Abstract
We have previously shown that repeated sequential administration of doxorubicin, followed 24 h later by zoledronic acid, inhibits tumour growth in models of established breast cancer bone metastasis. As breast cancer patients only receive zoledronic acid every 3–4 weeks, the aim of the current study was to establish the anti-tumour and bone effects of a single administration of doxorubicin/zoledronic acid combination therapy in a bone metastasis model.
MDA-MB-231-GFP cells were injected i.c. in 6-week-old nude mice. On day 2, animals received PBS, doxorubicin (2 mg/kg i.v.), zoledronic acid (100 μg/kg s.c.) or doxorubicin followed 24 h later by zoledronic acid. Anti-tumour effects were assessed on days 15/23 by quantification of apoptotic and proliferating cells and changes in expression of genes implicated in apoptosis, proliferation and bone turnover. Bone effects were assessed by μCT analysis, bone histomorphometry and measurement of serum markers. A tumour-free control group was included.
Combination treatment reduced bone tumour burden compared to single agent or PBS control and increased levels of tumour cell apoptosis on day 15, but this was no longer detectable on day 23. Animals receiving zoledronic acid had increased bone density, without evidence of tumour-induced lesions. Bone histomorphometry showed that zoledronic acid caused a decrease in osteoblast and osteoclast numbers and an increase in osteoclast size, in both tumour-free and tumour-bearing animals.
Our data show that although zoledronic acid modifies the bone microenvironment through effects on both osteoblasts and osteoclasts, this does not result in a significant anti-tumour effect in the absence of doxorubicin.
Abbreviations: Oc, Osteoclast; Ob, Osteoblast; PINP, Procollagen type I N-terminal propeptide; μCT, Microcomputed tomography; ELISA, Enzyme-linked immunoassay; H&E, Haematoxylin and eosin; TRAP, Tartrate-resistant acid phosphatase; GFP, Green fluorescent protein; NBP, Nitrogen-containing bisphosphonate
Keywords: Bone metastasis, Breast cancer, Bisphosphonate, Combination therapy
1. Introduction
Patients with breast cancer bone metastasis may be treated with a combination of chemotherapy, often an anthracyclin, in addition to the potent anti-resorptive bisphosphonate (BP) zoledronic acid. The optimisation of combination therapy in this setting has been the subject of a number of in vitro and in vivo studies. The majority of these have shown beneficial effects of BPs, alone or in addition to standard therapy, of a variety of tumours in and outside bone (reviewed in [1], [2]). A comprehensive comparison of the bone studies suggests that improved outcome may require earlier initiation of BP treatment schedule than that currently recommended for patients with metastatic bone disease [1]. We have found that even small tumour colonies induce major changes to the microenvironment before overt osteolysis is detected [3]. Early intervention may therefore be necessary to inhibit homing and growth of the tumour cells at the metastatic site.
The clinical relevance of bisphosphonates as anti-cancer agents has been subject to considerable debate and results relating to anti-tumour effects from clinical trials of bisphosphonates have been conflicting, with some reporting direct anti-tumour effects [4], [5] and some not [6]. However, the recently reported AZURE trial demonstrated that adjuvant zoledronic acid combined with chemotherapy in high-risk breast cancer patients did not result in increased overall survival in all patients [7]. Surprisingly, post-menopausal women did benefit from adjuvant zoledronic acid, with approximately 30% increase in overall survival compared to those receiving chemotherapy alone, suggesting that the endocrine environment plays a key role in determining the response to therapy. The cellular and molecular mechanisms responsible for this differential effect remain to be established, and there is clearly a need for more detailed investigations of effects of therapy on the bone microenvironment.
In order to treat metastatic bone disease effectively all steps involved in the metastatic cascade need to be taken into account as for example the interactions between primary and secondary sites, tumour cell–bone cell cross talk and osteomimicri [8]. Therefore, concomitant targeting of both the tumour and the bone microenvironment is likely to be required. Bisphosphonates have been combined with chemotherapy agents in models of bone metastasis from both breast and prostate cancer (reviewed in [2], [9]). An intensive dosing regimens of docetaxel and risedronate (4 mg/kg docetaxel twice a week, 150 μg/kg risedronate 5 times a week), has been shown to eliminate osteolytic bone disease and cause a substantial inhibition of tumour growth in a MDA-231luc model, compared to the single agents [10]. Similarly, Brubaker et al. found that repeated administration of zoledronic acid and docetaxel (100 μg/kg zoledronic acid twice a week and 20 mg/kg docetaxel every 2 weeks) was superior to the single agents at inhibiting growth of established LuCaP 23.1 prostate cancer bone metastases [11].
Using clinically achievable dosing regimens we have previously demonstrated a substantial, sequence-dependent, anti-tumour activity of doxorubicin (2 mg/kg) and zoledronic acid (100 μg/kg) in vivo. Six cycles of weekly administration of the two agents induced significant and sustained reduction in the growth of s.c. MDA-MB-436 xenografts [12], [13], osseous breast tumours [14] and inhibition of development of spontaneous mouse mammary tumours [15]. The clinical relevance of these studies is unclear, as patients currently receive zoledronic acid only once every 3–4 weeks, compared to the 6 weekly doses, and agents are routinely given on the same day rather than after a 24-hour gap. In a model of advanced bone metastasis with established lytic lesions, we have shown that a single administration of zoledronic acid given 24 h after the first cycle of weekly doxorubicin does reduce intra-osseous tumour growth [16]. However, starting treatment at this late stage of the disease did not have major impact on progression or survival, suggesting that earlier interventions are required to achieve significantly improved outcomes. In the present study we have therefore investigated whether administration of a single round of clinically achievable combination therapy at an early stage of bone metastasis development can prevent tumour progression in bone. Due to the increasing importance of the microenvironment in osseous tumour growth [17], we have also performed a detailed analysis of the effects of the therapeutic agents on bone cells, both in the presence and absence of tumour. Our data show that a single dose of zoledronic acid modifies both osteoblasts and osteoclasts, regardless of the presence of tumours. Despite zoledronic acid causing sustained increase in trabecular bone volume, there was no reduction in bone tumour burden without the addition of doxorubicin. Early administration of combination therapy caused a substantial but transient suppression of tumour growth, indicating that repeated courses of treatment are required for optimal anti-tumour effects.
2. Materials and methods
2.1. Maintenance of cell line and tumour growth model
Human MDA-MB-231 breast cancer cells stably expressing green fluorescent protein (GFP) were maintained in RPMI-1640 (GIBCO) supplemented with 10% foetal calf serum and 1% penicillin/streptomycin (PAA Laboratories) and grown at 37 °C and 5% CO2.
