Abstract
Nitrous oxide (N2O) is a powerful greenhouse gas and a key catalyst of stratospheric ozone depletion. Yet, little data exist about the sink and source terms of the production and reduction of N2O outside the well-known oxygen minimum zones (OMZ). Here we show the presence of functional marker genes for the reduction of N2O in the last step of the denitrification process (nitrous oxide reductase genes; nosZ) in oxygenated surface waters (180–250 O2 μmol.kg-1) in the south-eastern Indian Ocean. Overall copy numbers indicated that nosZ genes represented a significant proportion of the microbial community, which is unexpected in these oxygenated waters. Our data show strong temperature sensitivity for nosZ genes and reaction rates along a vast latitudinal gradient (32°S-12°S). These data suggest a large N2O sink in the warmer Tropical waters of the south-eastern Indian Ocean. Clone sequencing from PCR products revealed that most denitrification genes belonged to Rhodobacteraceae. Our work highlights the need to investigate the feedback and tight linkages between nitrification and denitrification (both sources of N2O, but the latter also a source of bioavailable N losses) in the understudied yet strategic Indian Ocean and other oligotrophic systems.
Introduction
Emissions of nitrous oxide (N2O) are of an eminent concern as the greenhouse warming power is 300 times stronger than CO2 [1,2]. N2O is the precursor of nitric oxide (NO) radicals and the single most destructive source of ozone-depleting [3]. Marine N2O production is predicted to increase under global warming scenarios including ocean acidification, sea surface warming and coastal eutrophication [1]. Yet limited data exists on potential feedback systems in the marine environment.
N2O production occurs during nitrification both during the formation of hydroxylamine from NH4+ and during the formation of NO3- from NO2- (Fig 1). The production of N2O is oxygen sensitive and nitrification rates are predicted to increase by the expansion of low oxygen waters [4]. Hypoxic and suboxic waters (<50 μmol.L-1 and <5 μmol.L-1 Voss et al. [5],[6]) have been expanding over a 50 year period in regions from the subarctic [7] to the tropical oceans [8] and are hot spots for nitrification and denitrification (fixed N removal) processes. In oxygen minimum zones (OMZ) such as those in the Arabian Sea [9,10], off the coast of Peru [11] and in the Benguela upwelling waters [4] the respiratory activity of heterotrophic denitrifying bacteria have been shown to contribute up to 35% of the N2O budget [12].
Fig 1. N–cycle emphasising the production of N2O through denitrification (grey arrows) and nitrification (red arrows).
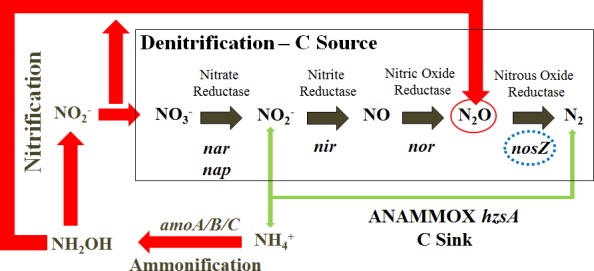
The production of N2 through anammox is presented with green arrows. Arrow width indicates suggested pathway importance in the south-eastern Indian Ocean. Marker genes for the different steps of the nitrogen cycle are in italics. The nitrous oxide reductase gene (nosZ) is highlighted with a dashed blue circle. Heterotrophic denitrification (nir/nor genes) is highlighted as a C source while the autotrophic anammox process (hzsA genes) is highlighted as a C sink. Note: dissimilatory reduction of NO2− to NH4+ and N2O is not presented on this figure. Figure adapted from Throbäck et al. [53]
Waite et al. [13] noted that the surface waters at low-latitude in the South Indian Ocean are depleted in oxygen (“NO” values as low as 175 μmol kg-1) compared to the open Atlantic and Pacific [14]. These lower oxygenated waters and their predicted expansion are a hot spot for nitrification [15] and consequently for N2O production. Nitrogen inputs, including the redistribution of fixed N2 through ammonification [16] and nitrification [17] have been shown to alter over relatively short timescales and fuel primary productivity on a regional scale in the south-eastern Indian Ocean [18,19]. The pivotal role of nitrification in this ocean basin leaves a big question mark on the magnitude of N2O production under current and future climate scenarios.
The only known metabolic pathway that converts the destructive N2O gas into the inert N2 gas is through the copper-containing enzyme nitrous oxide reductase (nosZ). The nosZ enzyme is found in most denitrifying organisms and also in a few non-denitrifying bacteria, such as Vibrio succinogenes [20]. If non-denitrifying bacteria are dominant then the south-eastern Indian Ocean can act as sink for N2O gas. Denitrification and the consequent bio-available N-losses can still occur when the former dominate the ecosystem. The significance of accurately quantifying nosZ genes are crucial to give us a better insights in the sinks and sources terms of N2O production and reduction.
