journal of Managed Care Pharmacy. 2011;17:621–643. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van de Wetering de Rooij L. Safety, pharmacokinetics and efficacy of anti-RANKL nanobody ALX-0141 in healthy postmenopausal wome. Annals of the Rheumatic Diseases. 2011;70(suppl 3):136. [Google Scholar]
- 118.Heymann D., Ory B., Gouin F., Green J., Rédini F. Bisphosphonates: new therapeutic agents for the treatment of bone tumors. Trends in Molecular Medicine. 2004;10:337–343. doi: 10.1016/j.molmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Ory B., Moriceau G., Rédini F., Heymann D. mTOR inhibitors (rapamycin and derivatives) and nitrogen-bisphosphonates: bi-functional compounds for the treatment of bone tumors. Current Medicinal Chemistry. 2007;14:1381–1387. doi: 10.2174/092986707780831159. [DOI] [PubMed] [Google Scholar]
- 120.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49:71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 121.Coleman R. The use of bisphosphonates in cancer treatment. Annals of the New York Academy of Sciences. 2011;1218:3–14. doi: 10.1111/j.1749-6632.2010.05766.x. [DOI] [PubMed] [Google Scholar]
- 122.Ory B. Zoledronic acid suppresses lung metastases and extends overall survival of osteosarcoma of osteosarcoma-bearing mice. Cancer. 2005;104:2522–2529. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 123.Gouin F., Rédini F., Ory B., Heymann D. Zoledronic acid slows down rat chondrosarcoma progression, increases overall survival and delays tumor recurrence after intralesional curettage. International Journal of Cancer. 2006;119:980–984. doi: 10.1002/ijc.21951. [DOI] [PubMed] [Google Scholar]
- 124.Ory B., Blanchard F., Battaglia S., Gouin F., Rédini F., Heymann D. Zoledronic acid activates the DNA S phase checkpoint and induces osteosarcoma cell death characterized by AIF and EndoG translocation in dependently of p53 and Rb status. Molecular Pharmacology. 2007;71:333–343. doi: 10.1124/mol.106.028837. [DOI] [PubMed] [Google Scholar]
- 125.Holen I., Coleman R.E. Bisphosphonates as treatment of bone metastases. Current Pharmaceutical Design. 2010;16:1262–1271. doi: 10.2174/138161210791034003. [DOI] [PubMed] [Google Scholar]
- 126.Lamoureux F. Relevance of a new rat syngenic model of osteoblastic metastases from prostate carcinoma for pre-clinical studies using zoledronic acid. International Journal of Cancer. 2008;122:751–760. doi: 10.1002/ijc.23187. [DOI] [PubMed] [Google Scholar]
- 127.Moriceau G. Therapeutic approach of primary bone tumors by bisphosphonates. Current Pharmaceutical Design. 2010;16:2981–2987. doi: 10.2174/138161210793563554. [DOI] [PubMed] [Google Scholar]
- 128.Ory B., Moriceau G., Redini F., Heymann D. mTOR inhibitors (rapamycin and its derivatives) and nitrogen containing bisphosphonates: bi-functional compounds for the treatment of bone tumours. Current Medicinal Chemistry. 2007;14:1381–1387. doi: 10.2174/092986707780831159. [DOI] [PubMed] [Google Scholar]
- 129.Heymann D. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 130.Moriceau G. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Research. 2010;70:10329–10339. doi: 10.1158/0008-5472.CAN-10-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Battaglia S. Impact of oncopediatric dosing regimen of zoledronic acid on bone growth: preclinical studies and case report of an osteosarcoma pediatric patient. Journal of Bone and Mineral Research. 2011;26:2439–2451. doi: 10.1002/jbmr.453. [DOI] [PubMed] [Google Scholar]
- 132.Daubiné F., Le Gall C., Gasser J., Green J., Clézardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. Journal of the National Cancer Institute. 2007;99:322–330. doi: 10.1093/jnci/djk054. [DOI] [PubMed] [Google Scholar]
- 133.Ottewell P.D., Mönkkönen H., Jones M., Lefley D.V., Coleman R.E., Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. Journal of the National Cancer Institute. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 134.Heymann D., Fortun Y., Redini F., Padrines M. Osteolytic bone diseases:physiological analogues of bone resorption effectors as alternative therapeutic tools. Drug Discovery Today. 2005;10:242–247. doi: 10.1016/S1359-6446(04)03265-9. [DOI] [PubMed] [Google Scholar]
- 135.Heymann D. Novel targeted therapies of bone tumors and future directions. Future Medicine Ltd., E book. In: Picci P, Ruggieri P, editors. Advances in bone metastasis management. 2012. p. 124–133. 10.2217/9781780840307.