All experiments were carried out in accordance with local guidelines and with Home Office approval under project licence 40/2972 held by Professor N.J. Brown, University of Sheffield, United Kingdom. MDA-MB-231-GFP cells (1×105) were injected into the left heart ventricle of 6 week old female balb/c nu/nu mice under general anaesthesia induced by 100 mg/kg ketamine (Fort Dodge Animal Health Ltd) and 15 mg/kg xylazine (Bayer plc). A total of n=15 (sacrificed on day 15) and n=10 (sacrificed on day 23) tumour-bearing mice/group were used in addition to 24 tumour-free animals (n=3/group, sacrificed on day 15). All animals were randomised into 4 treatment groups: (1) PBS, (2) doxorubicin (2 mg/kg i.v.), (3) zoledronic acid (100 μg/kg s.c.) and (4) doxorubicin followed 24 h later with zoledronic acid. Treatment was initiated 2 days post tumour cell injection (Fig. 1). At the end of the protocol, food was removed and some animals were injected intraperitoneally with 400 mg/kg bromodeoxyuridine (BrdU; Sigma-Aldrich, 3 h before sacrifice) before blood was collected under deep non-recovery anaesthesia. For mice sacrificed on day 15, hind legs were fixed in PFA, scanned by μCT prior to decalcification. On day 23, right hind legs were processed as above while bone marrow and tumours of the left hind legs were used for gene expression analysis.
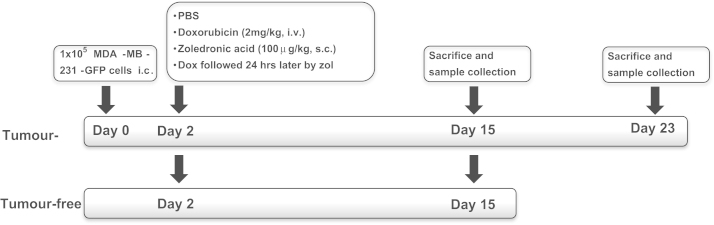
Fig. 1.
Study protocol assessing treatment-effects on tumour-free and tumour-bearing bones. For the tumour study, balb/c nude mice (female, 6 weeks) were injected with MDA-MB-231-GFP cells on day 0. All animals (including tumour-free mice) were treated on day 2 with PBS, doxorubicin (2 mg/kg i.v.), zoledronic acid (100 μg/kg s.c.) or doxorubicin followed 24 h later with zoledronic acid, animals were sacrificed on day 15 or 23.
2.2. Measurement of tumour area
Histological sections (3 μm) of decalcified hind legs were stained with H&E and analysis was carried out using Osteomeasure software. Tumour area (mm2) was measured on 2–3 non-serial sections per sample by interactively measuring all visible tumour foci. Intra- and extraosseous tumour areas were scored separately. Sections stained using Goldner's trichrome staining kit (Merck) was performed for exemplatory visualisation purposes only.
2.3. Immunohistochemistry
For immunohistochemical analysis of tumour cell apoptosis and proliferation, two non-serial sections per tumour sample were stained for BrdU (day 15), Ki67 (day 23) and caspase-3 (day 15 and 23) as previously described in [13], [16]. Caspase-3 staining was performed using rabbit polyclonal anti-active caspase-3 (AF835, R&D Systems; 1:750) followed by a biotin-conjugated anti-rabbit secondary antibody (BA-1000, Vector Laboratories; 1:200). Staining for the proliferation marker Ki67 was carried out using an anti-human Ki67 mouse monoclonal antibody (MIB-1, DakoCytomation; 1:125) followed by a biotin-conjugated anti-mouse secondary antibody (BA-2000, Vector Laboratories, 1:200). Immunohistochemistry for BrdU was done using mouse anti-human BrdU (Clone Bu20a, M0744, DakoCytomation; 1:175) and a biotin conjugated anti-mouse secondary antibody (BA-2000, Vector Laboratories; 1:200). The number of DAB positive cells per mm2 tumour area was scored using a Leica BMRB upright microscope and Osteomeasure software with a 10x objective. Intra- and extra-osseous tumour area were scored separately.
2.4. Tartate-resistant acid phosphatase (TRAP) staining and bone cell scoring
TRAP staining was used for all osteoclast (Oc) quantifications. Briefly, dewaxed sections were incubated in acetate–tartrate buffer at 37 °C for 5 min followed by incubation in naphthol AS–BI phosphate, dimethylformamide in acetate–tartrate buffer for 30 min at 37 °C. Finally sections were placed in a solution containing sodium nitrite, pararosaniline and acetate–tartrate buffer for 15 min at 37 °C, before counterstaining in haematoxylin. The number of TRAP stained osteoclasts per millimetre trabecular bone surface was scored using a Leica RMRB upright microscope with a 10x objective and OsteoMeasure software (Osteometrics). In the same sections, osteoblasts were scored per millimetre trabecular bone surface in line with previously published studies [18], [19], [20], [21]. At least two non-serial sections were analysed per sample and all trabecular bone surfaces were scored. For measurements of osteoclast size, the area of TRAP stained osteoclasts was analysed using OsteoMeasure software. Data are expressed as mean osteoclast size in mm2.
2.5. Microcomputed tomography imaging
Microcomputed tomography analysis was carried out using a Skyscan 1172 X-ray-computed microtomograph (Skyscan). Imaging was carried out at a voltage of 50 kV and a currency of 200 μA with a medium camera resolution of 2000×1024, an aluminium filter of 0.5 mm and pixel size was set to a dimension of 4.3 μm. Scanning was initiated from the proximal tibia or distal femur. For each sample, images were reconstructed with NRecon software. The volume of interest (VOI) was then specified by interactively drawing on the two-dimensional acquisition images. For trabecular bone measurements, the VOI was composed only of cancellous bone, and the cortices were excluded. Trabecular bone volume (BV/TV in %, which is defined as the percentage of the volume of interest occupied by binarised solid objects) was calculated covering 1 mm, starting from the lowest part of the growth plate.
2.6. Statistical analysis
Prism GraphPad (Version 5.0a) was used for all statistical analysis. Analysis was by t-test or one-way ANOVA followed by Tuckey post-test. The applied test is indicated in each figure legend. All data are shown as mean±SEM and differences have been interpreted as being significant at p≤0.05.
3. Results
This study used an established model of bone metastasis where intracardiac injection of MDA-MB-231 breast cancer cells result in tumour growth in the long bones of nude mice. As shown in Fig. 1, treatment was administered on days 2–3 following tumour cell injection, to allow tumour cell homing and initial colonisation of skeletal sites. Animals were sacrificed either on day 15 or day 23. A parallel experiment was performed using animals without tumours, terminated on day 15, to allow assessment of treatment-induced effects in a tumour-free bone microenvironment.