Material and Methods
PCR, real-time PCR and clone library analysis
Samples were collected during two regional voyages in the south-eastern Indian Ocean aboard the RV Southern Surveyor (SS) in August (SS2012_V04) and September (2012 SS2012_T06; Fig 2). No additional specific permissions were required for any of the voyages. Associated biogeochemical meta and underway data can be downloaded from http://www.imos.org.au/. Associated biogeochemical meta and underway data can be downloaded from http://www.imos.org.au/. For DNA analysis, 2 L of seawater was filtered through Sterivex capsules (0.2 μm pore size) with a peristaltic pump. A modified organic (phenol:chloroform:isoamyl based) DNA extraction protocol was used alongside extraction columns from the PowerWater DNA isolation kit (Mo Bio Laboratories, USA). DNA extraction protocol has been described in Raes et al. [21]. Functional genes encoding for nitrous oxide reductase (nosZ) were quantified in technical triplicates by real-time PCR (qPCR) using a 7500 real-time PCR system (Applied Biosystems, Foster City, USA). nosZ genes were amplified using nosZ-F and nosZ 1622R primers [22]. The 15-μl reactions contained 0.15μL 100x BSA, 0.1 μL forward and reverse primers,7.5 μl 2 x SensiFAST™ master mix and 2 μL template DNA (final concentration between 10-25ng template DNA). Cycling conditions were 1 cycle at 96°C for 3 min, followed by 40 cycles at 96°C for 45s, followed by 30s at the annealing temperature of 55°C, followed by 30s at 72°C and 34s at the fluorescent acquisition temperature of 82°C. Dissociation curves were run at 95°C for 15s, followed by 1 min at 55°C and 15s at 95°C. Standards for nosZ gene quantification using qPCR were prepared by amplifying a constructed plasmid containing the respective gene fragment from an environmental clone. Standards for qPCR were made up using a serial dilution (10−1>>10−6 ng.μL-1) of known copies of PCR fragments.
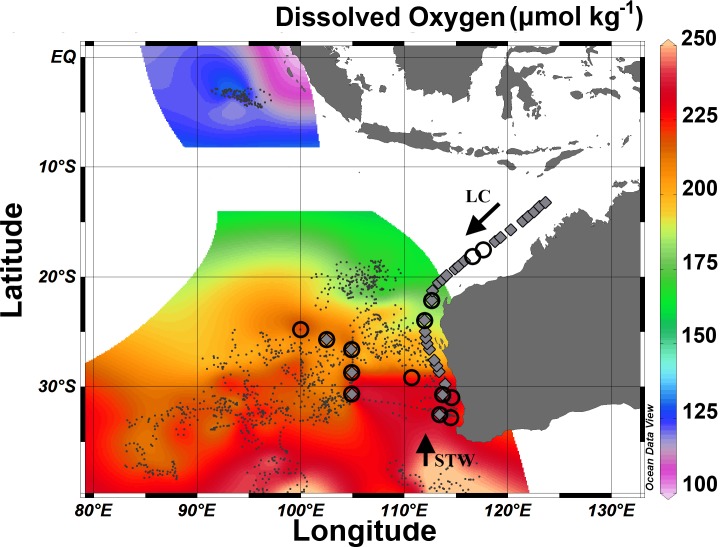
Fig 2. Spatial extent of oxygen concentrations (integrated from the surface to 200m depth).
Data (black squares) sourced from all available Argo floats with oxygen sensor in the region and cruise voyages conducted on the RV Southern Surveyor in 2010, 2011, 2012 and 2013. Low dissolved oxygen (<125 μmol.kg-1) stretches from the tropics to the subtropics. Black circles highlight sampling stations for functional N genes (nosZ and hzsA). Grey diamonds denote sampling station for heterotrophic microbial counts. Regional water masses Subtropical waters (STW) and Leeuwin Current waters (LC) are denoted by black arrows.
Polymerase chain reactions (PCR) for hzsA genes were set up using hzsA1597F and hzsA1857R [23]. Cycling conditions were 1 cycle at 96°C for 3 min, followed by 40 cycles at 96°C for 45s, followed by 30s at the annealing temperature of 55°C, followed by 30s at 72°C.
PCR products were purified from the reaction mix using magnetic beads (Agincourt, Beverly, MA, USA).Clone libraries were setup using the Invitrogen TOPO TA Cloning® Kit according to the manufacturer’s instructions. nosZ and hzsA gene fragments were sequenced using an ABI 3130XL genetic analyzer (Applied Biosystems) and aligned using Geneious® and Arb software package (http://www.arb-home.de/). Total microbial abundance was measured using a Beckman Coulter Gallios flow cytometer counter and has been described in detail in Raes et al. [21] (S1 Table).
