Abstract
Purpose
To evaluate bone density changes at the level of normal bone and bone metastases after zoledronic acid (ZA) treatment in oncologic patients.
Materials and methods
We retrospectively evaluated 72 consecutive adult patients with histologically confirmed solid tumors with at least 1 newly diagnosed bone metastatic lesion. Bone metastases were diagnosed by bone scans and confirmed with computed tomography (CT). Patients received intravenous ZA, 4 mg, by 15-min infusion every 28 day through a peripheral or a central venous access and were monitored for at least 3 months and a maximum of 24 months. Bone density was determined at the level of bone metastases and at the level of normal trabecular and cortical bone using a ROI-based approach.
Results
A significant increase was demonstrated at the level of normal trabecular bone of the calvarium and the femoral neck. No significant increase of density was observed at the level of the normal cortical bone. Bone metastases showed a significant increase in CT density as compared to baseline up to 24 months after zoledronic acid.
Conclusion
We have found that long term treatment with ZA increases trabecular bone density in oncologic patients whereas normal cortical bone changes are not detectable.
Keywords: Bone metastasis, Cortical bone, Trabecular bone, CT Density, Zoledronic acid, Osteolytic
1. Introduction
Patients with advanced breast, prostate, lung and colo-rectal cancer frequently develop bone metastases (BMs). These lesions may be asymptomatic or may cause pain, pathologic fractures, malignant hypercalcemia, epidural spinal cord compression and/or shorten survival [1]. The underlying pathophysiology of BMs involves reciprocal interactions between tumor cells and the bone micro-environment that lead to the disruption of the balanced physiological activity between osteoblasts and osteoclasts. Loss of this critical balance results in a spectrum of osteolytic to osteoblastic bone lesions [2].
Computed tomography (CT) provides accurate morphological images of bone, allowing visualization of cortical and trabecular bone, tumor margins and dimensions [2]. Anatomical extension of lesions is depicted on CT studies as well as the presence of sclerosis in the context of lytic lesions [3]. Whole-body CT scans are diffusely used to detect osteolytic, mixed or osteoblastic bone lesions in the staging, follow-up and re-staging studies of oncological patients. CT scans are also considered crucial in the assessment of the bone response to therapy [2], [4], [5]. In osteolytic bone metastases, indeed, an increase of density is considered an indicator of response to therapy [2].
As potent inhibitors of osteoclast function, biphosphonates are being incorporated into the management of metastatic bone disease [6], with beneficial effects on skeletal complications [7], [9], bone pain [9], quality of life [7], [8], [9], particularly in advanced breast cancer [7], [10], multiple myeloma [8], [11], and more recently in lung, prostate and kidney cancer [12], [13]. Zoledronic acid (ZA) is a potent third generation nitrogen-containing biphosphonate, which has been widely used in the treatment of Paget's disease of bone [14], hypercalcemia [15], multiple myeloma [16], breast cancer BMs [16], prostate cancer BMs [17], lung cancer BMs [18] and osteolytic BMs [19], [20].
In patients with BMs, decrease of bone density is the consequence of several factors, not only of pathologic mechanisms at bone metastatic sites, but also of the normal ageing process, of concurrent postmenopausal or drug-related osteoporosis, or of androgen-deprivation therapy in men with prostate cancer [21].
In multicenter randomized controlled trials, once yearly injection of 5 mg of ZA has been demonstrated to significantly reduce the risk of vertebral and hip fractures in postmenopausal women [22] and the risk of recurrence of clinical fractures in men and women with a recent hip fracture [23].
Sclerosis of bone metastases has been documented by CT imaging after ZA treatment in studies [24], [25], [26] conducted on patients at an advanced stage of cancer. However, the CT changes of the normal bone after ZA treatment in oncological patients has not yet been established.
The primary aim of this study was to determine CT density changes of the normal trabecular and cortical bone tissue in oncological patients undergoing routine whole body CT examinations; as reference, in the same group of patients, the rate of sclerosis of bone metastases up to 24 months after the initiation of ZA treatment was evaluated.