3.1. A single dose of combination therapy inhibits tumour growth in bone
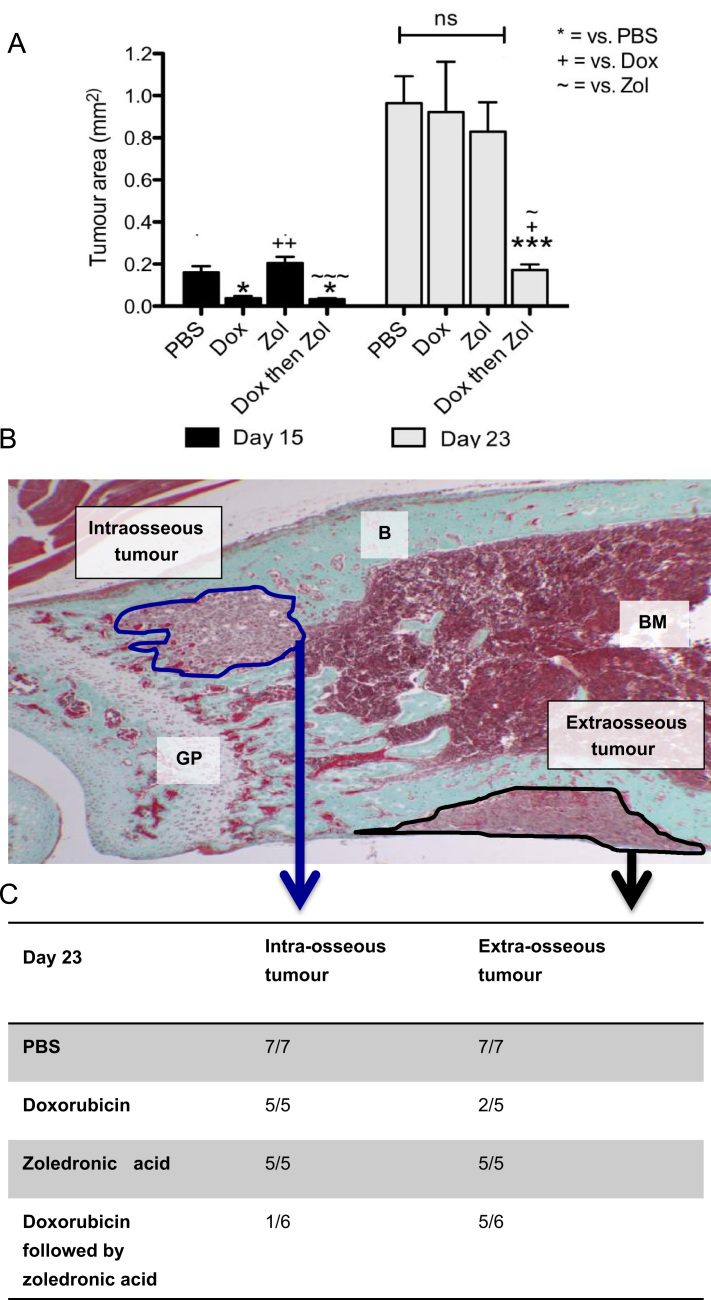
Previous in vivo studies have shown that repeated administration of doxorubicin followed 24 h later by zoledronic acid induced a significant reduction of breast tumour growth at various sites, including bone. As shown in Fig. 2A, a single, early dose of combination treatment was sufficient to effectively reduce bone associated tumour growth on day 15 compared to mice treated with PBS or zol alone (PBS 0.16 mm2 vs. dox then zol 0.03 mm2, p<0.05) and this was maintained on day 23 (PBS 0.97 mm2 vs. dox then zol 0.17 mm2, p<0.001). The combination treatment was the only therapeutic strategy used in this study that induced a sustained anti-tumour effect. Doxorubicin alone inhibited total tumour growth only transiently (day 15, PBS 0.16 mm2 vs. dox 0.04 mm2, p<0.05), but was not sufficient to affect later stages of tumour progression (day 23, PBS 0.95 mm2 vs. dox 0.92 mm2, p>0.05). Differential analysis of intra- vs. extra-osseous tumour area showed that the combination treatment had a major effect on intraosseous tumour growth, with only 1 animal out of 6 having detectable tumour foci in the bone marrow cavity on day 23 (Fig. 2C). In addition, extraosseous tumour growth in the combination group at day 15 was reduced by 29% (p<0.05) compared to that in animals receiving PBS (data not shown).
Fig. 2.
Effects of combination therapy on tumour burden. (A) Analysis of total tumour area (mm2) performed on a minimum of 2 non-serial sections per sample using a 4x objective. One way ANOVA and Tuckey post test, * is p<0.05, ** is p<0.01, *** is p<0.001, ns=not significant. (B) Example Goldner's trichrome stained section depicting extra- and intra-osseous tumour foci. (C) Number of mice on day 23 with intra- and extra-osseous tumour colony detected by histology.
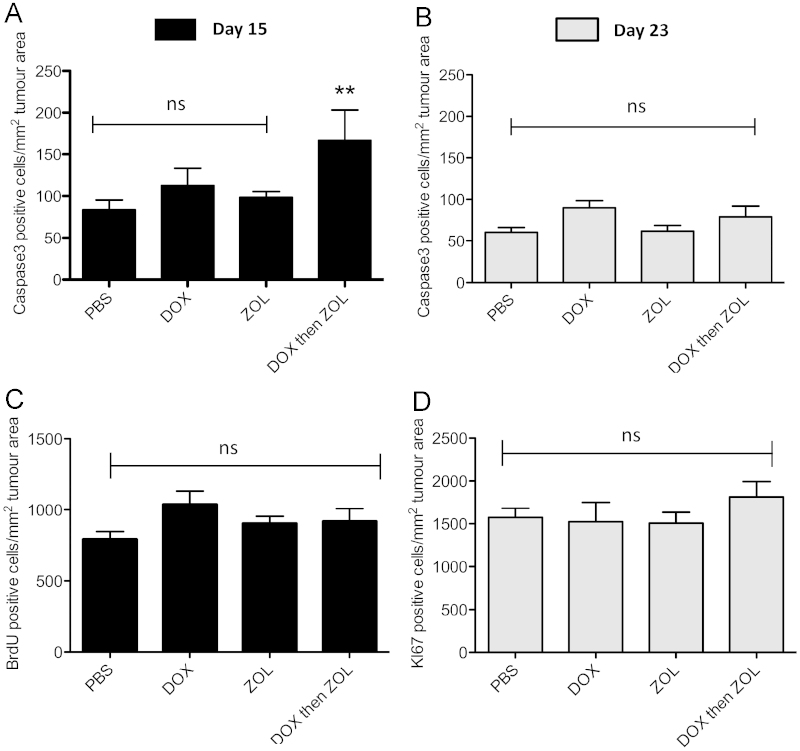
To determine whether the observed reduction in tumour size was due to treatment-induced changes in apoptosis and/or proliferation, caspase-3, Ki67 and BrdU immunohistochemistry were performed. Quantification of active caspase-3 positive tumour cells on day 15 showed that only the combination treatment caused a significant increase in the number of apoptotic tumour cells (dox then zol 166.62 vs. PBS 83.64, p<0.01, Fig. 3A). This effect was lost over time as no difference between any of the groups was detected on day 23 (Fig. 3B). Differential analysis of intra- and extraosseous tumour areas showed that the increase in overall tumour cell death in the combination group was due to an increase in apoptosis in tumours growing outside the bone marrow cavity on day 15. The data are in agreement with the observed decrease in extraosseous tumour area on this day. In addition, doxorubicin mono-therapy increased the number of apoptotic tumour cells in the intra but not the extraosseous tumour foci, again mirroring the effects on tumour size on day 15 (Fig. 2). No change in the number of caspase-3 positive tumour cells was observed in any treatment group on day 23 indicating that the effect on tumour cell apoptosis was only transient. This was supported by a lack of change in apoptotic genes (BCL2, CRADD) in tumours isolated from this treatment group (data not shown). Tumour cell proliferation was not significantly reduced by any of the treatment schedules, with tumours at day 15 and 23 showing high levels of Ki67/BrdU positive cells, regardless of whether they were located inside or outside the bone marrow cavity (Fig. 3C and D).