Argo data
Oxygen data from 229 Argo float profiles were analysed from 2006 up to September 2014. These data were collected and made freely available by the International Argo Program and the national programs that contribute to it (http://www.argo.ucsd.edu, http://argo.jcommops.org). Argo floats numbers used were 4900483; 4900484; 4900485; 4900487; 4900487; 4900441; 5901310; 5901311; 5901313; 5901314; 5901369; 5901646; 5901697; 5902100; 5902105; 5903593. The Argo Program is part of the Global Ocean Observing System. Argo float data were sourced from the Integrated Marine Observing System (IMOS; http://imos.org.au/). Additional information on the oxygen data within the Argo data system can be found in the documentation of Processing Argo Oxygen data at the DAC level (http://www.argodatamgt.org/Documentation). For completion on the QC of the Argo data we note that Takeshita et al. [24] have reported a mean oxygen sensor error, relative to the World Ocean Atlas climatology data, of about 10 μmol.kg-1 in surface waters with sensors generally reading too low.
Results and Discussion
Here we present results describing the presence of the nitrous oxide reductase enzyme (nosZ) in cruise samples collected from well-oxygenated (180–250 μmol kg−1) photic zone waters in the south-eastern Indian Ocean. We detected the presence nosZ genes with an average concentrations of 1.9x105±1.31x105 nosZ copies mL-1 (±SD, n = 18). Our data compliment the nosZ gene copy concentrations ranging between ~1 x 103 to 1 x 105 copies mL-1 at the oxygenated surface waters and the deeper hypoxic waters in the Arabian Sea reported by Wyman et al. [25]. These combined results show a wide biogeographical distribution of nosZ genes in both south-eastern and western parts of the Indian Ocean. Bacterial clone sequencing of the nosZ genes in the south-eastern Indian Ocean showed an overall dominance of Rhodobacteraceae, with the majority of the sequences belonging to uncultured nitrous oxide reductase bacteria clones 3–57 nosZ, 31-nosZ, 29-nosZ-LZB and 39-nosZ-LZB.
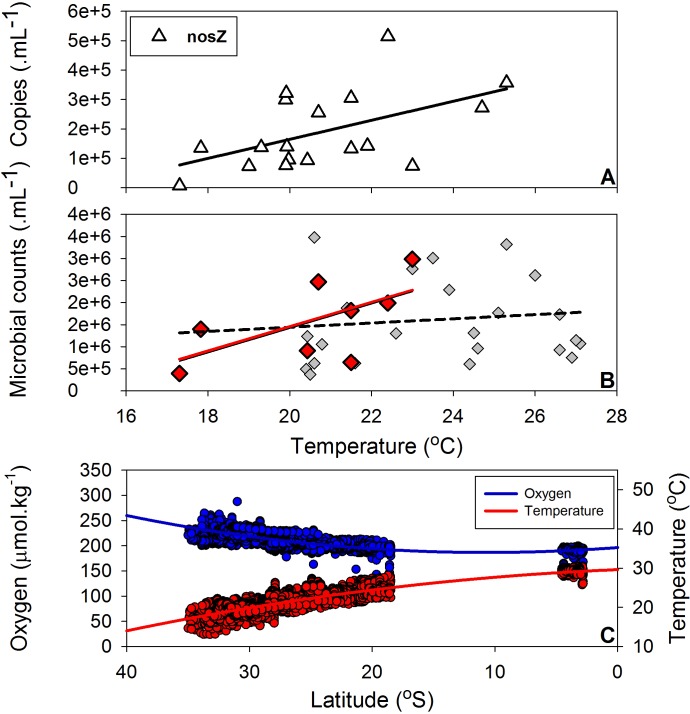
Our nosZ gene copy data correlated positively with increasing temperatures in the south-eastern Indian Ocean (Fig 3A). The total microbial abundance however showed no significant relationship with temperature and regionally averaged ~1.6 x 106 cells mL-1 (n = 31; Fig 3B, Fig 2 and S1 Table). Across the data set, a conservative estimate suggests that the organisms catalysing the reduction of N2O to the inert N2 gas could represent over 1% of the heterotrophic cell abundance (assuming up to 5 nosZ copies per cell), which is a significant proportion of the functional microbial community. Yet, these percentage drastically increase when we assume lower nosZ copy numbers per cell [26]. The positive environmental gradient of nosZ gene copy numbers and temperature, along with a decline in oxygen (Fig 3C and S1 Fig), suggests a preferential nitrous oxide reductase niche at higher temperatures and a potential and important sink for the harmful N2O gas in the warmer Tropical waters. Butler et al. [27] measured supersaturation of N2O around 20% with a maximum of 37%, near 8°S in the eastern Indian Ocean. The authors were able to link this supersaturation of N2O to upwelling events near the boundary of the equatorial counter current and the south equatorial current [28]. Dissolved N2O concentrations at depth in the eastern Indian Ocean have been reported by the former authors to be higher in the northern latitudes, where they have been shown to form a core of high N2O (~150-600m) north of the equator [27].