2. Materials and methods
2.1. Patients
We conducted a retrospective analysis of adult patients with histologically confirmed solid tumors with at least 1 newly diagnosed bone metastatic lesion. Bone metastases were diagnosed by bone scans and confirmed on CT images. The study was carried out according to the principles of the Declaration of Helsinki. Our institutional review board approved the study and all patients provided a written informed consent to receive ZA administration and to undergo CT examinations.
The patients underwent therapy with ZA (Zometa®, Novartis Pharma, USA) in the period between December 2004 and February 2010. We included patients with a total bilirubin level lower than 2 mg/dL, and serum creatinine level lower than 2 mg/dL, to avoid the effects of prolonged immobility and hepatic or renal osteodystrophy on bone metabolism. Patients with proven peptic ulcer, poor performance status unrelated to bone disease (WHO 3±4), Paget's disease, primary hyperparathyroidism, administration of calcitonin and any prior treatment with bisphosphonates were excluded from the study.
Concurrent cytotoxic, hormonal or steroid therapy was permitted. Each patient was treated with chemotherapy and/or radiotherapy, using an individualized therapeutic approach according to the current international recommendations and the institutions' practice. Patients with metastatic breast cancer were allowed to receive hormonal therapy with tamoxifen or aromatase inhibitors in case of estrogen and/or progesterone receptor positive disease. Patients with castration-sensitive metastatic prostate cancer as well as patients with castration refractory disease were allowed to continue androgen deprivation therapy with luteinizing hormone-releasing hormone (LHRH) analogs and/or anti-androgens. Patients received intravenous ZA, 4 mg, by 15-min infusion every 28 day through a peripheral or a central venous access and were monitored for at least 3 months and a maximum of 24 months. According to standard procedures, supplementation with vitamin D (400 Units/die) and calcium (500 mg/die) was added.
All patients were monitored for skeletal related events (SREs) by physical examination and by diagnostic imaging techniques (X-rays, CT scan, or magnetic resonance imaging scan) at any symptom or sign indicating skeletal disease progression. The definition of SRE included pathologic fractures, surgery or radiotherapy to bone to treat or prevent an impending fracture, palliative radiotherapy to bone, spinal cord compression, malignant hypercalcemia, and changes in antineoplastic therapy because of worsening bone pain.
2.2. CT scans
CT images were obtained using a 16 and a 64 slice CT scanner (Somatom Sensation, Siemens, Erlangen, Germany). Whole body CT scans were acquired at baseline (prior to treatment) and every 3 and/or 6 and/or 12 months, according to the staging and restaging needs of each patient, to the current international recommendations and to the institutions' practice. WBCT used for the analysis were performed up to 24 months after the initiation of treatment with ZA. Images were obtained using the whole body protocol (KV 120, 140 mAs, B30 kernel) and were reformatted at 5 mm section thickness, before and after bolus administration of non-ionic iodinated contrast agent, at the concentration of 350 mg/mL (Iobitridol, Xenetix®, Guerbet, France), injected intravenously with a power injector (EnVision; Medrad Italia, PV, Italy) (total volume=120 mL, flow=3 mL/min).
Image evaluation was conducted on a separate workstation applying bone-specific Hounsfield Units (HU) windows (width: 2500 HU; window level: 480 HU). By using a two-reader consensus, C.C.Q. and P.D. drew regions of interest (ROIs) on images obtained in the contrast-enhanced scan (60–70 s after contrast injection). After opening the CT scans on the eFilm workstation (MERGE-Healthcare, NL), images were anonymized with no access to the date of the examination. ROIs were chosen at the level of the bone metastases on the basis of concordant CT and bone scans at baseline. Bone lesions previously treated by radiotherapy were not considered for analysis.
ROIs were drawn at the level of normal trabecular and cortical bone in the occipital calvarial bone chosen as a non-weight-bearing, and in the left femoral neck chosen as a weight-bearing skeletal segment. At the level of the normal trabecular bone of the left femoral neck, negative values related to the high content of fatty bone marrow were not considered for analysis and alternative ROIs were drawn.