Fig. 3.
Effects on caspase-3 and Ki67/BrdU. Histological sections were stained for caspase-3 to detect apoptotic tumour cells. Data are expressed as number of positive cells per tumour area in mm2 for (A) day 15 and (B) day 23. Staining for (C) BrdU (day 15) or (D) Ki67 (day 23) was performed to detect tumour cell proliferation At least 2 non-serial sections per sample were analysed using a 10x objective. One way ANOVA and Tuckey post test, ** is p<0.01 vs. PBS control, ns=not significant.
These results show that a single dose of combination therapy is sufficient to reduce tumour growth inside the bone marrow cavity, most likely due to increased levels of tumour cell apoptosis in the first few days after administration.
3.2. Effects of therapy on naïve v.s. tumour bearing bone
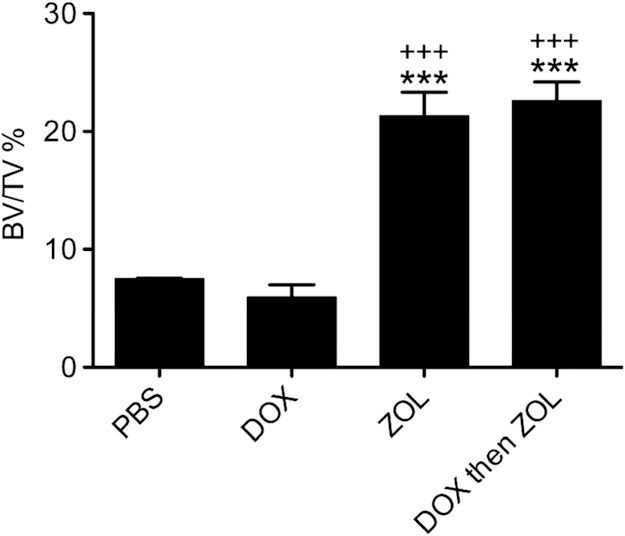
We next investigated the treatment-induced effects on bone structure, comparing bones from tumour-free and tumour-bearing animals. Analysis was performed in the regions of bone most commonly colonized by tumours in this model (proximal tibia/distal femur). This allowed us to identify the bone-specific effects of the different treatments, without the complication of tumour-induced bone disease. As shown in Fig. 4, a single administration of zoledronic acid, alone or in combination with doxorubicin, had profound effects on bone structure assessed by μCT. BV/TV was significantly increased compared to control (PBS 7.51% vs. zol 21.33% and dox followed by zol 22.60%, both p<0.001), mainly due to increased trabecular number. Doxorubicin did not affect trabecular bone structure compared to saline control, hence there was no chemotherapy-induced bone loss following the dose/schedule used in this study.
Fig. 4.
Effects on bone structure and integrity in the absence of tumour. Quantification of trabecular bone volume per tissue volume (BV/TV) for tumour-free animals sacrificed on day 15. One way ANOVA and Tuckey's post test, *** is p<0.001 compared to control and +++ is p<0.001 compared to doxorubicin.
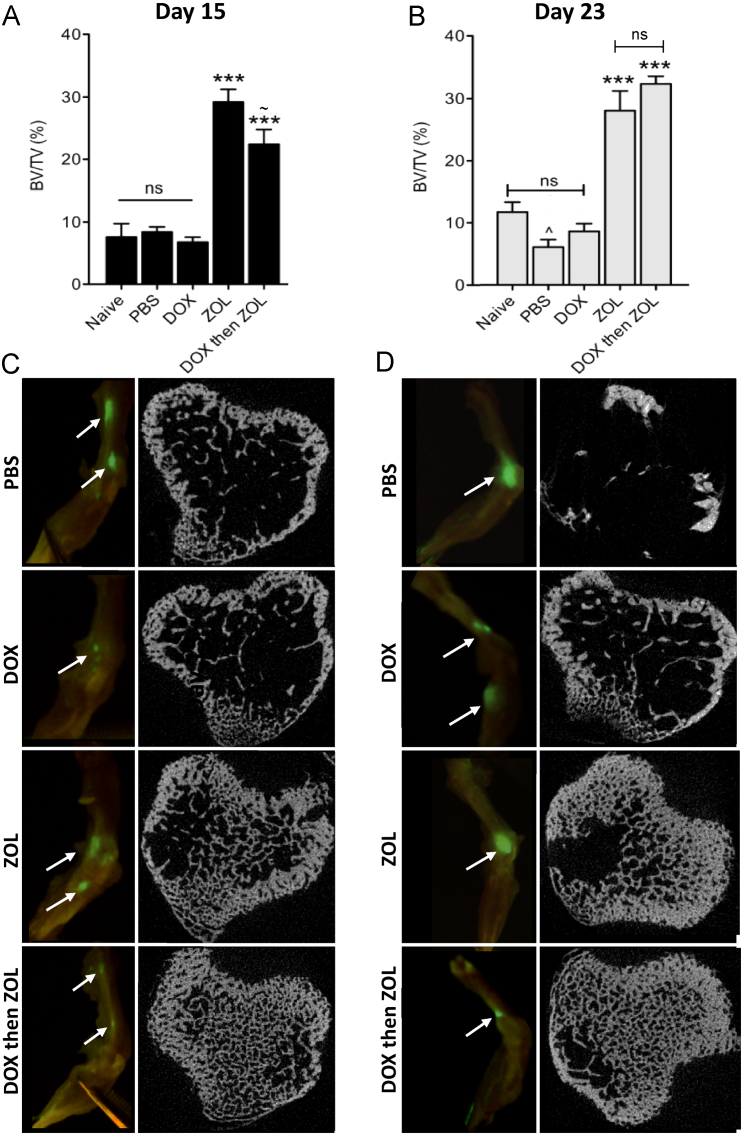
In tumour bearing animals, tumour growth was detected in hind legs of animals from all treatment groups, suggesting that none of the agents completely prevented the initial steps of tumour cell homing, colonisation and growth. Foci were generally located around the knee joint in the proximal tibia and/or distal femur (for examples see Fig. 5C and D), but were not associated with extensive bone destruction at termination on day 23. μCT analysis showed that a single dose of zoledronic acid administered two days post tumour cell injection significantly increased trabecular bone volume when compared to PBS or single agents (zol 29.24% and dox then zol 22.43% vs. PBS 8.4%, both p<0.001 on day 15; zol 28.09% and dox then zol 32.39% vs. PBS 6.1%, both p<0.001 on day 23, Fig. 5A and B). As was seen in tumour-free animals, doxorubicin did not reduce trabecular bone volume when compared to control at any time point. Further analysis revealed that the positive effects of treatment on bone volume were mainly due to an increase in trabecular number, rather than thickness. Examples of bone cross sections from each treatment group and time point are depicted in Fig. 5C and D.