Fig 3. (A) Nitrous oxide reductase (nosZ) gene copies (copies mL-1) plotted against in situ temperature data (°C); nosZ: r2 = 0.27, slope = 32381 copies.°C-1, coefficient standard error = 13259, p = 0.027. (B) Microbial counts (cells mL-1) versus in situ temperature data; red diamonds denote stations where nosZ genes were measured.
These microbial counts suggest a positive correlation with temperature (r2 = 0.39, p = 0.098). Grey diamonds present regional microbial abundance but do not suggest a temperature correlation (r2 = 0.016, p = 0.5); see Fig 2 for spatial coverage. Fig 3 C presents a latitudinal relationship of oxygen (r2 = 0.6, p<0.0001) and temperature (r2 = 0.8, p<0.0001). Data are capped from the surface to 50 m depth and sourced from all available Argo floats through the Australian Argo DAC as part of the IMOS portal and cruise voyages conducted on the Southern Surveyor in 2010, 2011, 2012 and 2013.
Raes et al. [21] showed that along the same temperature gradient, shown here, the total dissolved inorganic nitrogen (DIN) pool increased significantly from the subtropics (35°S) to the tropics (12°S), and that the highest DIN concentrations occurred at the highest NH4 +:NO3 − ratios. Along this latitudinal gradient the authors also noted that the microbial community in the subtropics (cooler waters) were associated with deep nutrient fluxes (preferential NO3− and PO43− concentrations) and that the microbial community in the tropics (warmer waters) were linked with an increase in NH4+ and NO2- concentrations. The Tropical warmer waters are shown to be subjected to rapid recycling of organic matter where primary productivity is controlled via ammonification and nitrification within the euphotic zone [21]. The positive slope between nosZ copy numbers and increasing temperature indicates a feedback between the production (nitrification) and reduction of N2O. Large blooms of Trichodesmium also occur in these warm Tropical waters. Trichodesmium spp. has been suggested as a potential host for denitrifying bacteria in oxygenated waters of the Arabian Sea [25]. The low oxygen habitats within Trichodesmium colonies and other marine aggregates are interesting niches for cryptic N-cycling process, and are under explored habitats for a range of N cycling genes.
Pearce and Feng [29] confirmed a warming trend of ~0.02°C year–1 in the south-eastern Indian Ocean since 1951 from in situ temperature measurements at a coastal monitoring station on the Western Australian continental shelf. Marine heat waves such as the one recorded in 2011 in the south-eastern Indian Ocean are linked with El Niño/Southern Oscillations and are predicted to increase in frequency as a result of global warming [30,31], yet their ecological impacts towards primary productivity are not well understood [32]. The increasing nosZ gene abundance with temperature raises the question of whether increased nitrification rates and the consequent enhanced production of N2O through extreme climatic warming events and warming sea surface temperatures could positively be balanced by the reduction of N2O.
Oceanic oxygen concentrations impact a suite of biogeochemical cycling parameters that will influence N-cycling processes and carbon sequestration in the tropical oceans [33]. Thompson et al (2011) proposed that shallow (100-200m) lower dissolved oxygen layers (~180 μmol kg−1) in the south-eastern Indian Ocean ~32°S are physically continuous with similar layers as far north as 6°S in the North Indian Ocean (~50 μmol kg−1). The analysis of 229 vertical Argo floats profiles and in situ oxygen data from 4 voyages in the south-eastern Indian Ocean confirmed this conceptual model (Figs 2–4). The tight linkage between N and O is further described by the conservative water mass tracer “NO” [14] where respiration of organic matter and O2 consumption are combined into a single parameter. Waite et al. [13] and Raes et al. [19] proposed that these lower oxygenated waters are a hotspot for a diverse range of N cycling processes that play a vital role in providing necessary inorganic N compounds through ammonification and nitrification thereby sustaining primary productivity in these oligotrophic waters. In this data set we show that nosZ gene copy numbers correlated with these lower “NO” and lower oxygenated waters.
Fig 4. Spatial extent of shallow (100-200m) low dissolved oxygen layers stretching from the tropics to the subtropics.