At each site, three circle-sized ROIs (0.1 cm2 for bone metastases and normal trabecular bone and 0.05 cm2 for normal cortical bone) were drawn on an eFilm workstation (MERGE-Healthcare, NL) and the average value was used for analysis (Fig. 1). Since mixed bone metastases are frequent in patients with advanced cancer, two groups of predominantly lytic or predominantly sclerotic metastases were classified according to an attenuation threshold value of 300 HU, as previously reported [27]. Measurements were obtained at baseline CT and at the CT scans performed 12 and 24 months after the initiation of ZA treatment.
Fig. 1.
Regions of interest (ROIs) (red spots) were drawn at the level of the trabecular and cortical occipital calvarium (A), trabecular and cortical left femoral neck (B) and in the context of bone metastatic lesions (C). Average of three values at each site was used for comparison between groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Statistical analysis
Patient demographic data, clinical data, primary tumor characteristics and CT density measurements were loaded on a database.
The absolute data were plotted and the relative increments to baseline were calculated.
Descriptive statistics (median, quartiles, ranges, average and standard deviation) was performed. The difference between groups was determined using the non-parametric Wilcoxon signed ranks test on the SPSS platform (SPSS, 14.0). The p=0.05 was considered as the threshold for a significant difference among groups.
3. Results
3.1. Patients
We included 72 consecutive patients (35 males and 37 females). The mean age at the diagnosis of bone metastases was 67±12 (±standard deviation) years. Demographic details are provided in Table 1. According to the CT density threshold of 300 HUs, 64% (46/72) of the patients were included in the group of osteolytic metastases and 36% (26/72) of the patients were included in the group of osteoblastic metastases.
Table 1.
Patients demographics and characteristics.
| Total | Breast ca. | Prostate ca. | Colon ca. | Lung ca. | Other | |
|---|---|---|---|---|---|---|
| Patients no. | 72 | 29 | 14 | 10 | 6 | 13 |
| M/F | 35/37 | 1/28 | 14/0 | 8/2 | 5/1 | 7/6 |
| Age (years) | 67±12 | 66±12 | 73±8 | 76±9 | 68±11 | 58±13 |
| Histotype | – | Ductal ca. 23 | Adenoca. 14 | Adenoca. 9 | Adenoca. 2 | |
| Infiltrative lobular ca. 6 | Undifferentiated ca. 1 | Small cell ca. 2 | ||||
| Squamous cell ca. 2 | ||||||
| No. of patients with lytic/blastic metastases at baseline | 46/26 | 21/8 | 6/8 | 7/3 | 4/2 | 8/5 |
The largest group was represented by patients with a diagnosis of breast cancer and a high prevalence of osteolytic metastases (72%, 21/29) at baseline.
An acute reaction after the first administration of ZA was observed in 7% of the cases (5/72); episodes of asymptomatic hypocalcemia in 9% of the cases (6/72); a slight increase of creatinine serum levels (inferior to 1.5 mg/dL) in 11% of the cases (8/72), osteonecrosis of the mandible in 3% of the cases (2/72).
Skeletal related events were not observed in 50% of the cases (36/72). Among the group of patients that experienced a skeletal related event, 30 patients underwent radiotherapy, 4 patients experienced bone fractures and 2 patients received orthopedic surgery. No atypical fractures were reported.
3.2. CT density of normal bone
Among the 72 patients included in the study, density measurements were available at baseline in a group of 55 patients for the occipital calvarium and a group of 57 patients for the left femoral neck, because of the presence of metastatic lesions, suspicious focal areas of altered density, or the presence of prosthesis at these sites. Details are presented in Table 2.
Table 2.