Fig. 5.
Effects on bone structure and integrity in tumour-bearing bones. Analysis of osteolytic bone disease was carried out for the proximal tibia and the distal femur in animals sacrificed on (A) day 15 and (B) 23. Data are expressed as trabecular bone volume per tissue volume (% BV/TV). One way ANOVA and Tuckey's post test, *** is p<0.001 compared to naïve, PBS or dox; ^ is p<0.05 vs. naïve and ∼ is p<0.05 vs. zol; ns=not significant. Cross sections taken from μCT scans of the proximal tibia and images of GFP foci in bone are shown in for (C) day 15 and (D) day 23.
3.3. Treatment-induced changes of the bone microenvironment—effects on osteoblasts and osteoclasts
Therapeutic schedules that include zoledronic acid are likely to cause alterations in the bone microenvironment, but it is unclear how combination treatment affects the different cellular compartments in the region of the bone–tumour interphase. We therefore compared the effects of both single agents and combination therapy on osteoblast (Ob) and osteoclast (Oc) numbers in a tumour-free and a tumour-bearing setting.
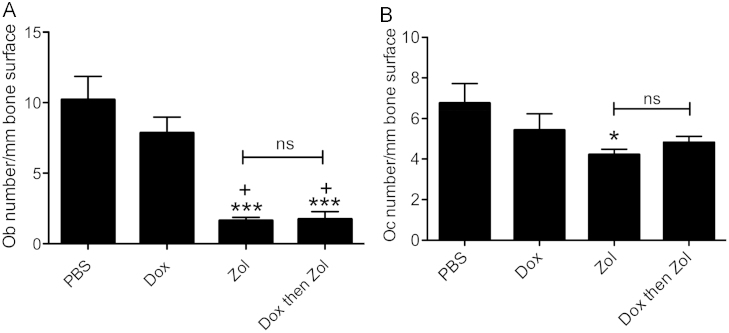
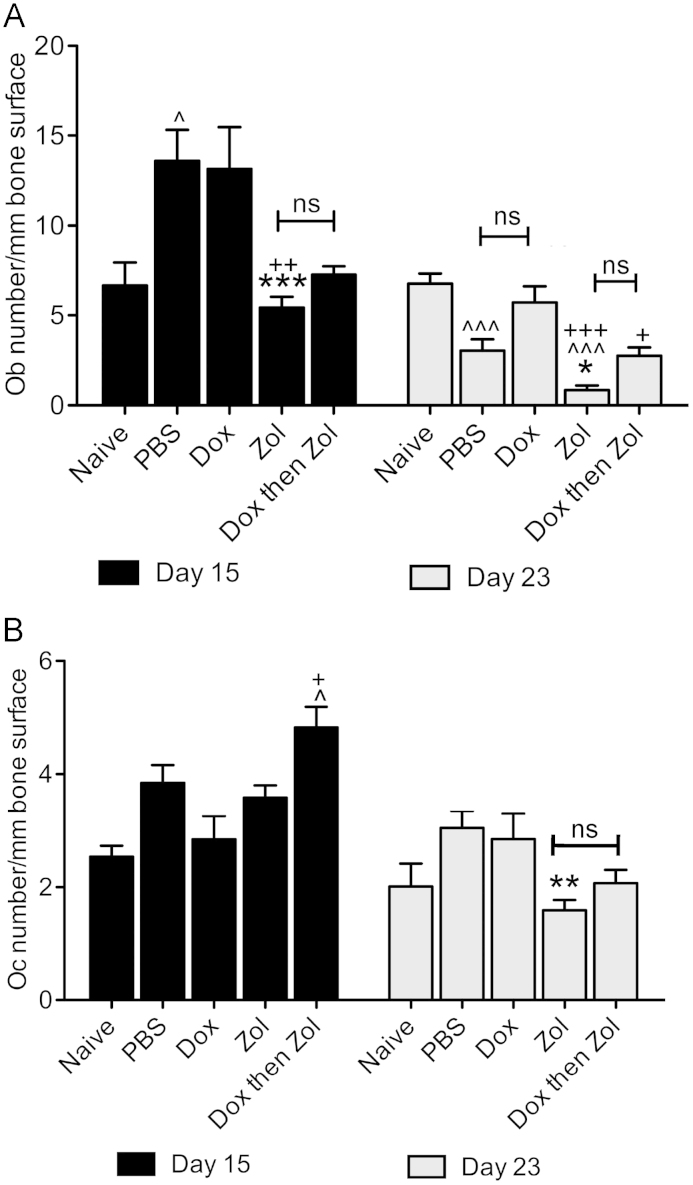
Detailed histomorphometric analysis of bones from tumour-free animals (day 15) showed a dramatic reduction in Ob number/mm bone following treatment with zol, either alone or following dox (PBS 10.21 vs. zol 1.63 and combination 1.74, both p<0.001, Fig. 6A). Similar effects were seen in tumour-bearing bones where zoledronic acid treatment inhibited the tumour-induced increase in osteoblast number seen in PBS control (zol 5.43 vs. PBS 13.59, p<0.001) and induced further reductions at day 23 (zol 0.86 vs. PBS 3.04, p<0.05, Fig. 7A). The effects of therapy on Ob numbers were reflected by a decrease in serum levels of the bone formation marker PINP on day 15 (PBS 42.17 ng/ml vs. zol 22.8 ng/ml, p<0.05). Differences in PINP levels were no longer detectable by 23 (data not shown), probably due to the 3-week interval between treatment and sample collection.
Fig. 6.
Treatment effects on osteoblast and osteoclast numbers in tumour-free bone. Animals were treated with PBS, 2 mg/kg dox, 100 μg/kg zol or combination therapy on day 2. Osteoblast (A) and osteoclast (B) numbers were analysed per mm trabecular bone surface on a minimum of 2 non-serial TRAP stained sections per sample. One way ANOVA and Tuckey post test, * is p<0.05 and *** is p<0.001 compared to PBS control; + is p<0.05 vs. dox; ns=not significant.
Fig. 7.
Treatment effects on osteoblast and osteoclast numbers in tumour bearing bone. Animals were treated with PBS, 2 mg/kg dox, 100 μg/kg zol or combination therapy on day 2. Osteoblast (A) and osteoclast (B) numbers were analysed per mm trabecular bone surface on a minimum of 2 non-serial TRAP stained sections per sample. One way ANOVA and Tuckey post test, * is p<0.05, ** is p<0.01 and *** is p<0.001 compared to PBS control; + is vs. dox and ^ is vs. naïve; ns=not significant.