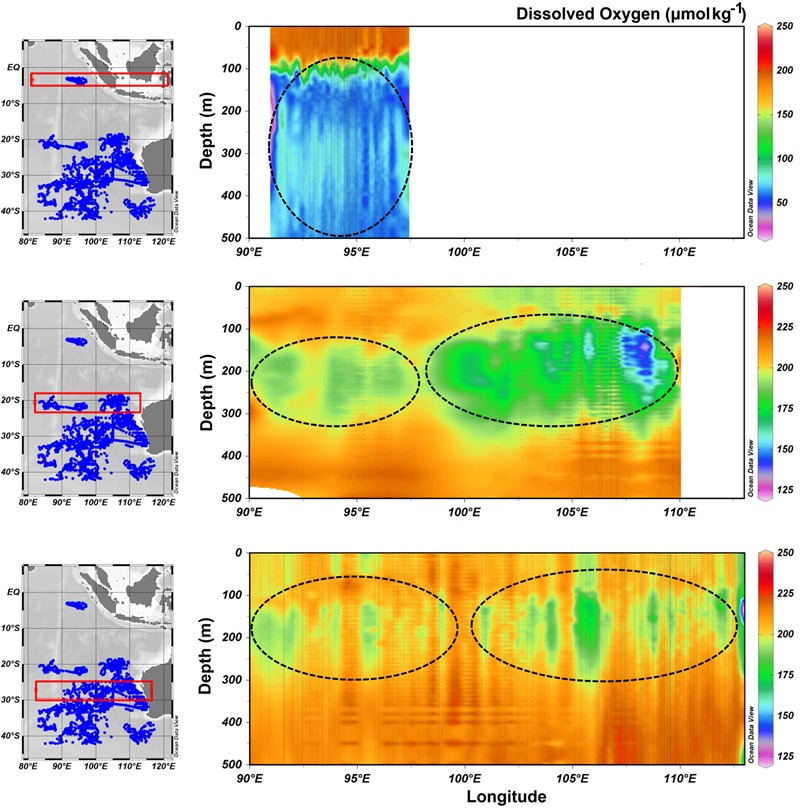
Data (blue dots) sourced from all available ARGO floats with oxygen in the region and cruise voyages conducted on the Southern Surveyor in 2010, 2011, 2012 and 2013. The red rectangle indicates the latitudinal area where a longitudinal slice through the water column has been analysed. Low dissolved oxygen layers (μmol kg-1) are highlighted by black circles.
To date, little information is available on the potential feedback between the reduction of N2O and denitrification (bio available N losses) outside oxygen minimum zones in this and many other regions of the global ocean. Nitrous oxide reductase does not catalyse denitrification, as denitrification is defined as the conversion of fixed/reactive nitrogen to N2O. We can therefore not assume a priori that organisms that carry nosZ genes also have other N reduction genes such as nir and nor genes. Yet, the dominance of Rhodobacteraceae in our samples suggests that the intermediate steps in the denitrification pathway (from NO3- to NO2- and NO) could also be present [34]. Li et al. [35] also shown a close coupling between NO3- deficits and active denitrification in the Indian Ocean. Many authors have highlighted a close coupling between N2 fixation and denitrification [36–38] while others have shown a significant correlation in the abundance of nosZ genes and denitrification rates [39–41]. The south-eastern Indian Ocean has relatively higher N2 fixation rates compared to the Atlantic and Pacific [21], which suggest a potential for denitrification rates. Furthermore, aerobic denitrification has theoretically been suggested [42] and empirically been shown [43] in the marine environment. A number of authors [44–46] have also highlighted active N losses associated with suboxic and anaerobic microhabitats, such as biofilms on marine aggregates in generally oxygenated waters. We therefore postulate that the south-eastern Indian Ocean is also subjected to denitrification (bio-available N-losses) in the photic zone. Most studies report a relationship between N loss gene copy numbers and N loss rates. In the OMZ waters of the Arabian Sea and the Peruvian upwelling, Jayakumar et al. [47] reported a range of denitrification gene copies from 2 to 6 x105 mL-1 which related to N2 loss rates up to 26 nmol.L-1 of N2 day-1. Although we lack rates for the above processes, the abundance of nosZ gene copies from our results are an interesting finding and the potential implications for bio available N-losses are worth further investigation.
It is crucial that we keep the biogeography principle in mind of: “Everything is everywhere but nature selects” [48]. Yet, we note that in all our samples we detected hydrazine synthase (hzsA) genes, which are the functional encoders for the anammox process. Clone sequencing from PCR products revealed that clones belonged to the genera Candidatus Scalindua, Jettenia and the species Brocadia fulgida. Uncultured anaerobic ammonium-oxidizing bacterial clones were also detected in our samples and closest sequence belonged to clones BS21, clone jwl2F/2R and clone I230-3. The finding of the anammox process in detectable quantities in these oxygenated waters is surprising and a novel finding.