CT density values and increments of normal bone at baseline, 12 months and 24 months after first administration of ZA.
| No. of observations | HUs median [25–75%] | Average of increment vs. baseline [±SEM] | pvalue [vs. baseline] | |
|---|---|---|---|---|
| Calvarial cortical bone | ||||
| 0 | 55 | 1536 [1327–1762] | – | – |
| 12 months | 55 | 1554 [1316–1906] | 14% [±8%] | 0.05 |
| 24 months | 37 | 1591 [1298–1954] | 22 [±15%] | 0.11 |
| Calvarial trabecular bone | ||||
| 0 | 55 | 1056 [878–1234] | – | – |
| 12 months | 55 | 1138 [890–1338] | 8 [±3%] | 0.01 |
| 24 months | 37 | 1146 [961–1506] | 11 [±4%] | 0.02 |
| Femoral cortical bone | ||||
| 0 | 57 | 726 [586–844] | – | – |
| 12 months | 57 | 715 [617–830] | 12% [±10%] | 0.21 |
| 24 months | 37 | 671 [587–846] | 5% [±4%] | 0.43 |
| Femoral trabecular bone | ||||
| 0 | 57 | 120 [89–153] | – | – |
| 12 months | 57 | 137 [00–179] | 23% [±14%] | 0.09 |
| 24 months | 37 | 137 [100–173] | 18% [±6%] | 0.005 |
After 12 months and 24 months of ZA treatment, we observed a significant increase of density at the level of normal trabecular bone of the occipital calvarium, as compared to baseline (p<0.05) (Fig. 2). The average increment of bone density was higher at the level of the left femoral neck as compared to the occipital calvarium. However, the increase of density at the femoral neck reached the significance threshold only after 24 months since the initiation of ZA treatment (p<0.001) (Fig. 2).
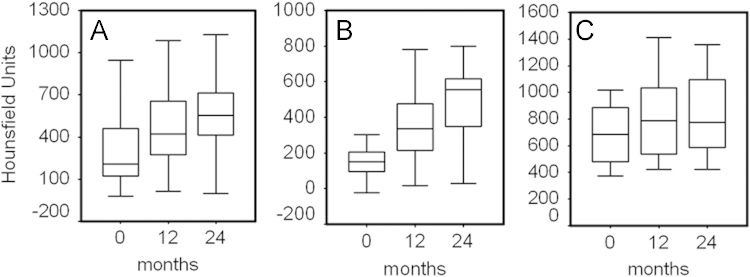
Fig. 2.
Box plots represent median, quartiles, minimum and maximum values of absolute Hounsfield units of calvarium (A and C) and femoral (D and E) normal bone. NCB=normal cortical bone; NTB=normal trabecular bone.
At the level of the normal cortical bone, we did not observe significant increases of density both in the occipital calvarium and in the left femoral neck (Table 2).
3.3. CT density of bone metastases
CT density at the level of BMs increased during treatment (Table 3 and Fig. 3). Patients showed an average increment of 98% after 12 months of treatment. Density values were 1.75 times higher than baseline after 24 months of treatment. As shown in Table 2, the relative increment of density was higher for osteolytic metastases (1.34 times at 12 months and 2.56 times at 24 months) than for osteoblastic metastases (35% at 12 months and 31% at 24 months). The density of bone osteolytic metastases significantly increased at 24 months in comparison to the 12 months time point (Fig. 4).
Table 3.
CT density values and increments of bone metastatic lesions at 12 months and 24 months after first administration of ZA. HU=Hounsfield units; SEM=standard error of the mean; BMs=bone metastases.
| No. of observations | HUs median [25–75%] | Average of increment vs. baseline [±SEM] | pvalue [vs. baseline] | |
|---|---|---|---|---|
| Total patients | ||||
| 0 | 72 | 260 [154–466] | – | – |
| 12 months | 54 | 525 [323–725] | 98% [±20%] | <0.0001 |
| 24 months | 35 | 555 [413–712] | X1.75 [±37%] | <0.0001 |
| Patients with lytic BMs | ||||
| 0 | 46 | 185 [103–251] | – | – |
| 12 months | 34 | 371 [222–541] | X1.34 [±27%] | <0.0001 |
| 24 months | 23 | 554 [348–617] | X2.56 [±40%] | <0.0001 |
| Patients with blastic BMs | ||||
| 0 | 26 | 514 [463–779] | – | – |
| 12 months | 20 | 747 [611–1007] | 35% [±6%] | 0.001 |
| 24 months | 11 | 774 [588–1096] | 31% [±7%] | 0.03 |
Fig. 3.