In line with the reported anti-resorptive activity, zoledronic acid caused a reduction in osteoclast numbers in animals without tumour on day 15 (zol 4.22 vs. PBS 6.75, p<0.05, Fig. 6B). However, this effect was not seen in animals with tumours in bone on day 15 (Fig. 7B). This was surprising considering the zol-induced increase in trabecular bone volume detected by μCT at this time point (Fig. 5). At the later time point (day 23), osteoclast numbers in mice treated with zol or the combination treatment were reduced, with a significant change in the zol treated group compared to PBS control (zol 1.59 vs. 3.04, p<0.01, Fig. 7B). In agreement with the μCT data, doxorubicin did not affect the numbers of osteoblast or osteoclasts at either time point when compared to PBS control.
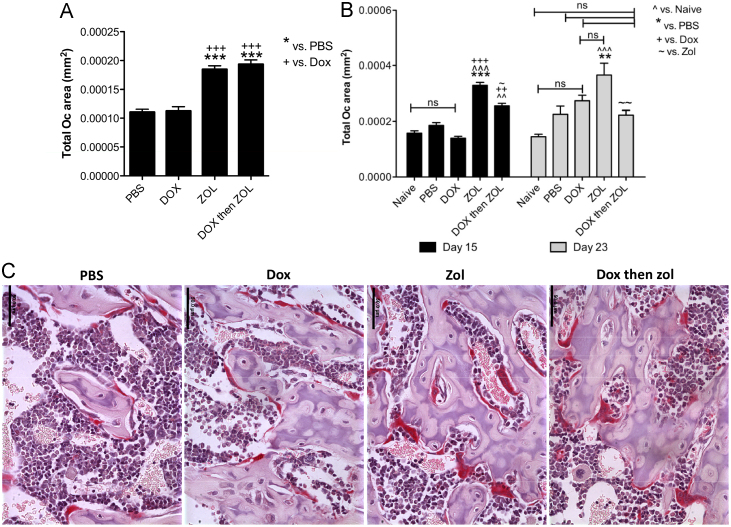
Although alterations in bone cell numbers are expected to indicate major treatment-induced effects, changes in cell morphology and activity may be equally important. We therefore performed a comprehensive analysis to establish the effects of the different treatments on osteoclast size, both in the presence and absence of tumour. In the tumour-free group, bones from zoledronic acid treated animals had significantly larger osteoclasts (measured in mm2) compared to animals in the PBS control group (PBS 1.1×10−4 mm2 vs. Zol 1.9×10−4 mm2 and vs. combination 1.9×10−4 mm2, both p<0.001, Fig. 8A). This effect was also observed at both time points in the tumour-bearing animals (PBS 1.9×10−4 mm2 vs. Zol 3.3×10−4 mm2 on day 15, p<0.001 and PBS 2.3×10−4 mm2 vs. Zol 3.7×10−4 mm2 on day 23, p<0.01, Fig. 8B). As illustrated in Fig. 8C, the majority of the osteoclasts in the zoledronic acid treated animals displayed a changed morphology compared to control, appearing bigger, thicker and covering large areas of bone. However, there were no differences in the levels of circulating CTX (bone resorption marker) between any of the treatment groups, probably due to the interval between treatment and serum sample collection (data not shown). Collectively the data indicate an initial effect of zoledronic acid on osteoclast activity, rather than by causing a decrease in osteoclast number.
Fig. 8.
Effects on osteoclast size. Measurement of osteoclast size (expressed as mean area in mm2) for osteoclasts in (A) tumour-free mice and (B) tumour-bearing animals. Data are shown as mean±SEM, One way ANOVA and Tuckey post test, * is p<0.05, ** is p<0.01, *** is p<0.001, ns=not significant. Black columns: grey columns: day 23. (C) Example images of TRAP stained osteoclasts (red) for each treatment group on day 15, scale bar =50μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This is the first report to show that a single administration of doxorubicin followed by zoledronic acid at an early time point (day 2 following tumour cell injection) is sufficient to cause significant inhibition of breast tumour growth in bone. The experiments were designed to allow comparison of treatment-induced effects over time and to establish whether these were sustained in the 3 weeks following administration of therapy. The inclusion of both tumour free and tumour-bearing animals is unique to our study, allowing us to identify and compare treatment-induced effects on bone in the presence and absence of tumour.
Our data show that, despite zoledronic acid having a substantial effect on bone, tumour growth was only reduced in animals that received combination therapy. This is in agreement with a number of studies reporting a lack of anti-tumour effects after bisphosphonate monotherapy. The basis of combination therapy is to effectively reduce tumour growth by targeting both the tumour and the microenvironment at the metastatic site. In agreement with previous studies [12], [13], [14], [16], we found that only tumours from the combination group exhibited significant differences in apoptosis on day 15, mirroring the reduction in tumour area. No changes in active tumour cell proliferation were detected at either time point. In addition, genes regulating apoptosis and proliferation showed to be significantly altered following 6 weekly cycles of combination therapy [14] were unchanged after the single cycle given in the present study. This is probably due to differences in treatment scheduling and sample collection points, as in the earlier investigations tumours were collected for analysis 24 h after the final administration of repeated cycles of combination therapy. The data suggest that apoptotic cell death and reduction in proliferation is likely to be an acute effect of combination therapy, and therefore no longer detectable 2–3 weeks following administration of treatment. These results clearly demonstrate that repeated cycles of combination therapy is required to maintain an anti-tumour in bone effect beyond 1–2 weeks.
The effects of zoledronic acid are most likely due to impairment of the tumour-driven alterations to the bone microenvironment. This may render the bone marrow cavity unfavourable for metastatic growth, potentially redirecting tumour cells to other sites, including the periosteal surface. Whereas combination therapy substantially reduced tumour growth inside the bone marrow cavity, growth in the periosteum was unaffected. There is preclinical evidence that bisphosphonate treatment exhibits stronger anti-tumour effects in the bone marrow cavity compared to the extraosseous bone-associated soft tissue when given alone or in combination with cytotoxic agents. Studies investigating the effects of bisphosphonates as a single agent have reported either no effect on extraosseous tumour growth after pamidronate or olpadronate treatment [22] or increased bone-associated soft tissue tumours after risedronate [23] or the third generation bisphosphonate YH529 [24]. Collectively the data from these studies suggest that the bisphosphonate-induced inhibition of bone remodelling may redirect tumour growth to extraosseous sites. We have previously shown that doxorubicin given 24 h after zoledronic acid induced significantly increased levels of tumour cell apoptosis and reduced proliferation in intraosseous parts of large MDA-MB-231/B02 tumours, whereas the extraosseous parts of the same tumour were unaffected [16]. The present data are in agreement with these reports, showing that a single dose of combination treatment effectively reduced tumour growth inside the bone marrow cavity, but did not eliminate extraosseous tumour growth. Importantly, the tumour colonies growing along the periosteal surface were not part of a large, intraosseous tumour spilling through osteolytic lesions but were separate tumour colonies. This could suggest that during the process of tumour cell dissemination, the cells seed to multiple sites but remain dormant until triggered to proliferate or that cells previously settled in the bone marrow cavity disseminated to other sites at relatively early stages of tumour progression (prior to day 15). There are few and opposing published reports relating to the effects of bisphosphonates on the cells of the periosteum, with the majority of studies focusing on characterisation of endosteal bone surfaces [25]. Risedronate and alendronate are reported to inhibit periosteal osteoblast activity independently of bone resorption [26]. In contrast, risedronate, alendronate or zoledronic acid were found to have no significant effect on periosteal bone formation in oestrogen-deficient rats [27]. The role of the periosteal microenvironment in development and progression of bone metastasis, as well as in response to therapy, therefore remains to be established. Our data suggest that development of improved therapeutic interventions must include control of tumour colonisation of periosteal sites, in addition to growth in the bone marrow cavity.