- 136.Engleman V.W. A peptidomimetic antagonist of the alpha (v)beta3 inhibits bone resorption in vitro and prevents osteoporosis in vivo. The Journal of Clinical Investigation. 1997;99:2284–2292. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng X. Disabling of RANK receptor complex by novel osteoprotegerin like peptidomimetics restores bone loss in vivo. The Journal of Biological Chemistry. 2004;279:8269–8277. doi: 10.1074/jbc.M309690200. [DOI] [PubMed] [Google Scholar]
- 138.Aoki K. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. The Journal of Clinical Investigation. 2006;116:1525–1534. doi: 10.1172/JCI22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heath D.J. An osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myeloma. Cancer Research. 2007;67:202–208. doi: 10.1158/0008-5472.CAN-06-1287. [DOI] [PubMed] [Google Scholar]
- 140.Penolazzi L. Peptide nucleic acid-DNA decoy chimeras targeting NF-kappa B transcription factors: induction of apoptosis in human primary osteoclasts. International Journal of Molecular Medicine. 2004;14:145–152. [PubMed] [Google Scholar]
- 141.Penolazzi L. Decoy oligonucleotides targeting NF-κB transcription factors: induction of apoptosis in human primary osteoclasts. Biochemical Pharmacology. 2003;66:1189–1198. doi: 10.1016/s0006-2952(03)00470-2. [DOI] [PubMed] [Google Scholar]
- 142.Clohisy J.C. NF-κB signaling blockade abolishes implant particle-induced osteoclastogenesis. Journal of Orthopaedic Research. 2004;22:13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 143.Clohisy J.C. Direct inhibition of NF-kappa B blocks bone erosion associated with inflammatory arthritis. Journal of immunology. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- 144.Carron C.P. Peptidomimetic antagonists of alphavbeta3 inhibit bone resorption ny inhibiting osteoclast bone resorptive activity,not osteoclast adhesion to bone. The Journal of Endocrinology. 2000;165:587–598. doi: 10.1677/joe.0.1650587. [DOI] [PubMed] [Google Scholar]
- 145.Bakewell S.J. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proceedings of the National Academy of Sciences of the USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harms J.F. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MD-435 skeletal metastasis. Clinical & Experimental Metastasis. 2004;21:119–128. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 147.van der Horst G. Targeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13:516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao Y. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Research. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 149.Wadas T.J., Deng H., Sprague J.E., Zheleznyak A., Weilbaecher K.N., Anderson C.J. Targeting the alphavbeta3 integrin for small-animal PET/CT of osteolytic bone metastases. Journal of Nuclear Medicine. 2009;50:1873–1880. doi: 10.2967/jnumed.109.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harms J.F. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clinical & Experimental Metastasis. 2004;21:119–128. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 151.Ishibashi O., Niwa S., Kadoyama K., Inui T. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sciences. 2006;79:1657–1660. doi: 10.1016/j.lfs.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 152.Costa A.G., Cusano N.E., Silva B.C., Cremers S., Bilezikian J.P. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nature Reviews Rheumatology. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- 153.Sturge J., Caley M.P., Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nature Review Clinical Oncology. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 154.Ochi Y. Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone. 2011;49:1351–1356. doi: 10.1016/j.bone.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 155.Le Gall C. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Research. 2007;67:9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- 156.Xiang A. Changes in micro-CT 3D bone parameters reflect effects of a potent cathepsin K inhibitor (SB-553484) on bone resorption and cortical bone formation in ovariectomized mice. Bone. 2007;40:1231–1237. doi: 10.1016/j.bone.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 157.Jensen A.B. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: results of a 4-week, double-blind, randomized, controlled trial. Clinical Breast Cancer. 2010;10:452–458. doi: 10.3816/CBC.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 158.Weilbaecher K.N., Guise T., Mc Cauley L.K. Cancer to Bone: a fatal attraction. Nature Reviews Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]