(A–C) A 65-year-old woman with diagnosis of ductal breast carcinoma. CT images of a predominantly lytic lesion (arrow in A) observed at baseline (A), 12 months (B) and 24 months (C) after initiation of ZA treatment; (D, E) A 75-year-old man with diagnosis of prostate adenocarcinoma. CT images of a predominantly blastic lesion (arrow in D) observed at baseline (D), 12 months (E) and 24 months (F) after initiation of ZA treatment.
Fig. 4.
Box plots represent median, quartiles, minimal and maximal values of absolute Hounsfield units of all (A), predominantly lytic (B) and predominantly blastic (C) bone metastases measured at baseline, 12 months and 24 months after initiation of ZA treatment.
4. Discussion
We found that long-term treatment with ZA increases trabecular bone density in oncologic patients, whereas normal cortical bone changes are not detectable. These findings may have important implications in tumor treatment and in the management of osteoporotic patients who are treated with much lower doses of ZA.
Biphosphonates are known to reduce the incidence of skeletal complications in patients with BMs and delay the onset of SREs and the progression of skeletal disease. ZA is a third generation bisphosphonate that has been shown to be more effective than other biphosphonates [27] and significantly reduces skeletal related complications compared with placebo in patients with BMs [28].
This study shows increase of bone density at the level of the normal trabecular bone in oncologic patients. In non-oncologic postmenopausal osteoporotic patients, once yearly injections of ZA (5 mg/year vs. much higher doses in oncology) significantly increase bone mineral density (BMD) of the trabecular compartment at the spine, femoral neck, trochanter and hip, when compared with placebo, as measured by both dual-energy X-ray absorptiometry (DXA) and quantitative computed analysis (QCT) [29].
In oncologic patients with bone metastases, bone resorption may be linked to several factors such as the normal ageing process, concurrent postmenopausal or drug-related osteoporosis, androgen-deprivation therapy in men with prostate cancer; in our group, even in the occurrence of these phenomena, we still observed an increase of bone density after 12 months (occipital calvarium) and 24 months (occipital calvarium and left femoral neck) of ZA treatment.
We observed a different response to ZA treatment over time: the results were different at the femoral neck as compared to the calvarium. Normal trabecular bone showed a significant increase after 12 months of ZA treatment at femoral neck but not at the calvarium. This could be explained either by a different effect of ZA in different skeletal segments or, alternatively, by concomitant higher bone turnover of the trabecular femoral neck.
At the 24 months time-point, the increase of density of the femoral neck showed a higher increase rate as compared to the calvarium: we interpreted these results possibly as a slow but progressive effect of weight-bearing at the femoral neck.
Density measures of the cortical bone did not yield, in respect to a trend toward a density increase, a significant change up to 24 months: still, these results may be explained by a different effect of ZA on the cortical bone in comparison to that on the trabecular bone or by HU measurements at a saturation level in the cortical compartment, so that slight increases could not be detected. These results, thus, confirm previous reports of a slight but not significant increase of cortical density after ZA treatment [29].
We, moreover, confirmed previous evidence of a net increase of CT density of bone metastases after ZA treatment and demonstrated that the changes persist at a 24 months follow-up. This study and previous reports [24], [25], [26] have demonstrated significant sclerotic changes of BMs, more evident in patients with osteolytic lesions. The current data show that changes persist after 24 months of treatment in both osteolytic and osteoblastic metastases. The CT density of osteolytic metastases shows a progressive increase at 24 months in comparison to the 12 months time-point, suggesting that the effect does not reach a plateau level, even after 24 months of treatment. These data support and confirm a dominant effect of ZA as an inhibitor of osteoclastic activity, even in the long-term treatment. Previous reports have shown the effect of concomitant ZA and radiation treatment on density of bone metastases [30]. Radiation therapy alone has been previously shown to increase density of bone metastases by means of quantitative computed tomography [31], [32], [33]; in our study, however, skeletal segments previously treated with radiation were not considered and excluded from analysis.