Conclusion
The reduction of the potent greenhouse gas N2O to the inert N2 gas is of a global concern. Here we showed that the Tropical waters of the south-eastern Indian Ocean can act as a potential sink for N2O. Our data suggest a close coupling between nitrification (the production of N2O) and denitrifiers (the reduction of N2O). We propose that the lower oxygenated waters of the south-eastern Indian Ocean can both act as a sink and a source of N2O emissions. As a new testable hypothesis we suggest that we are underestimating N-losses in this and many other marine ecosystems. Future work which would allow us to fully understand the ecological role of the nosZ gene and even anammox bacteria in the lower oxygenated surface water of the south eastern Indian Ocean could include stable isotope probing [49], reverse transcriptive activities of nosZ genes [50] and quantification of nitrification and denitrification rates [51]. The inclusion of stations from the OMZ in the Arabian Sea would allow a greater understanding of the sink and source terms of N2O [52] and nosZ gene activity under aerobic and anaerobic environments within the same Ocean basin. In summary our data highlights the need to further investigate key process in the marine nitrogen cycle in this understudied yet strategic Ocean basin as sink and source terms are predicted to alter under global change scenarios such as ocean warming and decreasing oxygen concentrations.
Supporting Information
See Fig 1 for CTD stations. Note: Elevated NO2_ concentrations up to 0.3 μmol.L-1 in relative oxygenated surface waters.
(TIF)
(DOCX)
Acknowledgments
We thank the captain and Marine National Facility crew of the RV. Southern Surveyor for their technical assistance while at sea. We would like to thank the anonymous reviewers for their time and insights.
Data Availability
Physical, biogeochemical data and metadata to support this article can be accessed through the Integrated Marine Observing System (IMOS http://www.imos.org.au/). ARGO float data are available by the International Argo Program and the national programs that contribute to it (http://www.argo.ucsd.edu, http://argo.jcommops.org). Microbial data data are presented in S1 Table.
Funding Statement
E. J. Raes received an Australian Postgraduate scholarship from the University of Western Australia and a CSIRO Wealth from Oceans postgraduate top-up scholarship.
References
- 1.Codispoti LA (2010) Interesting times for marine N2O. Science 327: 1339–1340. 10.1126/science.1184945 [DOI] [PubMed] [Google Scholar]
- 2.Naqvi S, Bange HW, Farias L, Monteiro P, Scranton M, Zhang J (2010) Marine hypoxia/anoxia as a source of CH4 and N2O. Biogeosciences 7: 2159–2190. [Google Scholar]
- 3.Montzka S, Reimann S, Engel A, Krüger K, O’Doherty S, Sturges W, et al. (2011) Ozone depleting substances (ODS’s) and related chemicals, Chapter 1 in: scientific assessment of ozone depletion: 2010. Global Ozone Research and Monitoring Project World Meteorological Organization, Geneva, Switzerland: 516. [Google Scholar]
- 4.Kuypers MM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R, et al. (2005) Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proceedings of the National Academy of Sciences of the United States of America 102: 6478–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss M, Bange HW, Dippner JW, Middelburg JJ, Montoya JP, Ward B (2013) The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change. Phil Trans R Soc B: Biological Sciences 368: 20130121 10.1098/rstb.2013.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naqvi S, Naik H, Narvekar P (2003) The Arabian Sea. Biogeochemistry. [Google Scholar]
- 7.Whitney FA, Freeland HJ, Robert M (2007) Persistently declining oxygen levels in the interior waters of the eastern subarctic Pacific. Prog Oceanogr 75: 179–199. [Google Scholar]
- 8.Stramma L, Schmidtko S, Levin LA, Johnson GC (2010) Ocean oxygen minima expansions and their biological impacts. Deep Sea Research Part I: Oceanographic Research Papers 57: 587–595. [Google Scholar]
- 9.Ward BB, Devol AH, Rich JJ, Chang BX, Bulow SE, Naik H, et al. (2009) Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 461: 78–81. 10.1038/nature08276 [DOI] [PubMed] [Google Scholar]