Studies of therapeutic agents in xenograft models of bone metastases are often limited to assessment of tumour burden (by imaging) and gross bone integrity (by μCT). To fully understand the effects of therapeutic interventions, we also characterised the treatment-induced changes of the key cell populations in the host bone microenvironment. In the absence of tumour, a single dose of zoledronic acid, given alone or in combination with doxorubicin, caused a significant reduction of both osteoclast and osteoblast numbers on day 15. Surprisingly, the effect on osteoblasts was the most pronounced, indicating that zoledronic acid either caused inhibition of osteoblast development, induction of osteoblast apoptosis, or both. Further studies specifically designed to distinguish between these options are needed. Evidence that bisphosphonates can be internalised by osteoblasts in vivo is reported by Idris et al. [28] who detected unprenylated Rap1a in bone forming cells derived from mouse calvaria 24 h after administration of 0.1 mg/kg alendronate. In agreement with this, Orriss et al. [29] demonstrated that zoledronic acid affects osteoblast development and function in vitro. Although the literature is frequently questioning the in vivo effect of bisphosphonates on cells other than osteoclasts [30], [31], the data shown in the present study support that a single, clinically achievable dose of zoledronic acid clearly affects osteoblasts. However, we were unable to determine whether the observed effects were due to zoledronic acid affecting osteoblasts directly, or whether the effects were downstream of the osteoclast effects. Well-recognised feedback loops exist between these cell types as a part of normal bone homoeostasis. As the anti-resorptive effect of zoledronic acid is evident soon after administration of a single dose [32] it is most likely that the effects on actively resorbing osteoclasts precede the osteoblast effects.
μCT analysis of tumour bearing bones showed significantly increased bone density in animals receiving zoledronic acid, alone or in combination with doxorubicin, compared to PBS control. Our results confirmed that a single dose of zoledronic acid caused sustained bone effects throughout the experiment (until day 23). Despite this, the effects on bone cell numbers in the tumour-bearing environment were less prominent than those detected in the tumour-free bones, possibly due to the strong influence the tumour cells have on bone turnover at the metastatic site. No reduction in osteoclast number was detected on day 15 although μCT analysis clearly showed a dramatic increase in bone volume. In agreement with our previous study using a 6-week regimen in established, intratibial MDA-MB-436 tumours [14], zoledronic acid, as well as combination therapy, caused a decrease in osteoclast numbers compared to PBS control on day 23. Zoledronic acid could not only cause a reduction in the number of osteoclasts, but more importantly reduce their activity. We suggest that the morphological changes observed in osteoclasts from the zoledronic acid treated animals reflect this, as was shown by scoring of mean osteoclast area. The presence of “giant” osteoclasts has also been reported after long-term alendronate treatment in an osteoporotic setting [33]. The authors proposed that aminobisphosphonates effectively inhibited osteoclast activity and prolonged the lifespan of the cells, ultimately leading to an increase in cell size due to continuous fusion of the osteoclast with mononuclear precursors. In addition, apoptosis was detected in about 30% of the “giant” cells [34]. The data therefore suggest that bisphosphonate therapy decreases osteoclast activity and induces changes cell morphology before reducing viability. Prolonged/repeated treatment may not be required to generate this effect, as we have consistently noted larger osteoclasts in samples from zoledronic acid treated animals compared to control. A direct link between osteoclast size and anti-resorptive activity could not be established in the present study, as serum CTX levels did not reflect the treatment-induced changes in bone structure. This was most likely due to the 2–3 week time gap between treatment and the collection of serum.
Similar to the results observed in the tumour-free bones, albeit less extensive, was that the main bone cell affected by zoledronic acid in tumour-bearing bone was the osteoblast, with major reductions in cell number and activity (PINP) detectable on day 15. Zoledronic acid treatment may therefore effectively counteract the tumour-induced changes in both osteoblast and osteoclast numbers evident in bones from control animals treated with PBS. The effects on the bone microenvironment were caused by zoledronic acid, as doxorubicin treatment had no effect on bone cell numbers and there was no synergistic effect following combination treatment.
In summary, we have shown that administration of a single round of combination therapy significantly reduces tumour burden and preserves bone integrity, but is not sufficient to eliminate tumour growth in bone. Our results are in agreement with the established clinical effects of zoledronic acid in the advanced cancer setting, showing a dampening of disease rather than elimination of bone metastasis [35]. Our data show that although zoledronic acid modifies the bone microenvironment through effects on both osteoblasts and osteoclasts, this does not result in a significant anti-tumour effect in the absence of doxorubicin. As indicated by the recently published data from the AZURE trial [7], additional components of the bone microenvironment linked to menopausal status may be the key to the development of tumour growth in bone and response to combination therapy.
Acknowledgements
This study was supported by the Weston Park Hospital Cancer Charity, Sheffield UK and the Breast Cancer Campaign, UK. Zoledronic acid used in this study was a kind gift from Novartis Pharma.