Since concurrent antiblastic and/or hormonal treatments were not discontinued, we cannot rule out an effect on density due to other drugs or to their interactions with ZA. However, bone density increase was seen both in extra-skeletal “responder” and “non-responder” patients, as previously shown [25]. In rat models, ZA alone produces dose-dependent increases in cancellous bone volume and connectivity, 100 times more effectively than pamidronate, and decreases bone resorption [34]. As such, due to the inclusion of patients with different primary tumors and different chemotherapeutic schedules, it is sufficiently safe to claim, on the basis of our and previous reported data, a direct and independent effect of ZA on the increase of density of bone metastases in our patients. This effect is also supported by several reports showing that biphosphonate administration may significantly decrease levels of osteolytic [35], [36] and osteoblastic [37], [38] bone markers. Moreover, up-regulation of NF-κB ligand (RANKL) and osteoprotegerin levels [39], critical for the regulation of osteoclasts maturation, function, and survival, are known to occur following biphosphonates treatment [40], [41].
Osteosclerosis was also significant at the level of osteoblastic bone metastases. The effect, in this case, seemed to reach a plateau level since the difference between the 24 and the 12 months time point was not significant in this subgroup. Residual osteoclastic activity is present even in sclerotic metastases as it is suggested by some evidence of increased bone resorption in osteosclerotic metastases of prostate cancer [42], [43]. Yi et al. [44] have shown in an animal model of osteoblastic metastases that an initial phase of bone destruction is followed by extensive formation of bone. Their data suggest that bone resorption precedes bone formation in the development of osteoblastic metastases and that osteoclast activation plays an important role even in the course of osteoblastic metastases [44], [45]. The plateau of the increment of density, indeed, could be explained either by a minor effect of ZA on osteoblastic metastases or even by the reaching of HU saturation levels.
A limitation of the study is represented by the lack of quantitative computed tomography measurements [29]. Moreover, given the thin thickness of the cortical bone at the femoral neck, analysis at this site may be biased. However, on this regard, our method of analysis can be applied on routine CT images to evaluate responses to ZA therapy in oncologic patients without needs of additional softwares or phantoms. As such, measurements of response to therapy may be conducted in the standard clinical setting without additional tools.
In conclusion, these results suggest that long-term treatment with ZA increases the bone density both at the level of the normal bone and at the level of bone metastases, with a potential effect on reduction of the incidence of SRE in oncologic patients.
References
- 1.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Reviews Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Hamaoka T., Madewell J.E., Podoloff D.A., Hortobagyi G.N., Ueno N.T. Bone imaging in metastatic breast cancer. Journal of Clinical Oncology. 2004;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 3.Hayward J.L., Carbone P.P., Heusen J.C. Assessment of response to therapy in advanced breast cancer. British Journal of Cancer. 1977;35:292–298. doi: 10.1038/bjc.1977.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy G.R. Mechanisms of bone metastasis. Cancer. 1997;80(Suppl. 8):1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Shih L.Y., Shih H.N., Chen T.H. Bone resorption activity of osteolytic metastatic lung and breast cancers. Journal of Orthopaedic Research. 2004;22(6):1161–1167. doi: 10.1016/j.orthres.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Body J.J. Clinical trials in metastatic breast cancer to bone: past–present—future. Canadian Journal of Oncology. 1995;5(Suppl. 1):16–27. [PubMed] [Google Scholar]