- 10.Villanueva l, Speth DR, Vanalen T, Hoischen A, Jetten M (2014) Shotgun metagenomic data reveals signifcant abundance but low diversity of “Candidatus Scalindua” marine anammox bacteria in the Arabian Sea oxygen minimum zone. Frontiers in Microbiology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, et al. (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci 106: 4752–4757. 10.1073/pnas.0812444106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freing A, Wallace DW, Bange HW (2012) Global oceanic production of nitrous oxide. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1245–1255. 10.1098/rstb.2011.0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waite AM, Rossi V, Roughan M, Tilbrook B, Thompson PA, Feng M, et al. (2013) Formation and maintenance of high-nitrate, low pH layers in the eastern Indian Ocean and the role of nitrogen fixation. Biogeosciences 10: 5691–5702. [Google Scholar]
- 14.Broecker WS (1974) “NO”, a conservative water-mass tracer. Earth Planet Sci Lett 23: 100–107. [Google Scholar]
- 15.Thompson P, Wild-Allen K, Lourey M, Rousseaux C, Waite A, Feng M, et al. (2011) Nutrients in an oligotrophic boundary current: Evidence of a new role for the Leeuwin Current. Prog Oceanogr 91: 345–359. [Google Scholar]
- 16.Bouskill NJ, Eveillard D, Chien D, Jayakumar A, Ward BB (2012) Environmental factors determining ammonia-oxidizing organism distribution and diversity in marine environments. Environmental microbiology 14: 714–729. 10.1111/j.1462-2920.2011.02623.x [DOI] [PubMed] [Google Scholar]
- 17.Yool A (2011) Modeling the Role of Nitrification in Open Ocean Productivity and the Nitrogen Cycle. Methods in Enzymology 486: 3 10.1016/B978-0-12-381294-0.00001-8 [DOI] [PubMed] [Google Scholar]
- 18.Waite AM, Beckley LE, Guidi L, Landrum J, Holliday D, Montoya J, et al. (2015) Cross-shelf transport, oxygen depletion and nitrate release within a forming mesoscale eddy in the eastern Indian Ocean. Limnol Oceanogr (in press). [Google Scholar]
- 19.Raes EJ, Thompson PA, McInnes AS, Nguyen HM, Hardman‐Mountford N, Waite AM (2015) Sources of new nitrogen in the Indian Ocean. Global Biogeochemical Cycles. [Google Scholar]
- 20.Yoshinari T (1980) N2O reduction by Vibrio succinogenes. Applied and Environmental Microbiology 39: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raes EJ, Waite AM, McInnes AS, Olsen H, Nguyen HM, Hardman-Mountford N, et al. (2014) Changes in latitude and dominant diazotrophic community alter N2 fixation. Mar Ecol Prog Ser. [Google Scholar]
- 22.Kloos K, Mergel A, Rösch C, Bothe H (2001) Denitrification within the genus Azospirillum and other associative bacteria. Functional Plant Biology 28: 991–998. [Google Scholar]
- 23.Harhangi HR, Le Roy M, van Alen T, Hu B-l, Groen J, Kartal B, et al. (2012) Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria. Applied and Environmental Microbiology 78: 752–758. 10.1128/AEM.07113-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshita Y, Martz TR, Johnson KS, Plant JN, Gilbert D, Riser SC, et al. (2013) A climatology‐based quality control procedure for profiling float oxygen data. Journal of Geophysical Research: Oceans 118: 5640–5650. [Google Scholar]
- 25.Wyman M, Hodgson S, Bird C (2013) Denitrifying alphaproteobacteria from the Arabian Sea that express nosZ, the gene encoding nitrous oxide reductase, in oxic and suboxic waters. Applied and Environmental Microbiology 79: 2670–2681. 10.1128/AEM.03705-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61: 533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler JH, Elkins JW, Thompson TM, Egan KB (1989) Tropospheric and dissolved N2O of the west Pacific and east Indian Oceans during the El Nino Southern Oscillation event of 1987. Journal of Geophysical Research: Atmospheres (1984–2012) 94: 14865–14877. [Google Scholar]
- 28.Wyrtki K (1973) Physical oceanography of the Indian Ocean The biology of the Indian Ocean: Springer; pp. 18–36. [Google Scholar]
- 29.Pearce A, Feng M (2007) Observations of warming on the Western Australian continental shelf. Marine and Freshwater Research 58: 914–920. [Google Scholar]
- 30.Pearce A, Feng M (2011) The" marine heat wave" off Western Australia during the summer of 2010/11: Western Australian Fisheries and Marine Research Laboratories. [Google Scholar]
- 31.Cai W, Borlace S, Lengaigne M, van Rensch P, Collins M, Vecchi G, et al. (2014) Increasing frequency of extreme El Nino events due to greenhouse warming. Nature Clim Change 4: 111–116. [Google Scholar]
- 32.Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, de Bettignies T, et al. (2012) An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change. [Google Scholar]
- 33.Stramma L, Johnson GC, Sprintall J, Mohrholz V (2008) Expanding oxygen-minimum zones in the tropical oceans. Science 320: 655 10.1126/science.1153847 [DOI] [PubMed] [Google Scholar]