References
- 1.Brown H.K., Holen I. Anti-tumour effects of bisphosphonates—what have we learned from in vivo models? Current Cancer Drug Targets. 2009;9:807–823. doi: 10.2174/156800909789760339. [DOI] [PubMed] [Google Scholar]
- 2.Syddall S.P., Ottewell P.D., Holen I. Combined therapies of bone disease with bisphosphonates. Current Pharmaceutical Design. 2010;16:2988–2997. doi: 10.2174/138161210793563590. [DOI] [PubMed] [Google Scholar]
- 3.Brown H.K., Ottewell P.D., Evans C.A., Holen I. Location matters: osteoblast and osteoclast distribution is modified by the presence and proximity to breast cancer cells in vivo. Clinical and Experimental Metastasis. 2012 doi: 10.1007/s10585-012-9481-5. [DOI] [PubMed] [Google Scholar]
- 4.Diel I.J., Solomayer E.F., Costa S.D., Gollan C., Goerner R., Wallwiener D. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. The New England Journal of Medicine. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 5.Diel I.J., Jaschke A., Solomayer E.F., Gollan C., Bastert G., Sohn C. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Annals of Oncology. 2008;19:2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saarto T., Blomqvist C., Virkkunen P., Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19:10–17. doi: 10.1200/JCO.2001.19.1.10. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M. Breast-cancer adjuvant therapy with zoledronic acid. The New England Journal of Medicine. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 8.Santini D., Vincenzi B., Pantano F., Tonini G., Bertoldo F. Targeting bone metastases starting from the preneoplastic niche: home sweet home. Breast Cancer Research. 2011;13:111. doi: 10.1186/bcr2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holen I., Coleman R.E. Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Research. 2010;12:214. doi: 10.1186/bcr2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Beek E.R., Lowik C.W., van Wijngaarden J., Ebetino F.H., Papapoulos S.E. Synergistic effect of bisphosphonate and docetaxel on the growth of bone metastasis in an animal model of established metastatic bone disease. Breast Cancer Research and Treatment. 2009;118:307–313. doi: 10.1007/s10549-008-0236-6. [DOI] [PubMed] [Google Scholar]
- 11.Brubaker K.D., Brown L.G., Vessella R.L., Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006;6:15. doi: 10.1186/1471-2407-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottewell P.D., Lefley D.V., Cross S.S., Evans C.A., Coleman R.E., Holen I. Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. International Journal of Cancer. 2010;126:522–532. doi: 10.1002/ijc.24756. [DOI] [PubMed] [Google Scholar]
- 13.Ottewell P.D., Monkkonen H., Jones M., Lefley D.V., Coleman R.E., Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. Journal of the National Cancer Institute. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 14.Ottewell P.D., Woodward J.K., Lefley D.V., Evans C.A., Coleman R.E., Holen I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Molecular Cancer Therapeutics. 2009;8:2821–2832. doi: 10.1158/1535-7163.MCT-09-0462. [DOI] [PubMed] [Google Scholar]
- 15.Ottewell P., Brown H., Jones M., Rogers T., Cross S., Brown N. Combination therapy inhibits development and progression of mammary tumours in immunocompetent mice. Breast Cancer Research and Treatment. 2011 doi: 10.1007/s10549-011-1782-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Ottewell P.D., Deux B., Monkkonen H., Cross S., Coleman R.E., Clezardin P. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clinical Cancer Research. 2008;14:4658–4666. doi: 10.1158/1078-0432.CCR-07-1545. [DOI] [PubMed] [Google Scholar]
- 17.Santini D., Pantano F., Vincenzi B., Tonini G., Bertoldo F. The role of bone microenvironment, vitamin D and calcium. Recent Results in Cancer Research. 2012;192:33–64. doi: 10.1007/978-3-642-21892-7_2. [DOI] [PubMed] [Google Scholar]
- 18.Chantry A.D., Heath D., Mulivor A.W., Pearsall S., Baud'huin M., Coulton L. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. Journal of Bone and Mineral Research. 2010;25:2633–2646. doi: 10.1002/jbmr.142. [DOI] [PubMed] [Google Scholar]
- 19.Deleu S., Lemaire M., Arts J., Menu E., Van Valckenborgh E., Vande Broek I. Bortezomib alone or in combination with the histone deacetylase inhibitor JNJ-26481585: effect on myeloma bone disease in the 5T2MM murine model of myeloma. Cancer Research. 2009;69:5307–5311. doi: 10.1158/0008-5472.CAN-08-4472. [DOI] [PubMed] [Google Scholar]
- 20.Heath D.J., Chantry A.D., Buckle C.H., Coulton L., Shaughnessy J.D., Jr., Evans H.R. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. Journal of Bone and Mineral Research. 2009;24:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 21.Mohanty S.T., Kottam L., Gambardella A., Nicklin M.J., Coulton L., Hughes D. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Research and Therapy. 2010;12:R149. doi: 10.1186/ar3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Pluijm G., Que I., Sijmons B., Buijs J.T., Lowik C.W., Wetterwald A. Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Research. 2005;65:7682–7690. doi: 10.1158/0008-5472.CAN-04-4188. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki A., Boyce B.F., Story B., Wright K.R., Chapman M., Boyce R. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Research. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 24.Sasaki A., Kitamura K., Alcalde R.E., Tanaka T., Suzuki A., Etoh Y. Effect of a newly developed bisphosphonate, YH529, on osteolytic bone metastases in nude mice. International Journal of Cancer. 1998;77:279–285. doi: 10.1002/(sici)1097-0215(19980717)77:2<279::aid-ijc18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Allen M.R., Hock J.M., Burr D.B. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Iwata K., Li J., Follet H., Phipps R.J., Burr D.B. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone. 2006;39:1053–1158. doi: 10.1016/j.bone.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Feher A., Koivunemi A., Koivunemi M., Fuchs R.K., Burr D.B., Phipps R.J. Bisphosphonates do not inhibit periosteal bone formation in oestrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone. 2010;46:203–207. doi: 10.1016/j.bone.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Idris A.I., Rojas J., Greig I.R., Van't Hof R.J., Ralston S.H. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcified Tissue International. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 29.Orriss I.R., Key M.L., Colston K.W., Arnett T.R. Inhibition of osteoblast function in vitro by aminobisphosphonates. Journal of Cellular Biochemistry. 2009;106:109–118. doi: 10.1002/jcb.21983. [DOI] [PubMed] [Google Scholar]
- 30.Brown H.K., Ottewell P.D., Coleman R.E., Holen I. The kinetochore protein Cenp-F is a potential novel target for zoledronic acid in breast cancer cells. Journal of Cellular and Molecular Medicine. 2011;15:501–513. doi: 10.1111/j.1582-4934.2009.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roelofs A.J., Coxon F.P., Ebetino F.H., Lundy M.W., Henneman Z.J., Nancollas G.H. Fluorescent risedronate analogs reveal bisphosphonate uptake by bone marrow monocytes and localization around osteocytes in vivo. Journal of Bone and Mineral Research. 2009 doi: 10.1359/jbmr.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini D., Vincenzi B., Hannon R.A., Brown J.E., Dicuonzo G., Angeletti S. Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncology Reports. 2006;15:1351–1357. [PubMed] [Google Scholar]
- 33.Jain N., Weinstein R.S. Giant osteoclasts after long-term bisphosphonate therapy: diagnostic challenges. Nature Reviews Rheumatology. 2009;5:341–346. doi: 10.1038/nrrheum.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein R.S., Roberson P.K., Manolagas S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. The New England Journal of Medicine. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini D., Virzi V., Fratto M.E., Bertoldo F., Sabbatini R., Berardi R. Can we consider zoledronic acid a new antitumor agent? Recent evidence in clinical setting. Current Cancer Drug Targets. 2010;10:46–54. doi: 10.2174/156800910790980223. [DOI] [PubMed] [Google Scholar]