- 7.Hortobagyi G.N., Theriault R.L., Porter L. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. New England Journal of Medicine. 1996;335(24):1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 8.Berenson J.R., Lichtenstein A., Porter L. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. New England Journal of Medicine. 1996;334(8):488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 9.Purohit O.P., Anthony C., Radstone C.R., Owen J., Coleman R.E. High-dose intravenous pamidronate for metastatic bone pain. British Journal of Cancer. 1994;70:554–558. doi: 10.1038/bjc.1994.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson A.H., Powles T.J., Kanis J.A., McCloskey E., Hanson J., Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. Journal of Clinical Oncology. 1993;11(1):59–65. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Lahtinen R., Laakso M., Palva I., Virkkunen P., Elomaa I. Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia Group. Lancet. 1992;340(8827):1049–1052. doi: 10.1016/0140-6736(92)93075-x. [DOI] [PubMed] [Google Scholar]
- 12.Saad F., Lipton A. ZA is effective in preventing and delaying skeletal events in patients with bone metastases secondary to genitourinary cancers. BJU International. 2005;96(7):964–969. doi: 10.1111/j.1464-410X.2005.05740.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown J.E., Cook R.J., Major P. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. Journal of the National Cancer Institute. 2005;97(1):59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 14.Silverman S.L. Paget disease of bone: therapeutic options. Journal of Clinical Rheumatology. 2008;14(5):299–305. doi: 10.1097/RHU.0b013e318188b1f3. [DOI] [PubMed] [Google Scholar]
- 15.Kawada K., Minami H., Okabe K. A multicenter and open label clinical trial of ZA 4 mg in patients with hypercalcemia of malignancy. Japanese Journal of Clinical Oncology. 2005;35(1):28–33. doi: 10.1093/jjco/hyi005. [DOI] [PubMed] [Google Scholar]
- 16.Reed S.D., Radeva J.I., Glendenning G.A., Coleman R.E., Schulman K.A. Economic evaluation of ZA versus pamidronate for the prevention of skeletal-related events in metastatic breast cancer and multiple myeloma. American Journal of Clinical Oncology. 2005;28(1):8–16. doi: 10.1097/01.coc.0000138966.66780.3e. [DOI] [PubMed] [Google Scholar]
- 17.James N.D., Sydes M.R., Clarke N.W. Systemic therapy for advancing or metastatic prostate cancer [STAMPEDE]: a multi-arm, multistage randomized controlled trial. BJU International. 2009;103(4):464–469. doi: 10.1111/j.1464-410X.2008.08034.x. [DOI] [PubMed] [Google Scholar]
- 18.Langer C.J. Selected clinical trials in advanced non-small-cell lung cancer. Clinical Lung Cancer. 2010;11(5):358–359. [Google Scholar]
- 19.Rosen L.S., Gordon D.H., Dugan W., Jr ZA is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100(1):36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 20.Body J.J. Rationale for the use of bisphosphonates in osteoblastic and osteolytic bone lesions. Breast. 2003;12(Suppl. 2):37–44. doi: 10.1016/s0960-9776(03)80162-5. [DOI] [PubMed] [Google Scholar]
- 21.Saad F., Abrahamsson P.A., Miller K. Preserving bone health in patients with hormone-sensitive prostate cancer: the role of bisphosphonates. BJU International. 2009;104(11):1573–1579. doi: 10.1111/j.1464-410X.2009.08952.x. [DOI] [PubMed] [Google Scholar]
- 22.Black D.M., Delmas P.D., Eastell R. Once-yearly ZA for treatment of postmenopausal osteoporosis. New England Journal of Medicine. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 23.Lyles K.W., Colón-Emeric C.S., Magaziner J.S. ZA and clinical fractures and mortality after hip fracture. New England Journal of Medicine. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quattrocchi C.C., Piciucchi S., Sammarra M. Bone metastases in breast cancer: higher prevalence of osteosclerotic lesions. Radiologia Medica. 2007;112(7):1049–1059. doi: 10.1007/s11547-007-0205-x. [DOI] [PubMed] [Google Scholar]
- 25.Quattrocchi C.C., Santini D., Dell'aia P. A prospective analysis of CT density measurements of bone metastases after treatment with ZA. Skeletal Radiology. 2007;36(12):1121–1127. doi: 10.1007/s00256-007-0388-1. [DOI] [PubMed] [Google Scholar]
- 26.Amir E., Whyne C., Freedman O.C. Radiological changes following second-line ZA treatment in breast cancer patients with bone metastases. Clinical & Experimental Metastasis. 2009;26(5):479–484. doi: 10.1007/s10585-009-9247-x. [DOI] [PubMed] [Google Scholar]