- 34.Sabaty M, Schwintner C, Cahors S, Richaud P, Verméglio A (1999) Nitrite and nitrous oxide reductase regulation by nitrogen oxides in Rhodobacter sphaeroides f. sp. denitrificans IL106. Journal of Bacteriology 181: 6028–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y-H, Menviel L, Peng T-H (2006) Nitrate deficits by nitrification and denitrification processes in the Indian Ocean. Deep Sea Research Part I: Oceanographic Research Papers 53: 94–110. [Google Scholar]
- 36.Deutsch C, Gruber N, Key RM, Sarmiento JL, Ganachaud A (2001) Denitrification and N2 fixation in the Pacific Ocean. Global Biogeochem Cycles 15: 483–506. [Google Scholar]
- 37.Deutsch C, Sarmiento JL, Sigman DM, Gruber N, Dunne JP (2007) Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445: 163–167. [DOI] [PubMed] [Google Scholar]
- 38.Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451: 293–296. 10.1038/nature06592 [DOI] [PubMed] [Google Scholar]
- 39.Rich J, Heichen R, Bottomley P, Cromack K, Myrold D (2003) Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Applied and Environmental Microbiology 69: 5974–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iribar A, Hallin S, Pérez JMS, Enwall K, Poulet N, Garabétian F (2015) Potential denitrification rates are spatially linked to colonization patterns of nosZ genotypes in an alluvial wetland. Ecological Engineering 80: 191–197. [Google Scholar]
- 41.Ducey T, Miller J, Lang M, Szogi A, Hunt P, Fenstermacher D, et al. (2015) Soil Physicochemical Conditions, Denitrification Rates, and Abundance in North Carolina Coastal Plain Restored Wetlands. Journal of Environmental Quality 44: 1011–1022. 10.2134/jeq2014.09.0403 [DOI] [PubMed] [Google Scholar]
- 42.Codispoti L (2007) An oceanic fixed nitrogen sink exceeding 400 Tg N a? 1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4: 233–253. [Google Scholar]
- 43.Gao H, Schreiber F, Collins G, Jensen MM, Kostka JE, Lavik G, et al. (2010) Aerobic denitrification in permeable Wadden Sea sediments. The ISME journal 4: 417–426. 10.1038/ismej.2009.127 [DOI] [PubMed] [Google Scholar]
- 44.Klawonn I, Bonaglia S, Bruchert V, Ploug H (2015) Aerobic and anaerobic nitrogen transformation processes in N2-fixing cyanobacterial aggregates. ISME J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ploug H (2001) Small‐scale oxygen fluxes and remineralization in sinking aggregates. Limnology and Oceanography 46: 1624–1631. [Google Scholar]
- 46.Woebken D, Fuchs BM, Kuypers MM, Amann R (2007) Potential interactions of particle-associated anammox bacteria with bacterial and archaeal partners in the Namibian upwelling system. Applied and Environmental Microbiology 73: 4648–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayakumar A, Naqvi S, Ward BB (2009) Distribution and relative quantification of key genes involved in fixed nitrogen loss from the Arabian Sea oxygen minimum zone. Indian Ocean Biogeochemical Processes and Ecological Variability: 187–203. [Google Scholar]
- 48.O' Malley MA (2007) The nineteenth century roots of'everything is everywhere'. Nature Reviews Microbiology 5: 647–651. [DOI] [PubMed] [Google Scholar]
- 49.Radajewski S, Ineson P, Parekh NR, Murrell JC (2000) Stable-isotope probing as a tool in microbial ecology. Nature 403: 646–649. [DOI] [PubMed] [Google Scholar]
- 50.Nogales B, Timmis KN, Nedwell DB, Osborn AM (2002) Detection and diversity of expressed denitrification genes in estuarine sediments after reverse transcription-PCR amplification from mRNA. Applied and Environmental Microbiology 68: 5017–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naqvi S, Jayakumar D, Narvekar P, Naik H, Sarma V, D'souza W, et al. (2000) Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408: 346–349. [DOI] [PubMed] [Google Scholar]
- 52.Naqvi S, Yoshinari T, Jayakumar D, Altabet M, Narvekar P, Devol A, et al. (1998) Budgetary and biogeochemical implications of N2O isotope signatures in the Arabian Sea. Nature 394: 462–464. [Google Scholar]
- 53.Throbäck IN, Enwall K, Jarvis Å, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology 49: 401–417. 10.1016/j.femsec.2004.04.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See Fig 1 for CTD stations. Note: Elevated NO2_ concentrations up to 0.3 μmol.L-1 in relative oxygenated surface waters.
(TIF)
(DOCX)
Data Availability Statement
Physical, biogeochemical data and metadata to support this article can be accessed through the Integrated Marine Observing System (IMOS http://www.imos.org.au/). ARGO float data are available by the International Argo Program and the national programs that contribute to it (http://www.argo.ucsd.edu, http://argo.jcommops.org). Microbial data data are presented in S1 Table.