- 27.Lipton A. Bisphosphonates and breast carcinoma: present and future. Cancer. 2000;88(Suppl. 12):3033–3037. doi: 10.1002/1097-0142(20000615)88:12+<3033::aid-cncr20>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Kohno N., Aogi K., Minami H. ZA significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. Journal of Clinical Oncology. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 29.Eastell R., Lang T., Boonen S. HORIZON Pivotal Fracture Trial. Effect of once-yearly ZA on the spine and hip as measured by quantitative computed tomography: results of the HORIZON Pivotal Fracture Trial. Osteoporos International. 2010;21(7):1277–1285. doi: 10.1007/s00198-009-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassiliou V., Kardamakis D., Kalogeropoulou C. Clinical and radiologic response in patients with bone metastases managed with combined radiotherapy and bisphosphonates. Journal of Surgical Oncology. 2008;98(7):567–578. doi: 10.1002/jso.21145. [DOI] [PubMed] [Google Scholar]
- 31.Reinbold W.D., Wannenmacher M., Hodapp N., Adler C.P. Osteodensitometry of vertebral metastases after radiotherapy using quantitative computed tomography. Skeletal Radiology. 1989;18(7):517–521. doi: 10.1007/BF00351751. [DOI] [PubMed] [Google Scholar]
- 32.Chow E., Holden L., Rubenstein J. CT evaluation of breast cancer patients with osteolytic bone metastases undergoing palliative radiotherapy: a feasibility study. Radiotherapy and Oncology. 2004;70(3):291–294. doi: 10.1016/j.radonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Reinbold W.D., Wannenmacher M., Hodapp N., Adler C.P. Osteodensitometry of vertebral metastases after radiotherapy using quantitative computed tomography. therapeutic response in skeletal metastases using dual-energy x-ray absorptiometry: a prospective feasibility study in breast cancer patients. Cancer Investigation. 1999;17(8):566–574. [Google Scholar]
- 34.Pataki A., Müller K., Green J.R., Ma Y.F., Li Q.N., Jee W.S. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anatomical Record. 1997;249(4):458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Greenspan S.L., Bhattacharya R.K., Sereika S.M., Brufsky A., Vogel V.G. Prevention of bone loss in survivors of breast cancer: a randomized, double-blind, placebo-controlled clinical trial. Journal of Clinical Endocrinology and Metabolism. 2007;92(1):131–136. doi: 10.1210/jc.2006-1272. [DOI] [PubMed] [Google Scholar]
- 36.Clemons M.J., Dranitsaris G., Ooi W.S. Phase II trial evaluating the palliative benefit of second-line ZA in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. Journal of Clinical Oncology. 2006;24(30):4895–4900. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 37.Smith M.R., Cook R.J., Coleman R. Predictors of skeletal complications in men with hormone-refractory metastatic prostate cancer. Urology. 2007;70(2):315–319. doi: 10.1016/j.urology.2007.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman R.E., Major P., Lipton A. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate ZA. Journal of Clinical Oncology. 2005;23(22):4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 39.Mountzios G., Terpos E., Syrigos K. Markers of bone remodeling and skeletal morbidity in patients with solid tumors metastatic to the skeleton receiving the biphosphonate ZA. Translational Research. 2010;155(5):247–255. doi: 10.1016/j.trsl.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Theoleyre S., Wittrant Y., Tat S.K., Fortun Y., Redini F., Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine & Growth Factor Reviews. 2004;15(6):457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Grimaud E., Soubigou L., Couillaud S. Receptor activator of nuclear factor kappaB ligand [RANKL]/osteoprotegerin [OPG] ratio is increased in severe osteolysis. American Journal of Pathology. 2003;163(5):2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke N.W., McClure J., George N.J. Morphometric evidence for bone resorption and replacement in prostate cancer. British Journal of Urology. 1991;68(1):74–80. doi: 10.1111/j.1464-410x.1991.tb15260.x. [DOI] [PubMed] [Google Scholar]
- 43.Garnero P., Buchs N., Zekri J., Rizzoli R., Coleman R.E., Delmas P.D. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. British Journal of Cancer. 2000;82(4):858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi B., Williams P.J., Niewolna M., Wang Y., Yoneda T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Research. 2002;62(3):917–923. [PubMed] [Google Scholar]
- 45.Roodman G.D. Mechanisms of bone metastases. New England Journal of Medicine. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]